Systems Vaccinology for a Live Attenuated Tularemia Vaccine Reveals Unique Transcriptional Signatures That Predict Humoral and Cellular Immune Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Used and Specimen Collection
2.2. Microarray Experiments
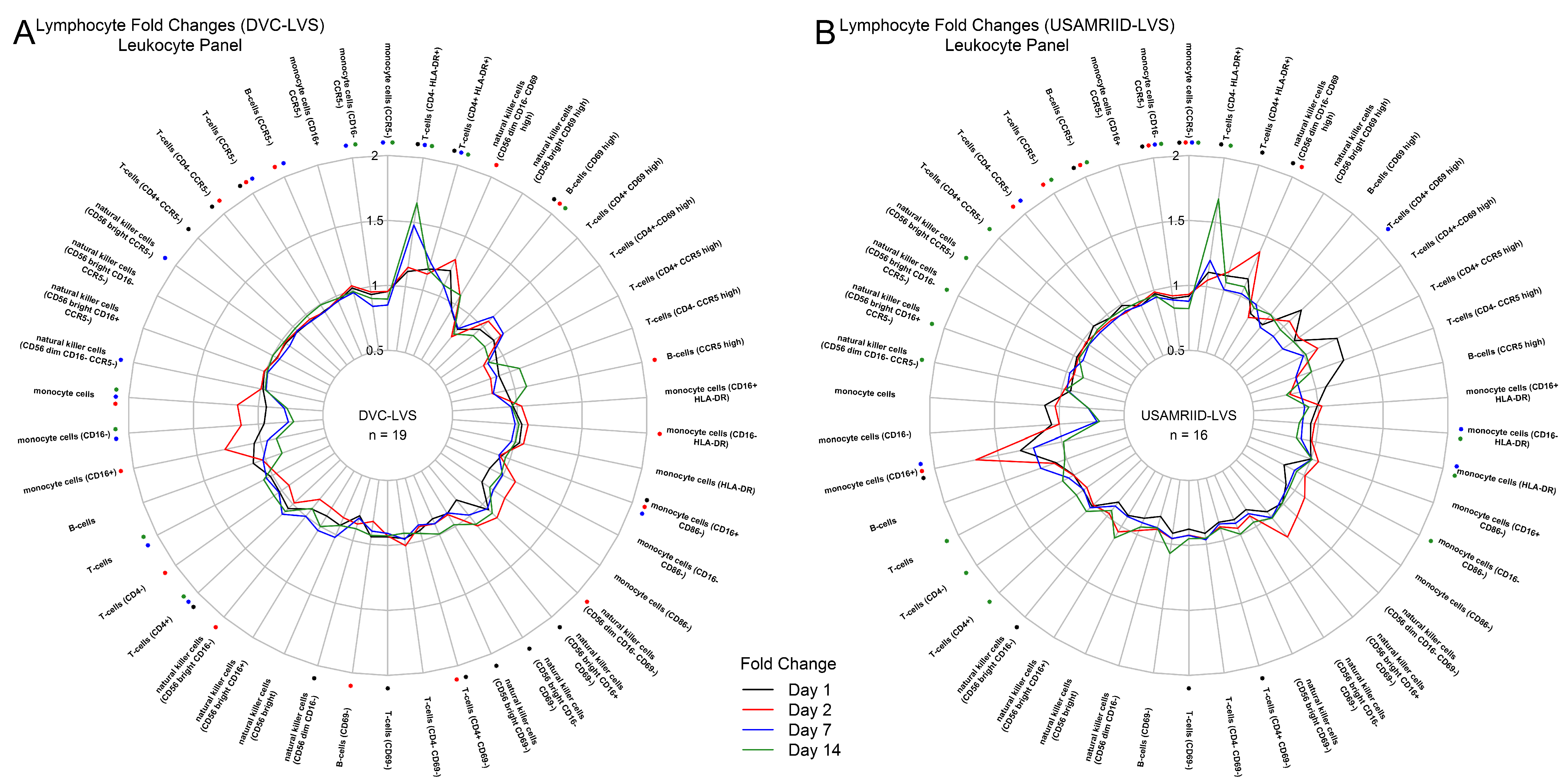
2.3. Cellular Phenotyping
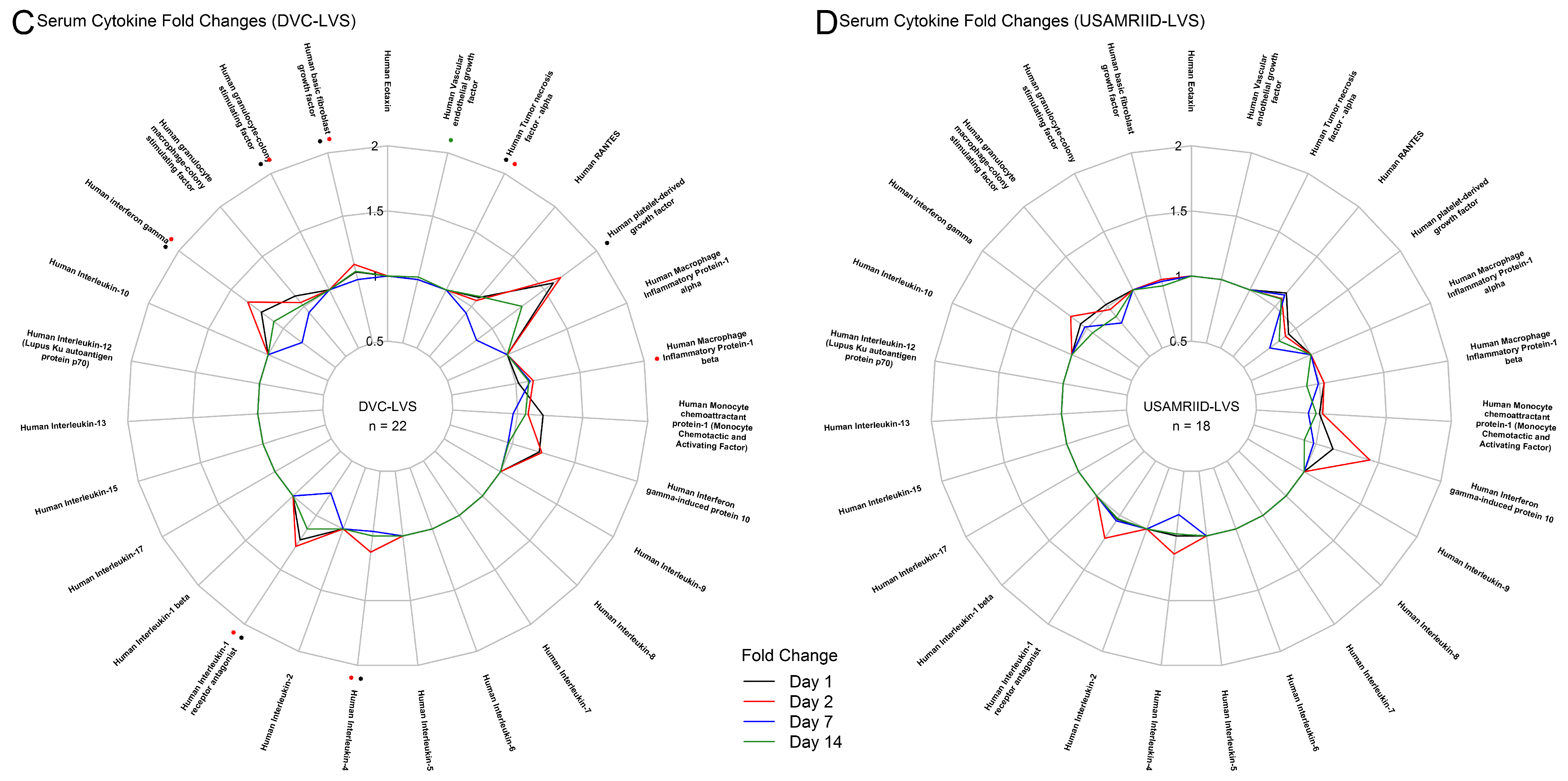
2.4. Plasma Cytokine Assay
2.5. Microagglutination Antibody Assay
2.6. Statistical Analyses
3. Results
3.1. Tularemia Vaccination Induced Peak Innate Responses at Day 2 and Peak Adaptive Responses at Day 14
3.2. DVC-LVS and USAMRIID-LVS Elicited Similar Gene Expression Profiles
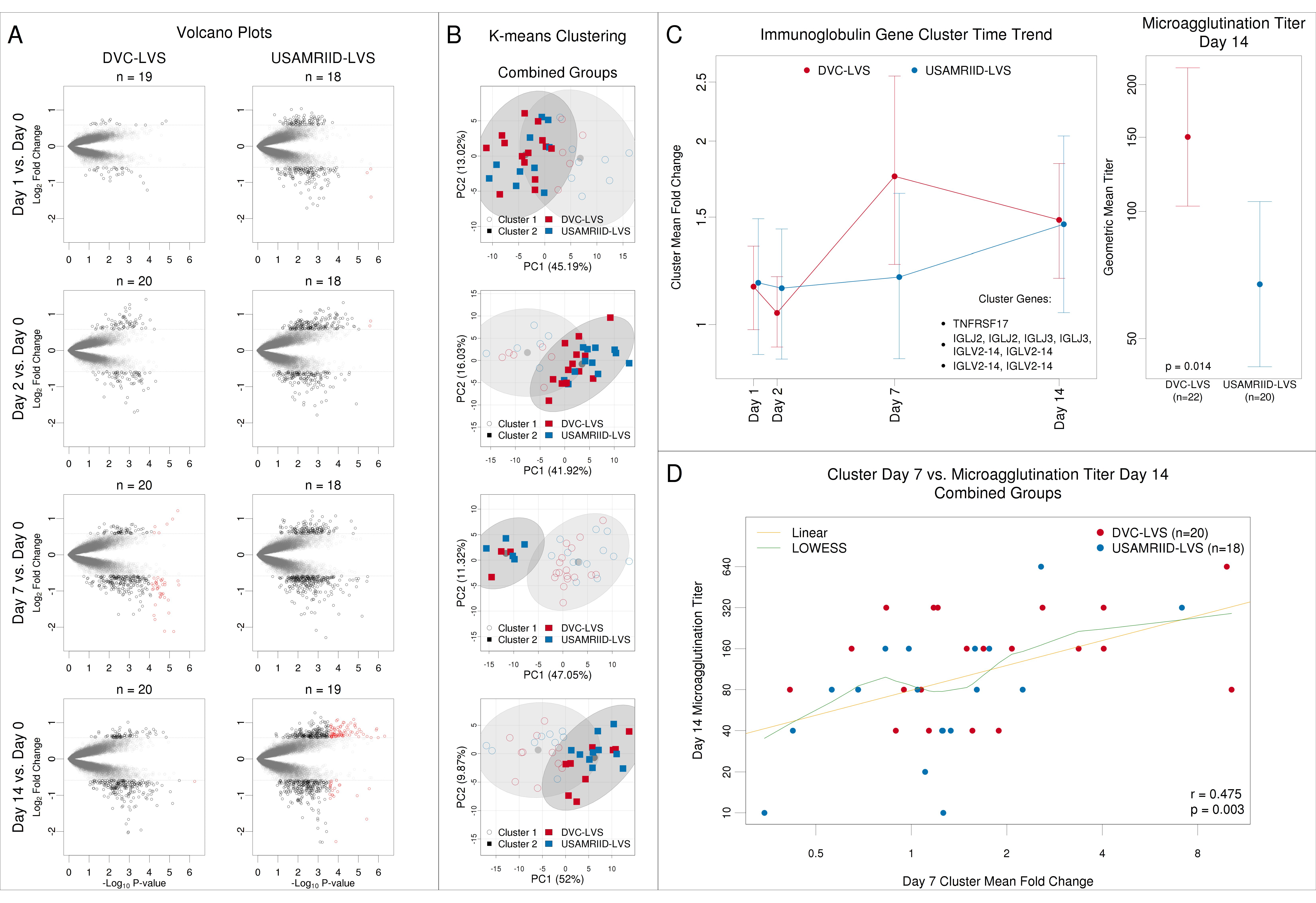
3.3. Gene Clustering Analysis Revealed Co-Expressed Genes That Were More Highly Upregulated at Day 7 in the DVC-LVS Group and Correlated with Increased Antibody Responses at Day 14
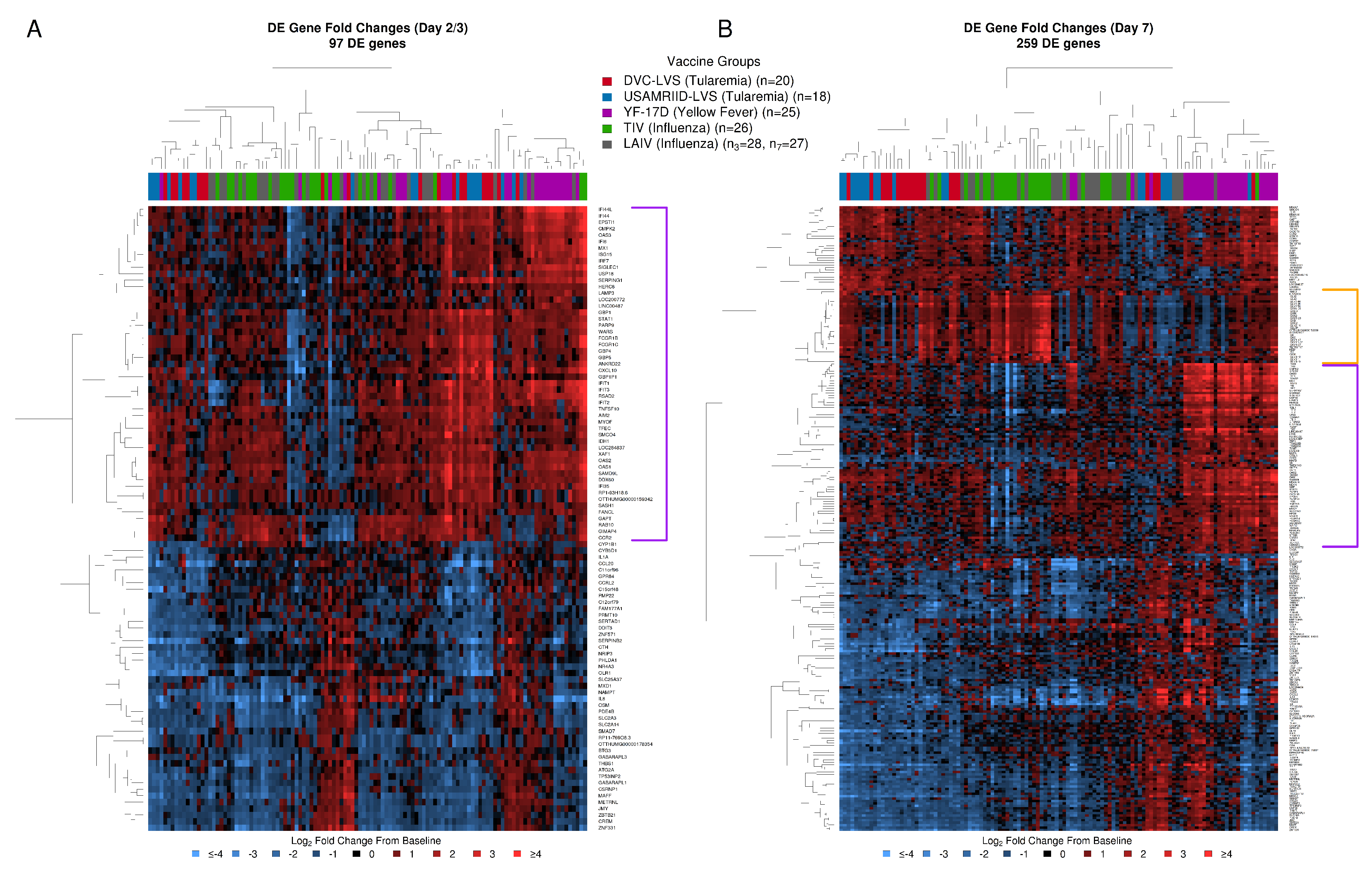
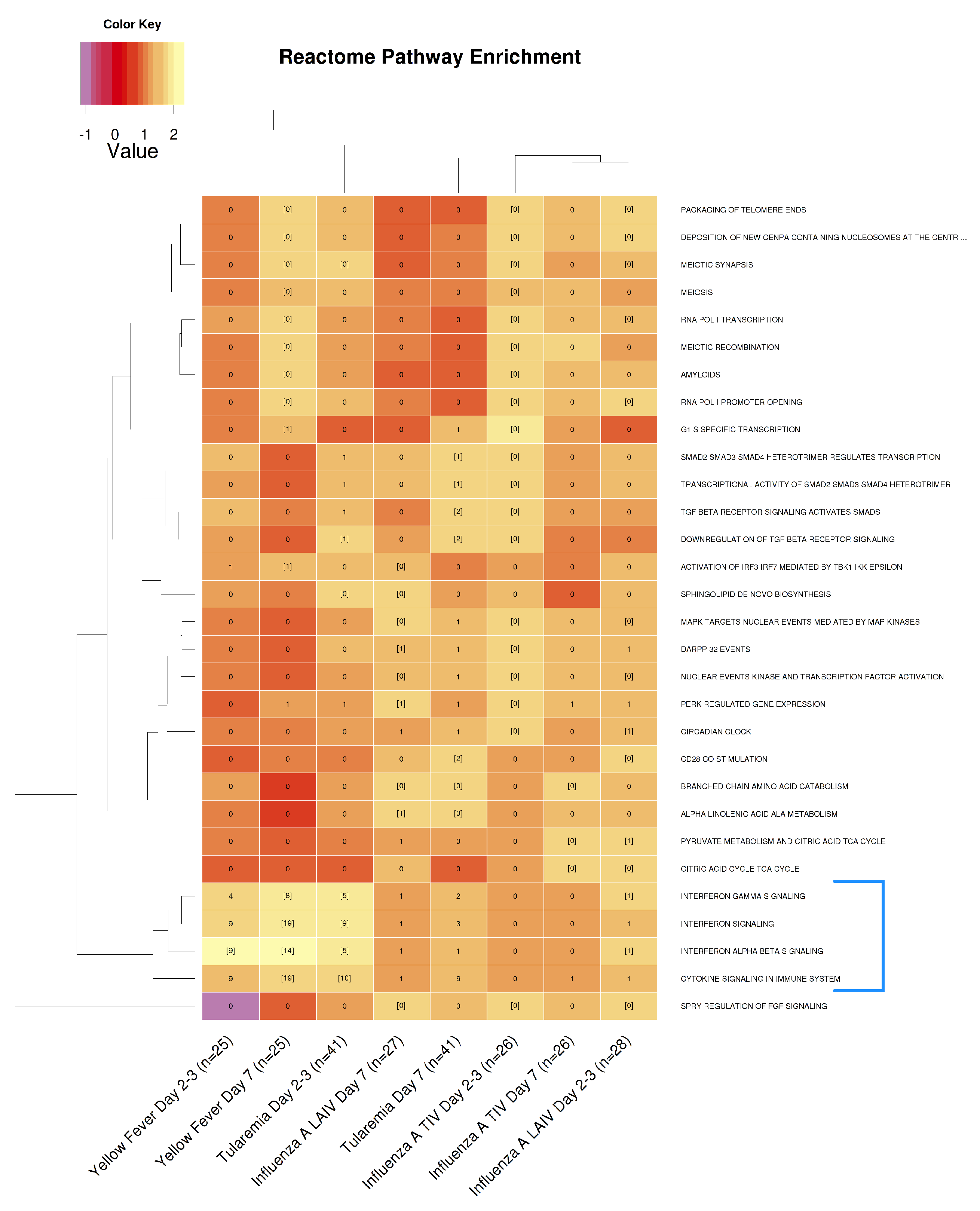
3.4. Comparative Analysis of Gene Expression Patterns Following Tularemia, Yellow Fever, and Influenza Vaccination
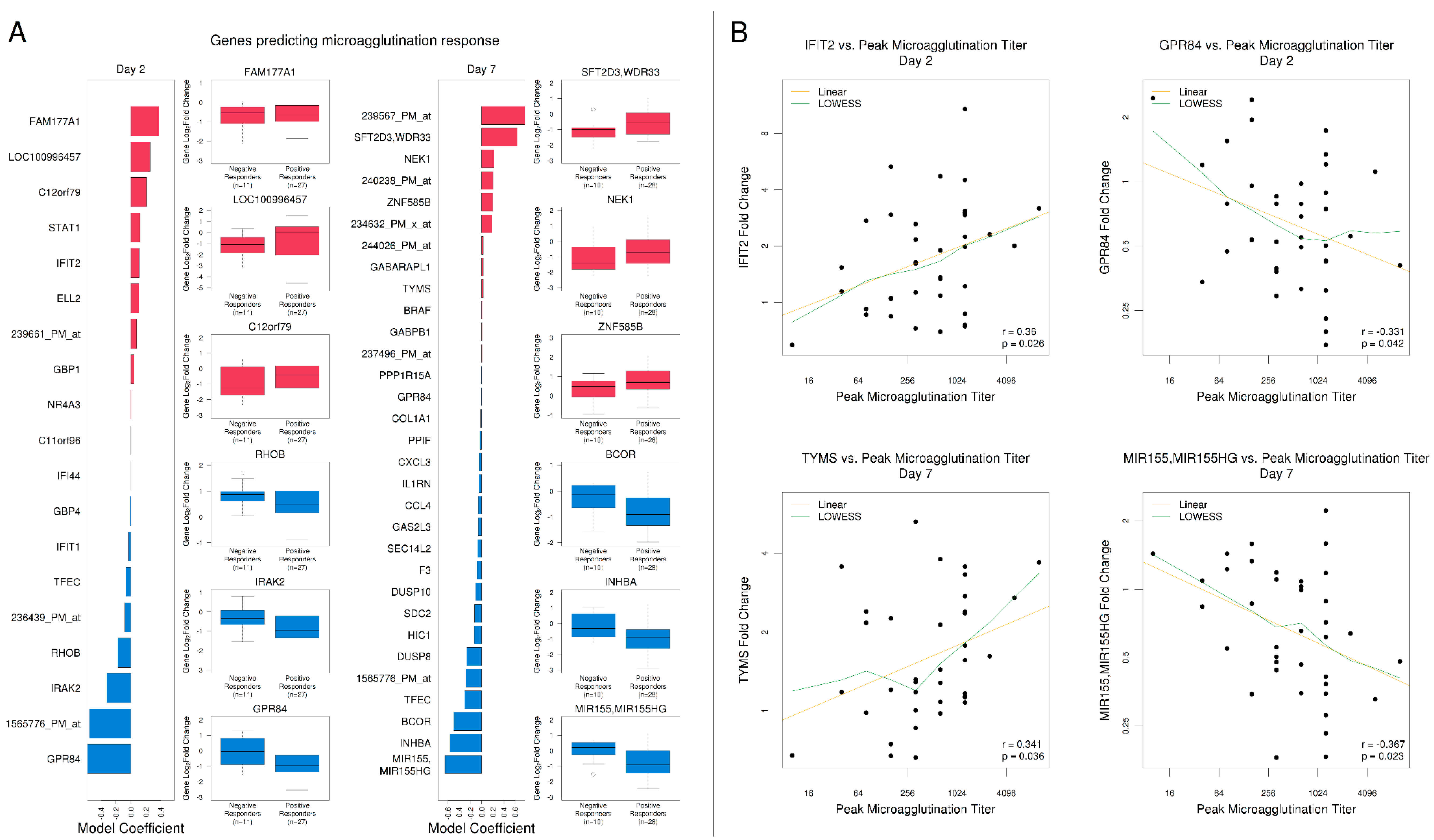
3.5. Gene Expression Signatures Predicted Antibody Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Francis, E. Tularemia. JAMA 1925, 84, 1243–1250. [Google Scholar] [CrossRef]
- Leber, A. Tularemia-Francisella tularensis. In Clinical Microbiology Procedures Handbook, Fourth Edition; ASM Press: Washington, DC, USA, 2016. [Google Scholar]
- Elkins, K.L.; Cowley, S.C.; Bosio, C.M. Innate and adaptive immunity to Francisella. Ann. N. Y. Acad. Sci. 2007, 1105, 284–324. [Google Scholar] [CrossRef] [PubMed]
- Kirimanjeswara, G.S.; Olmos, S.; Bakshi, C.S.; Metzger, D.W. Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol. Rev. 2008, 225, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Plzakova, L.; Kubelkova, K.; Krocova, Z.; Zarybnicka, L.; Sinkorova, Z.; Macela, A. B cell subsets are activated and produce cytokines during early phases of Francisella tularensis LVS infection. Microb. Pathog. 2014, 75, 49–58. [Google Scholar] [CrossRef] [PubMed]
- De Pascalis, R.; Mittereder, L.; Chou, A.Y.; Kennett, N.J.; Elkins, K.L. Francisella tularensis Vaccines Elicit Concurrent Protective T- and B-Cell Immune Responses in BALB/cByJ Mice. PLoS ONE 2015, 10, e0126570. [Google Scholar] [CrossRef] [PubMed]
- Wayne Conlan, J.; Shen, H.; KuoLee, R.; Zhao, X.; Chen, W. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an αβ T cell- and interferon gamma- dependent mechanism. Vaccine 2005, 23, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.M.; Crane, D.D.; Wehrly, T.D.; Fletcher, J.R.; Jones, B.D.; Bosio, C.M. Inclusion of Epitopes That Expand High-Avidity CD4+ T Cells Transforms Subprotective Vaccines to Efficacious Immunogens against Virulent Francisella tularensis. J. Immunol. 2016, 197, 2738. [Google Scholar] [CrossRef]
- Sjostedt, A.; Eriksson, M.; Sandstrom, G.; Tarnvik, A. Various membrane proteins of Francisella tularensis induce interferon-gamma production in both CD4+ and CD8+ T cells of primed humans. Immunology 1992, 76, 584–592. [Google Scholar]
- Cross, A.S.; Calia, F.M.; Edelman, R. From rabbits to humans: The contributions of Dr. Theodore E. Woodward to tularemia research. Clin. Infect. Dis. 2007, 45 (Suppl. 1), S61–S67. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Atmar, R.L.; Patel, S.M.; Wells, J.M.; Cate, T.; Ho, M.; Guo, K.; Pasetti, M.F.; Lewis, D.E.; Sztein, M.B.; et al. Safety, reactogenicity and immunogenicity of Francisella tularensis live vaccine strain in humans. Vaccine 2009, 27, 4905–4911. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Stapleton, J.T.; Keitel, W.A.; Frey, S.E.; Chen, W.H.; Rouphael, N.; Edupuganti, S.; Beck, A.; Winokur, P.L.; El Sahly, H.M.; et al. Tularemia vaccine: Safety, reactogenicity, “Take” skin reactions, and antibody responses following vaccination with a new lot of the Francisella tularensis live vaccine strain - A phase 2 randomized clinical Trial. Vaccine 2017, 35, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Pasetti, M.F.; Cuberos, L.; Horn, T.L.; Shearer, J.D.; Matthews, S.J.; House, R.V.; Sztein, M.B. An improved Francisella tularensis live vaccine strain (LVS) is well tolerated and highly immunogenic when administered to rabbits in escalating doses using various immunization routes. Vaccine 2008, 26, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.L.; Brittingham, K.C.; Hepburn, M.J.; Martin, J.W.; Petitt, P.L.; Pittman, P.R.; Bavari, S. Dominance of human innate immune responses in primary Francisella tularensis live vaccine strain vaccination. J. Allergy Clin. Immunol. 2006, 117, 1186–1188. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.L.; Brittingham, K.C.; Porter, M.W.; Hepburn, M.J.; Petitt, P.L.; Pittman, P.R.; Bavari, S. Transcriptome analysis of human immune responses following live vaccine strain (LVS) Francisella tularensis vaccination. Mol. Immunol. 2007, 44, 3173–3184. [Google Scholar] [CrossRef][Green Version]
- Akondy, R.S.; Monson, N.D.; Miller, J.D.; Edupuganti, S.; Teuwen, D.; Wu, H.; Quyyumi, F.; Garg, S.; Altman, J.D.; Del Rio, C.; et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J. Immunol. 2009, 183, 7919–7930. [Google Scholar] [CrossRef]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.; Marzolf, B.; et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar] [CrossRef]
- Nakaya, H.I.; Wrammert, J.; Lee, E.K.; Racioppi, L.; Marie-Kunze, S.; Haining, W.N.; Means, A.R.; Kasturi, S.P.; Khan, N.; Li, G.M.; et al. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 2011, 12, 786–795. [Google Scholar] [CrossRef]
- Li, S.; Rouphael, N.; Duraisingham, S.; Romero-Steiner, S.; Presnell, S.; Davis, C.; Schmidt, D.S.; Johnson, S.E.; Milton, A.; Rajam, G.; et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat. Immunol. 2014, 15, 195–204. [Google Scholar] [CrossRef]
- Sharma, M.; Krammer, F.; Garcia-Sastre, A.; Tripathi, S. Moving from Empirical to Rational Vaccine Design in the ’Omics’ Era. Vaccines 2019, 7, 89. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Rouphael, N.; Xu, Y.; Natrajan, M.S.; Beck, A.; Hart, M.; Feldhammer, M.; Feldpausch, A.; Hill, C.; Wu, H.; et al. Innate, T- and B-Cell Responses in Acute Human Zika Patients. Clin. Infect. Dis. 2018, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.D.; Swcf, A.J.; Dabney, A.; Robinson, D. Qvalue: Q-Value Estimation for False Discovery Rate Control; R Package Version 2018; The R Foundation: Vienna, Austria, 2018. [Google Scholar]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Tope, G.; Gillespie, M.; Vastrik, I.; D’Eustachio, P.; Schmidt, E.; de Bono, B.; Jassal, B.; Gopinath, G.R.; Wu, G.R.; Matthews, L.; et al. Reactome: A knowledgebase of biological pathways. Nucleic Acids Res. 2005, 33, D428–D432. [Google Scholar] [CrossRef]
- Querec, T.; Bennouna, S.; Alkan, S.; Laouar, Y.; Gorden, K.; Flavell, R.; Akira, S.; Ahmed, R.; Pulendran, B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 2006, 203, 413–424. [Google Scholar] [CrossRef]
- O’Gorman, W.E.; Huang, H.; Wei, Y.L.; Davis, K.L.; Leipold, M.D.; Bendall, S.C.; Kidd, B.A.; Dekker, C.L.; Maecker, H.T.; Chien, Y.H.; et al. The Split Virus Influenza Vaccine rapidly activates immune cells through Fcgamma receptors. Vaccine 2014, 32, 5989–5997. [Google Scholar] [CrossRef]
- Zhu, W.; Higgs, B.W.; Morehouse, C.; Streicher, K.; Ambrose, C.S.; Woo, J.; Kemble, G.W.; Jallal, B.; Yao, Y. A whole genome transcriptional analysis of the early immune response induced by live attenuated and inactivated influenza vaccines in young children. Vaccine 2010, 28, 2865–2876. [Google Scholar] [CrossRef]
- Vestal, D.J.; Jeyaratnam, J.A. The guanylate-binding proteins: Emerging insights into the biochemical properties and functions of this family of large interferon-induced guanosine triphosphatase. J. Interferon Cytokine Res. 2011, 31, 89–97. [Google Scholar] [CrossRef]
- Wallet, P.; Benaoudia, S.; Mosnier, A.; Lagrange, B.; Martin, A.; Lindgren, H.; Golovliov, I.; Michal, F.; Basso, P.; Djebali, S.; et al. IFN-gamma extends the immune functions of Guanylate Binding Proteins to inflammasome-independent antibacterial activities during Francisella novicida infection. PLoS Pathog. 2017, 13, e1006630. [Google Scholar] [CrossRef]
- Michalska, A.; Blaszczyk, K.; Wesoly, J.; Bluyssen, H.A.R. A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses. Front. Immunol. 2018, 9, 1135. [Google Scholar] [CrossRef]
- Howard, L.M.; Goll, J.B.; Jensen, T.L.; Hoek, K.L.; Prasad, N.; Gelber, C.E.; Levy, S.E.; Joyce, S.; Link, A.J.; Creech, C.B.; et al. AS03-Adjuvanted H5N1 avian influenza vaccine modulates early innate immune signatures in human peripheral blood mononuclear cells. J. Infect. Dis. 2018, 219, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.M.; Hoek, K.L.; Goll, J.B.; Samir, P.; Galassie, A.; Allos, T.M.; Niu, X.; Gordy, L.E.; Creech, C.B.; Prasad, N.; et al. Cell-Based Systems Biology Analysis Of Human As03-Adjuvanted H5n1 Avian Influenza Vaccine Responses: A Phase I Randomized Controlled Trial. PLoS ONE 2017, 12, e0167488. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.S.; Frey, S.E.; Yan, L.; Rothman, A.L.; Cruz, J.; Newman, F.K.; Orphin, L.; Belshe, R.B.; Ennis, F.A. Induction of human T cell-mediated immune responses after primary and secondary smallpox vaccination. J. Infect. Dis. 2004, 190, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Dolfi, D.V.; Mansfield, K.D.; Kurupati, R.K.; Kannan, S.; Doyle, S.A.; Ertl, H.C.; Schmader, K.E.; Wherry, E.J. Vaccine-induced boosting of influenza virus-specific CD4 T cells in younger and aged humans. PLoS ONE 2013, 8, e77164. [Google Scholar] [CrossRef]
- Akahoshi, T.; Sasahara, T.; Namai, R.; Matsui, T.; Watabe, H.; Kitasato, H.; Inoue, M.; Kondo, H. Production of macrophage inflammatory protein 3alpha (MIP-3alpha) (CCL20) and MIP-3beta (CCL19) by human peripheral blood neutrophils in response to microbial pathogens. Infect. Immun. 2003, 71, 524–526. [Google Scholar] [CrossRef]
- Liu, J.Y.; Li, F.; Wang, L.P.; Chen, X.F.; Wang, D.; Cao, L.; Ping, Y.; Zhao, S.; Li, B.; Thorne, S.H.; et al. CTL-vs Treg lymphocyte-attracting chemokines, CCL4 and CCL20, are strong reciprocal predictive markers for survival of patients with oesophageal squamous cell carcinoma. Br. J. Cancer 2015, 113, 747–755. [Google Scholar] [CrossRef]
- Sekiya, T.; Kashiwagi, I.; Yoshida, R.; Fukaya, T.; Morita, R.; Kimura, A.; Ichinose, H.; Metzger, D.; Chambon, P.; Yoshimura, A. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 2013, 14, 230–237. [Google Scholar] [CrossRef]
- Yamazaki, T.; Yang, X.O.; Chung, Y.; Fukunaga, A.; Nurieva, R.; Pappu, B.; Martin-Orozco, N.; Kang, H.S.; Ma, L.; Panopoulos, A.D.; et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 2008, 181, 8391–8401. [Google Scholar] [CrossRef]
- Le Borgne, M.; Etchart, N.; Goubier, A.; Lira, S.A.; Sirard, J.C.; van Rooijen, N.; Caux, C.; Ait-Yahia, S.; Vicari, A.; Kaiserlian, D.; et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 2006, 24, 191–201. [Google Scholar] [CrossRef]
- Sekiya, T.; Kashiwagi, I.; Inoue, N.; Morita, R.; Hori, S.; Waldmann, H.; Rudensky, A.Y.; Ichinose, H.; Metzger, D.; Chambon, P.; et al. The nuclear orphan receptor Nr4a2 induces Foxp3 and regulates differentiation of CD4+ T cells. Nat. Commun. 2011, 2, 269. [Google Scholar] [CrossRef]
- Min, F.; Wu, R.; Pan, J.; Huang, S.; Luo, Y.; Zhang, Y. Positive Correlation between IP-10 and IFN-gamma Levels in Rhesus Monkeys (Macaca mulatta) with Either Naturally Acquired or Experimental Infection of Mycobacterium tuberculosis. Biomed. Res. Int. 2017, 2017, 5089752. [Google Scholar] [CrossRef] [PubMed]
- Bosio, C.M.; Elkins, K.L. Susceptibility to secondary Francisella tularensis live vaccine strain infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity. Infect. Immun. 2001, 69, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Fu, N.; Guo, S.; Yan, Z.; Xu, Y.; Hu, H.; Menzel, C.; Chen, W.; Li, Y.; Zeng, R.; et al. Estimating accuracy of RNA-Seq and microarrays with proteomics. BMC Genom. 2009, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.; Moir, C.A.; Gillespie, Z.E.; Kusalik, A.; Mitchell, J.A.; Eskiw, C.H. Concordance between RNA-sequencing data and DNA microarray data in transcriptome analysis of proliferative and quiescent fibroblasts. R. Soc. Open Sci. 2015, 2, 150402. [Google Scholar] [CrossRef]
- Zhao, S.; Fung-Leung, W.P.; Bittner, A.; Ngo, K.; Liu, X. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS ONE 2014, 9, e78644. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, Y.; Hertwig, F.; Thierry-Mieg, J.; Zhang, W.; Thierry-Mieg, D.; Wang, J.; Furlanello, C.; Devanarayan, V.; Cheng, J.; et al. Comparison of RNA-seq and microarray-based models for clinical endpoint prediction. Genome. Biol. 2015, 16, 133. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natrajan, M.S.; Rouphael, N.; Lai, L.; Kazmin, D.; Jensen, T.L.; Weiss, D.S.; Ibegbu, C.; Sztein, M.B.; Hooper, W.F.; Hill, H.; et al. Systems Vaccinology for a Live Attenuated Tularemia Vaccine Reveals Unique Transcriptional Signatures That Predict Humoral and Cellular Immune Responses. Vaccines 2020, 8, 4. https://doi.org/10.3390/vaccines8010004
Natrajan MS, Rouphael N, Lai L, Kazmin D, Jensen TL, Weiss DS, Ibegbu C, Sztein MB, Hooper WF, Hill H, et al. Systems Vaccinology for a Live Attenuated Tularemia Vaccine Reveals Unique Transcriptional Signatures That Predict Humoral and Cellular Immune Responses. Vaccines. 2020; 8(1):4. https://doi.org/10.3390/vaccines8010004
Chicago/Turabian StyleNatrajan, Muktha S., Nadine Rouphael, Lilin Lai, Dmitri Kazmin, Travis L. Jensen, David S. Weiss, Chris Ibegbu, Marcelo B. Sztein, William F. Hooper, Heather Hill, and et al. 2020. "Systems Vaccinology for a Live Attenuated Tularemia Vaccine Reveals Unique Transcriptional Signatures That Predict Humoral and Cellular Immune Responses" Vaccines 8, no. 1: 4. https://doi.org/10.3390/vaccines8010004
APA StyleNatrajan, M. S., Rouphael, N., Lai, L., Kazmin, D., Jensen, T. L., Weiss, D. S., Ibegbu, C., Sztein, M. B., Hooper, W. F., Hill, H., Anderson, E. J., Johnson, R., Sanz, P., Pulendran, B., Goll, J. B., & Mulligan, M. J. (2020). Systems Vaccinology for a Live Attenuated Tularemia Vaccine Reveals Unique Transcriptional Signatures That Predict Humoral and Cellular Immune Responses. Vaccines, 8(1), 4. https://doi.org/10.3390/vaccines8010004




