Addressing Vaccine Hesitancy in China: A Scoping Review of Chinese Scholarship
Abstract
1. Introduction
2. Materials and Methods
2.1. Identifying Research Questions
2.2. Identifying Relevant Studies
2.3. Selection of Relevant Studies
2.4. Charting Data
2.5. Presentation of Results
3. Results
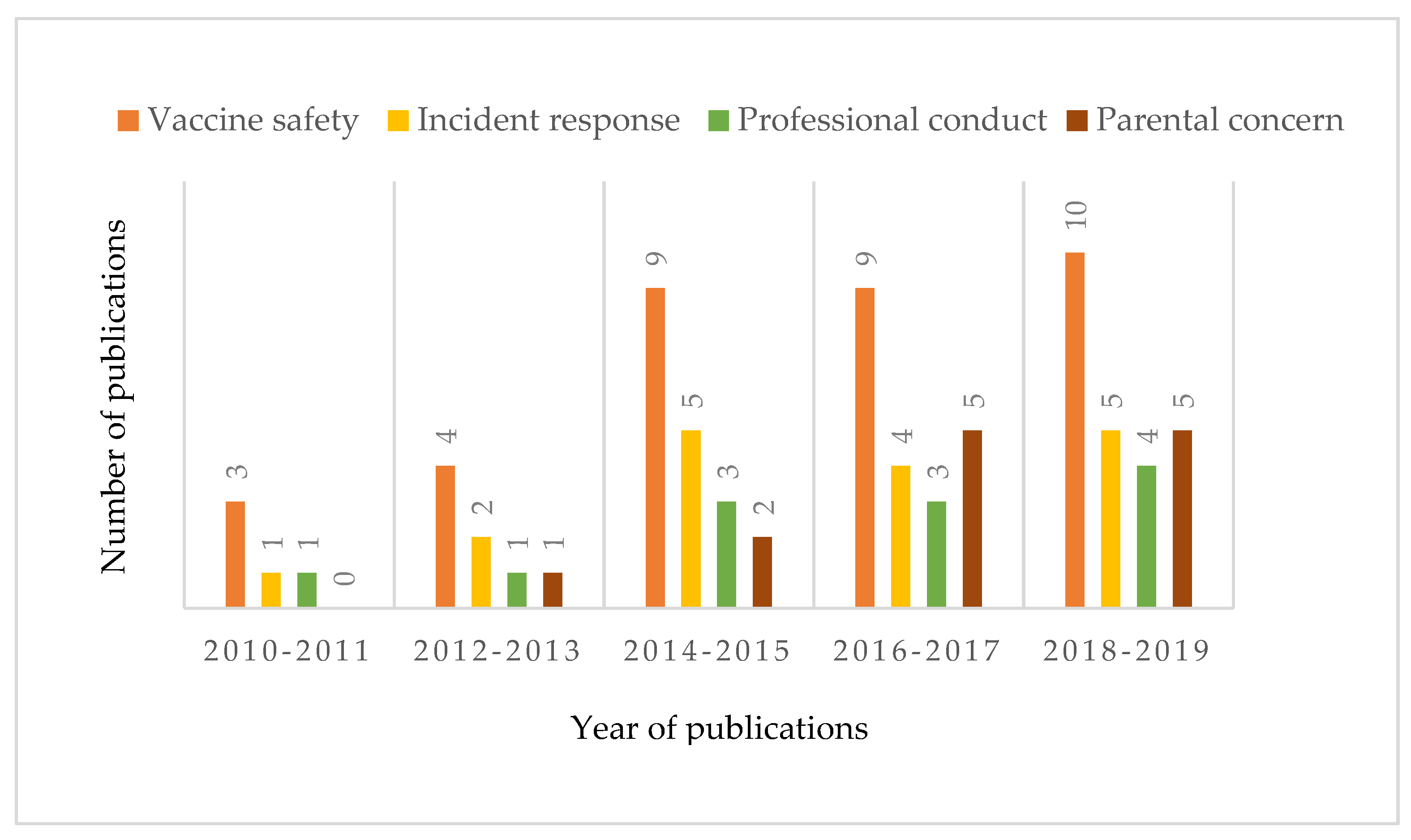
3.1. Selected Articles
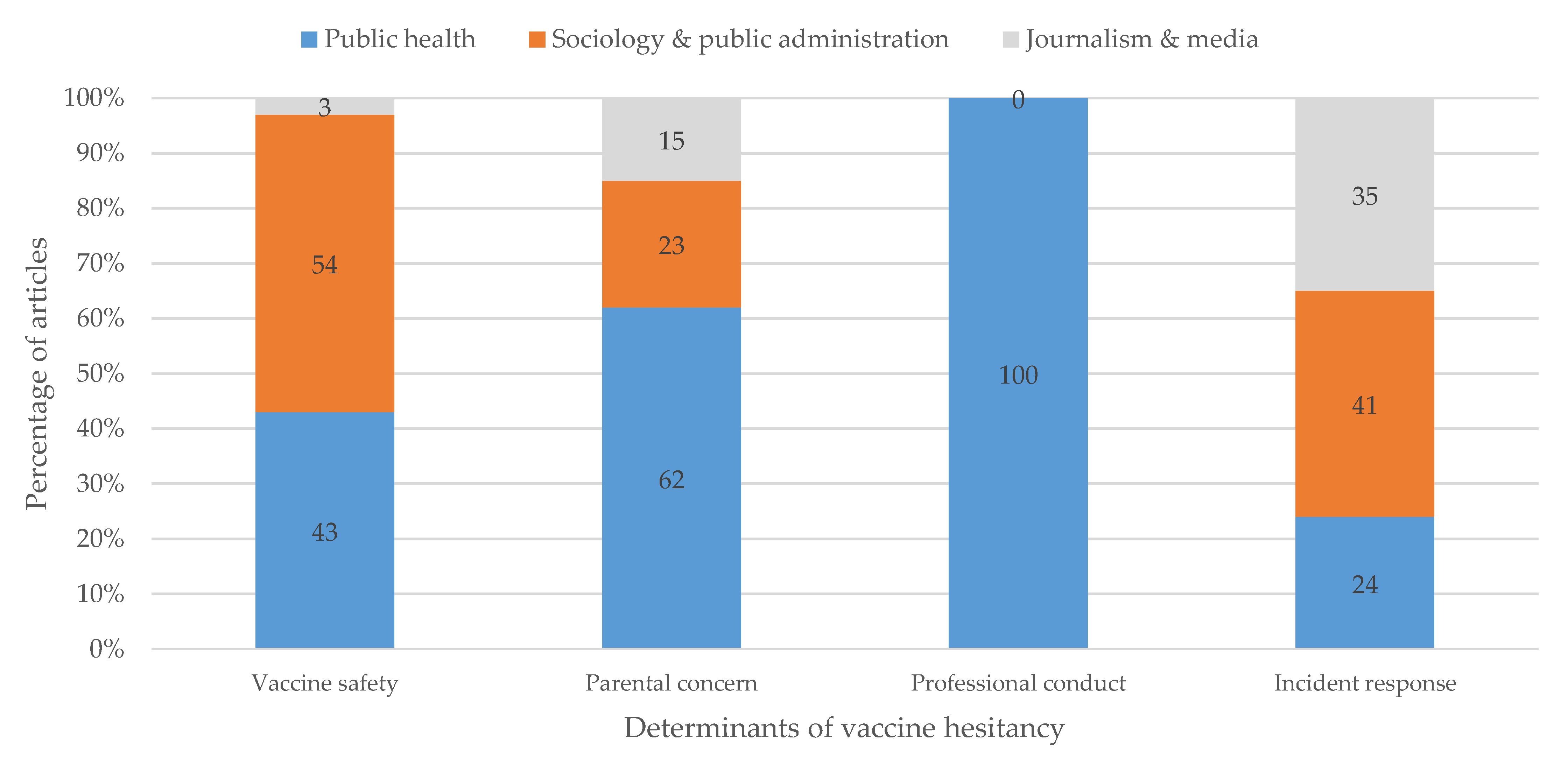
3.2. Defining the Problem of Vaccine Hesitancy
3.2.1. Vaccine Safety
3.2.2. Vaccine Incident Response
3.2.3. Professional Conduct of Vaccination
3.2.4. Parental Concern
3.3. Defining Solutions for Reducing Vaccination Hesitancy
3.4. Implementing New Policies
4. Discussion
Strengths and Limitations of Our Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Article (n = 77) | Expertise | Perspective |
|---|---|---|
| Chen T, 2017. [1] | Public health. | Professional conduct |
| Liu, X., Hu W. & Zhang S, 2018. [10] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Zhang Y, 2017. [12] | Public health (CDC). | Parental concern |
| Tong X, 2019. [14] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Zhou Q, Liu W, Chen L, 2018. [15] | Public health. | Vaccine safety |
| Peng Z, Wang D, Yang J, 2018. [16] | Public health (Epidemiology). | Parental concern |
| Wang & He, 2016. [25] | Sociology and Public administration (Sociology). | Vaccine safety |
| Hu Y, 2014. [26] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Liu X, Lin R, Yang C, Yu S, Zhang B, 2017. [29] | Public health. | Vaccine safety |
| CAMG, 2018. [30] | Sociology and Public administration (Sociology). | Vaccine safety |
| Li W, Chen W, Zhang J, 2016. [31] | Journalism and Media. | Vaccine safety |
| Sun Y, Xu L, Li S, 2015. [32] | Public health. | Incident response |
| Chen W, Gao Z, Li Y, 2016. [33] | Public health (epidemiology). | Vaccine safety |
| Di W, 2015. [34] | Sociology and Public administration (Sociology). | Vaccine safety |
| Shi L, 2017. [35] | Public health (CDC). | Vaccine safety |
| Sun W, 2014. [36] | Public health (Epidemiology). | Parental concern |
| Yu, W, et al., 2014. [37] | Public health (CDC). | Vaccine safety |
| Yuan & Li, 2017. [38] | Public health. | Vaccine safety |
| Zhang, K, 2017. [39] | Sociology and Public administration (Public Administration). | Parental concern |
| Ma J, Zhou L, Zhou L, 2015. [40] | Public health (CDC). | Vaccine safety |
| Meng &Xu, 2012. [41] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Lu Y, 2018. [42] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Wang & Yang, 2016. [43] | Public health (CDC). | Vaccine safety |
| Qian & Wang, 2012. [44] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Zhang Y, 2014. [45] | Sociology and Public administration (Sociology). | Vaccine safety |
| Zhou& Li, 2014. [46] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Jiang Y, Yu W, Zhang X, 2014. [47] | Public health (CDC). | Vaccine safety |
| Li H, 2019. [48] | Journalism and Media. | Incident response |
| Li & Chen, 2011. [49] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Zhang H, 2018. [50] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Han J, Zhou W, 2016. [51] | Sociology and Public administration (Sociology). | Incident response |
| Lai S, 2013. [52] | Sociology and Public administration (Public Administration). | Incident response |
| Qi &Cheng, 2015. [53] | Sociology and Public administration (Sociology). | Incident response |
| Sui X, 2014. [54] | Journalism and Media. | Incident response |
| Xiao X, 2017. [55] | Sociology and Public administration (Sociology). | Incident response |
| Song J, 2018. [56] | Journalism and Media. | Incident response |
| Song W. 2018. [57] | Journalism and Media. | Incident response |
| Huang, 2010. [58] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Zhao D, Li X, Lu L, 2018. [59] | Public health. | Professional conduct |
| Xiang F, 2012. [60] | Public health. | Incident response |
| Cheng M, Su Z, Lian Q, 2014. [61] | Public health. | Professional conduct |
| Liu F, 2014. [62] | Public health. | Professional conduct |
| Wang Y, 2012. [63] | Public health. | Professional conduct |
| Guo W, Wang J, Yu X, 2018. [64] | Public health. | Professional conduct |
| Qiao X, Wei Ji, Lu D, 2018. [65] | Public health (Epidemiology). | Professional conduct |
| Zhao X, Zhou L, Yang X, 2016. [66] | Public health. | Professional conduct |
| An L, Chen W Sun M, 2018. [68] | Public health. | Parental concern |
| Tang Z, 2018. [69] | Public health (Epidemiology). | Parental concern |
| Dai & Zhu, 2018. [70] | Journalism and Media. | Parental concern |
| Yang H,2017. [71] | Sociology and Public administration (Sociology). | Vaccine safety |
| Cao L, Wang H, and Zheng, 2012. [72] | Public health (CDC). | Parental concern |
| Ma G, 2016. [73] | Sociology and Public administration (Sociology). | Parental concern |
| Yu F, 2016. [74] | Public health (Epidemiology). | Parental concern |
| Wang Y, Sun L, Li M, 2019. [75] | Public health. | Professional conduct |
| Zhuang X, Wang R, 2016. [76] | Public health (Epidemiology). | Parental concern |
| Huang S, 2015. [77] | Journalism and Media. | Parental concern |
| Kunming CDC, 2018. [78] | Public health (CDC). | Vaccine safety |
| Xue & Li, 2018. [79] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Yu W, Ji S, Liu J, Cong B, Zhou Y, Zhang X, Cui F, Wang H, 2016. [80] | Public health (CDC). | Vaccine safety |
| Yi & Liao, 2013. [81] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Zhang & Chen, 2010. [82] | Public health (CDC). | Incident response |
| Yang & Ding, 2014. [83] | Public health. | Professional conduct |
| Wu Z, 2013. [84] | Public health (CDC). | Vaccine safety |
| Wang B, 2018. [85] | Journalism and Media. | Incident response |
| Feng B, 2014. [86] | Public health. | Professional conduct |
| Wen W, 2011. [87] | Public health (CDC). | Vaccine safety |
| Zhong X, Lu Z, Chen X, 2017. [88] | Public health. | Professional conduct |
| Song J, 2015. [89] | Sociology and Public administration (Public Administration). | Incident response |
| Jia X, 2016. [90] | Sociology and Public administration (Sociology). | Incident response |
| Lai H, 2018. [91] | Sociology and Public administration (Sociology). | Parental concern |
| Sun L, Cong Y, Wang Y, 2017. [92] | Public health (CDC). | Incident response |
| Yue D, Chang J, Hou Z, Wu Q, Meng Y, 2014. [93] | Public health. | Vaccine safety |
| Ye and Zhang, 2019. [94] | Sociology and Public administration (Public Administration). | Vaccine safety |
| Sun L, Guo J, Li J, 2018. [95] | Public Health (CDC). | Vaccine safety |
| Zhao Z, 2019. [96] | Sociology and Public administration (Public Administration). | Incident response |
| Li T, 2011. [97] | Journalism and Media. | Incident response |
| Zhang Z, 2014. [98] | Sociology and Public administration (Public Administration). | Vaccine safety |
References
- Chen, T. Investigation on medical staff’s knowledge on hepatitis B prevention. J. Tradit. Chin. Med. Manag. 2017, 12, 107–108. [Google Scholar]
- McLaughlin, K. Scandal clouds China’s global vaccine ambitions. Science 2016, 352, 506. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health: China’s Immunization Program Vaccination Rate Continues to Remain Above 90%. Available online: https://51jinke.com/news/5c739d48d42cbc28a9df0691 (accessed on 5 February 2019).
- Wagner, A.L.; Boulton, M.L.; Sun, X.; Mukherjee, B.; Huang, Z.; Harmsen, I.A.; Ren, J.; Zikmund-Fisher, B.J. Perceptions of measles, pneumonia, and meningitis vaccines among caregivers in Shanghai, China, and the health belief model: A cross-sectional study. BMC Pediatrics 2017, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Jarrett, C.; Eckersberger, E.; Smith, D.M.; Paterson, P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007–2012. Vaccine 2014, 32, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Gagnon, D.; Nickels, E.; Jeram, S.; Schuster, M. Mapping vaccine hesitancy—Country-specific characteristics of a global phenomenon. Vaccine 2014, 32, 6649–6654. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wagner, A.L.; Zheng, A.; Sun, X.; Boulton, M.L.; Huang, Z.; Zikmund-Fisher, B.J. The demographics of vaccine hesitancy in Shanghai, China. PLoS ONE 2018, 13, e0209117. [Google Scholar] [CrossRef]
- Yu, W.; Liu, D.; Zheng, J.; Liu, Y.; An, Z.; Rodewald, L.; Zhang, G.; Su, Q.; Li, K.; Xu, D.; et al. Loss of confidence in vaccines following media reports of infant deaths after hepatitis B vaccination in China. Int. J. Epidemiol. 2016, 45, 441–449. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, J.M.; Jiang, Z.; Shao, J.; Jiang, T.; Wang, Z.; Liu, K.; Tang, S.; Gu, H.; Jiang, J. Media and public reactions toward vaccination during the ‘hepatitis B vaccine crisis’ in China. Vaccine 2015, 33, 1780–1785. [Google Scholar] [CrossRef]
- Liu, X.; Hu, W.; Zhang, S. The trust degree of parents on the Shanxi vaccination progrma affected by the Shandong vaccine crisis in 2016. J. China Vaccine Immun. 2018, 24, 83–88. [Google Scholar]
- Vaccine Scandal in China Crosses “Moral Bottom Line”. Available online: https://thevaccinereaction.org/2018/07/vaccine-scandal-in-china-crosses-moral-bottom-line/ (accessed on 24 May 2019).
- Zhang, Y. Media reports that Chinese parents have decreased confidence in vaccination after hepatitis B vaccine causes infant death. Chin. J. Prev. Med. 2017, 51, 518. [Google Scholar]
- Wang, C.; Li, G.; Zhang, Y. Investigation on the status of vaccination among children aged 0–6 years in a county after the “Shandong Vaccine Incident”. J. Pract. Prev. Med. 2019, 26, 63–66. [Google Scholar]
- Tong, X. Investigation on the awareness of residents’ safety in Yangzhou City. China New Telecommun. 2019, 21, 239–240. [Google Scholar]
- Zhou, Q.; Liu, W.; Chen, L. The Impact of Shandong Illegal Vaccine Series on Vaccination Attitudes and Behaviors of Children in Shenzhen. Chin. J. Vaccines Immun. 2018, 2, 11–14. [Google Scholar]
- Peng, Z.; Wang, D.; Yang, J. Current status of influenza vaccine application and policy promotion of vaccination. Chin. J. Epidemiol. 2018, 39, 1045–1050. [Google Scholar] [CrossRef]
- Jarrett, C.; Wilson, R.; O’Leary, M.; Eckersberger, E.; Larson, H.J. Strategies for addressing vaccine hesitancy—A systematic review. Vaccine 2015, 33, 4180–4190. [Google Scholar] [CrossRef]
- Sadaf, A.; Richards, J.L.; Glanz, J.; Salmon, D.A.; Omer, S.B. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine 2013, 31, 4293–4304. [Google Scholar] [CrossRef]
- Geelen, E.; van Vliet, H.; de Hoogh, P.; Horstman, K. Taming the fear of voice: Dilemmas in maintaining a high vaccination rate in the Netherlands. Soc. Sci. Med. 2016, 153, 12–19. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Lu, Y.; Li, Q.; Chen, C.; Wang, D.; Li, M.; Li, Y.; Lu, J.; Chen, Z.; et al. Willingness and influential factors of parents to vaccinate their children with novel inactivated enterovirus 71 vaccines in Guangzhou, China. Vaccine 2018, 36, 3772–3778. [Google Scholar] [CrossRef]
- Yue, C.; Sun, X.; Wei, N.; Yu, W.; Cui, F.; Wang, H.; Li, L.; Zhang, L.; Shi, G.; An, Z. Quick assessment of the influence of Hepatitis B vaccine event on Children’s vaccination. Hum. Vaccines Immunother. 2016, 12, 2611–2615. [Google Scholar] [CrossRef][Green Version]
- Zhou, M.; Qu, S.; Zhao, L.; Kong, N.; Campy, K.S.; Wang, S. Trust collapse caused by the Changsheng vaccine crisis in China. Vaccine 2019, 37, 3419–3425. [Google Scholar] [CrossRef]
- Yuan, X. China’s vaccine production scare. Lancet 2018, 392, 371. [Google Scholar] [CrossRef]
- Liu, D.; Wu, W.; Li, K.; Xu, D.; Ye, J.; Li, L.; Wang, H. Surveillance of adverse events following immunization in China: Past, present, and future. Vaccine 2015, 33, 4041–4046. [Google Scholar] [CrossRef]
- Wang, Y.; He, R. The Dilemma of Vaccine Circulation Industry Chain Management and Multi-Center Management System of Vaccine Safety. Enterp. Manag. 2016, 6, 18–22. [Google Scholar]
- Hu, Y. Current Status and Improvement Countermeasures of Vaccine Supply and Supervision System in China. Chin. J. Drug Eval. 2014, 3, 175–179. [Google Scholar]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, R.; Yang, C.; Yu, S.; Zhang, B. Status, problems and countermeasures of vaccine safety supervision in China. China Public Health Manag. 2017, 2, 50–53. [Google Scholar]
- Chinese Administrative Management Group. Balancing state supervision and market: Challenge and countermeasures of vaccine safety. Chin. Adm. Manag. 2018, 400, 8–14.
- Li, W.; Chen, W.; Zhang, J. Is it safe to vaccinate? Innov. Era 2016, 5, 13–14. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Li, S. Evaluation of the application effect of real-time monitoring system for vaccine cold chain temperature. Chin. J. Vaccines Immun. 2015, 6, 675–679. [Google Scholar]
- Chen, W.; Gao, Z.; Li, Y. Investigation on the Impact of Illegal Vaccine Cases in Shandong on the Vaccination Attitudes and Behaviors of Children in Tianjin. China Public Health 2016, 32, 881–884. [Google Scholar]
- Di, W. C Company’s Quality Risk Analysis and Control Strategy for Refrigerated Drug Transportation. Ph.D. Thesis, East China University of Science and Technology, Shanghai, China, 2015. [Google Scholar]
- Shi, L. The Impact of “Shandong Vaccine Incident” on the Attitudes and Behaviors of Parents of Children in Two Counties of Henan Province. China Health Educ. 2017, 33, 255–257. [Google Scholar]
- Sun, W. Child Plan Immunization. In Proceedings of the 31st Academic Conference of the Chinese Society of Chinese Medicine Pediatrics, Beijing, China, 14 January 2014. [Google Scholar]
- Yu, W.; Li, F.; Zhang, Z. Investigation and analysis of the trustworthiness of vaccination among parents of some provinces after the hepatitis B vaccine incident in 2013. Chin. J. Vaccines Immun. 2014, 3, 233–236. [Google Scholar]
- Yuan, C.; Li, K. Analysis of Parents’ Trust of Vaccination. J. Mudanjiang Med. Coll. 2017, 2, 127–129. [Google Scholar]
- Zhang, K. The current problems and countermeasures of Drug Management in China. Rural Econ. Technol. 2017, 28, 115–116. [Google Scholar]
- Ma, J.; Zhou, L.; Zhou, L. The influence of negative information of hepatitis B vaccination on the vaccination trust of parents of urban and rural children in Ningxia. J. Ningxia Med. Univ. 2015, 37, 1046–1049. [Google Scholar]
- Meng, X.; Xu, L. Legal Mechanism, Institutional Investors and Corporate Governance—Based on Analysis of Chongqing Beer Hepatitis B Vaccine Project. Econ. Theory Bus. Manag. 2012, 32, 96–103. [Google Scholar]
- Lu, Y. Study on the Governance Issue of Vaccine Enterprises in China from the Perspective of Social Responsibility. Med. Law Sci. 2018, 10, 55–60. [Google Scholar]
- Wang, P.; Yang, F. Discussion on Vaccine and Cold Chain Management Experience. China Health Ind. 2016, 13, 187–189. [Google Scholar]
- Qian, X.; Wang, G. Analysis of China’s Vaccine Supervision System Based on Executive Force Perspective. Chin. Pharm. 2012, 37, 3474–3476. [Google Scholar]
- Zhang, Y. Third Party Participation in Risk Assessment for Major Events: Significance, Dilemma and Countermeasures. Inn. Mong. Soc. Sci. 2014, 35, 167–172. [Google Scholar]
- Zhou, W.; Li, H. Rent-seeking and Governance in Government Public Service Contract Outsourcing. Theor. Explor. 2014, 6, 87–91. [Google Scholar]
- Jiang, Y.; Yu, W.; Zhang, X. A telephone survey of public trust in vaccination after the hepatitis B vaccine incident in 2013. Chin. J. Vaccines Immun. 2014, 4, 314–317. [Google Scholar]
- Vaccination: The Public Should Not Hesitate. Available online: http://www.qstheory.cn/science/2019-04/29/c_1124431677.htm (accessed on 29 April 2019).
- Li, Y.; Chen, B. Reflection and Restatement of the Second Kind of Vaccine Supervision Mechanism from the Perspective of Constitutionalism. Med. J. China 2011, 24, 72–74. [Google Scholar]
- Zhang, H. Innovative concepts and measures, exploring a new model of vaccination supervision. Chin. J. Health Superv. 2018, 1, 15–18. [Google Scholar]
- Han, J.; Zhou, W. System Optimization of Third-Party Participation in Social Stability Risk Assessment of Major Events. J. Chongqing Univ. Sci. Technol. 2016, 11, 38–41. [Google Scholar]
- Lai, S. Accountability, Path Dependence and Disclosure: Local Government Behavior Research Based on 97 Public Crisis Cases. J. Public Adm. 2013, 10, 18–27. [Google Scholar]
- Qi, C.; Cheng, L. Construction of the ethical accountability mechanism for medical staff. In Proceedings of the 9th Annual Conference of the Shandong Medical Ethics Society and the 2nd Session of the 4th Council, Shandong, China, 29 April 2015. [Google Scholar]
- Penglai City Took the Lead to Establish a Social Risk Assessment Mechanism. Available online: http://www.dzwww.com/shandong/sdnews/201409/t20140902_10950508.htm (accessed on 24 May 2019).
- Xiao, X. Dilemma of Public Participation in Risk Assessment. Decis. Consult. 2017, 5, 81–85. [Google Scholar]
- The Vaccine Safety Management Law Seeks for Advice: Severely Punish Illegal Behavior and Implement Four Most Strict Standards. Available online: https://baijiahao.baidu.com/s?id=1616889356040775516&wfr=spider&for=pc (accessed on 25 May 2019).
- The Compensation Mechanism Needs to Be Established. Available online: http://www.cb.com.cn/zjssb/2018_0726/1248527.html (accessed on 25 May 2019).
- Huang, Y. Analysis of risk management deficits in the Shangxi vaccine crisis. Collect. Econ. 2010, 25, 39. [Google Scholar]
- Zhao, D.; Li, X.; Lu, L. Investigation of training and information transmission effects after introduction of new vaccines into immunization programs. Cap. Public Health 2018, 12, 138–141. [Google Scholar]
- Xiang, F. Case analysis of medical disputes involving hepatitis B vaccine and BCG-coupled death. Zhejiang Prev. Med. 2012, 24, 89. [Google Scholar]
- Cheng, M.; Su, Z.; Lian, Q. Study on the effect of vaccination informing on the rate of vaccination adverse reactions. Lab. Med. Clin. 2014, 5, 706–707. [Google Scholar]
- Liu, F. Discussion on the application of mother classroom in vaccination. Henan J. Prev. Med. 2015, 5, 100–101. [Google Scholar] [CrossRef]
- Wang, Y. How to screen contraindications before vaccination. J. Med. Front. 2012, 2, 311. [Google Scholar] [CrossRef]
- Guo, W.; Wang, J.; Yu, X. Investigation on the willingness and influencing factors of EV71 vaccine for vaccination medical staff in Dezhou City. J. Prev. Med. 2018, 3, 171–173. [Google Scholar]
- Qiao, X.; Wei, J.; Lu, D. Analysis of influencing factors of vaccination trust among grassroots medical staff. Int. J. Epidemiol. Infect. 2018, 45, 436–440. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Yang, X. Investigation on the influence of hepatitis B vaccine incident on hepatitis B vaccination rate and cognitive attitude of parents in hepatitis B vaccine in Jinan City. Chin. Community J. 2016, 4, 186–187. [Google Scholar]
- Zhou, L.; Yuan, H.; Wen, Y. Causes of refrigeration chain failure and improvement measures. Med. Equip. 2017, 30, 97–98. [Google Scholar]
- An, L.; Chen, W.; Sun, M. Analysis of vaccination status and influencing factors of varicella vaccine in children in Tianjin. Med. Anim. Control 2018, 34, 49–52. [Google Scholar]
- Tang, Z. Development and Preliminary Application of Children’s Parents’ Hesitation Questionnaire on Enterovirus 71 Inactivated Vaccine. Master’s Thesis, Chinese Center for Disease Control and Prevention, Beijing, China, 2018. [Google Scholar]
- Dai, W.; Zhu, Q. Research on Information Mechanism of Risk Amplification in Media Environment—Taking 2016 Shandong Vaccine Event as an Example. J. Southwest Univ. Natl. (Soc. Sci. Ed.) 2018, 39, 153–157. [Google Scholar]
- Yang, H. Research on the Drug Safety shifting from Administrative Supervision to Collaborative Governance in Shandong Vaccine Crisis. J. Tianjin Adm. Coll. 2017, 19, 8–15. [Google Scholar]
- Cao, L.; Wang, H.; Zheng, J. Investigation and Analysis of Vaccination Rate of Expanding National Immunization Program in China. Chin. J. Vaccines Immun. 2012, 5, 419–424. [Google Scholar]
- Ma, G. The Impact of the Health Belief Model on the Timely Vaccination of Migrant Children in the Community and Intervention Studies. Ph.D. Thesis, University of South China, Hengyang, China, 2016. [Google Scholar]
- Yu, F. Study on the influencing factors of immunization program immunization for migrant children. Chin. J. Health Nutr. 2016, 26, 11–14. [Google Scholar]
- Wang, Y.; Sun, L.; Li, M. Analysis of influencing factors of vaccination hepatolysis in a medical community in Beijing. Chin. J. Reprod. Health 2019, 30, 149–153. [Google Scholar]
- Zhuang, X.; Wang, R. Analysis of influencing factors of vaccination rate of children’s immunization program in Tongnan County. J. Taishan Med. Coll. 2016, 37, 1235–1237. [Google Scholar]
- Immunization Planning Experts Remind that do Not Hesitate to Inoculate. Available online: http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdys201512015 (accessed on 26 May 2019).
- History of Chinese Vaccine Development—Past and Present of Vaccination. Available online: http://www.sohu.com/a/229424002_99994855 (accessed on 25 April 2018).
- Xue, L.; Li, X. Deepening the Reform of Regulatory model and Promoting the Modernization of Market Supervision. China Adm. 2018, 398, 23–31. [Google Scholar]
- Yu, W.; Ji, S.; Liu, J.; Cong, B.; Zhou, Y.; Zhang, X.; Cui, F.; Wang, H. Continuity monitoring and analysis of the impact of illegal vaccines in Shandong on the vaccination of children’s parents. Chin. J. Vaccines Immun. 2016, 6, 601–605. [Google Scholar]
- Yi, H.; Liao, T. Administrative accountability of food and drug—Taking the food and drug safety supervision system reform and function transformation as the entry point. Food Sci. 2013, 34, 374–379. [Google Scholar]
- Zhang, D.; Chen, T. I know the truth about Shanxi vaccine. Communist Party Memb. 2010, 12, 26. [Google Scholar]
- Yang, M.; Ding, C. Discussion on the causes and countermeasures of adverse reactions of vaccination against DTP. Chin. J. Health Nutr. 2014, 7, 4035. [Google Scholar]
- Wu, Z. Current Situation and Management Countermeasures of Rural Children’s Vaccination. Chin. J. Health Nutr. 2013, 6, 85. [Google Scholar]
- Health Commission: Do a Follow-Up Observation and Counseling Service for Rabies Vaccinatees to Protect Their Legitimate Rights. Available online: http://www.xinhuanet.com/politics/2018-08/07/c_1123237162.htm (accessed on 7 August 2018).
- Feng, B. Design and Implementation of Vaccine Cold Chain Supervision System. Master’s Thesis, University of Electronic Science and Technology of China, Chengdu, China, 2014. [Google Scholar]
- Wen, W. Discussion on Scientific Training and Assessment of Grassroots Vaccination Workers. Chin. J. Sch. Dr. 2011, 25, 390–391. [Google Scholar]
- Zhong, X.; Lu, Z.; Chen, X. Investigation of trust in the vaccination abnormal response monitoring by vaccination personnel and parent. China Public Health 2017, 6, 874–878. [Google Scholar]
- Song, J. Case Study of Kangtai Hepatitis B Vaccine Trust Crisis in 2013. J. Natl. Sch. Adm. 2015, 5, 84–88. [Google Scholar]
- Jia, X. Problems and Countermeasures in the Relief System of Abuse Response Abnormality in China. Med. J. China 2016, 29, 50–53. [Google Scholar]
- Lai, H. Research on Legal Issues of Vaccine Damage; Law Press: Beijing, China, 2018. [Google Scholar]
- Sun, L.; Cong, Y.; Wang, Y. Public Attitude Analysis of Commercial Insurance Compensation Model for Abnormal Vaccination in Hebei Province. Chin. J. Vaccines Immun. 2017, 3, 278–281. [Google Scholar]
- Yue, D.; Chang, J.; Hou, Z.; Wu, Q.; Meng, Y. International vaccination anomaly response compensation mechanism for reference. China Health Econ. 2014, 1, 93–96. [Google Scholar]
- Ye, L.; Zhang, X. Vaccine Management Law will be Enforced on 1 December 2019. Med. Soc. 2019, 7, 66. [Google Scholar]
- Sun, L.; Guo, J.; Li, J. Comparative analysis of factors affecting the knowledge and belief of divalent polio vaccine by vaccination doctors and parents. Med. Anim. Control 2018, 8, 715–721. [Google Scholar]
- Zhao, Z. Second trial of the draft vaccine management law: Further strengthen the management of vaccination and ensure the safety of vaccines. Chin. Peoples Congr. 2019, 477, 20–21. [Google Scholar]
- Why Is it Difficult for Doctors to Be Accused of “Medical Accidents”? Available online: http://zqb.cyol.com/html/2011-09/02/nw.D110000zgqnb_20110902_2-07.htm (accessed on 26 May 2019).
- Zhang, Z. Research on Accountability of Medical Security Management in China. Chin. Med. Manag. Sci. 2014, 4, 13–18. [Google Scholar]
- Wang, Y. Strengthening the public health management of medical institutions is the key to improving the quality and level of disease control in China. Chin. J. Med. 2007, 87, 512–514. [Google Scholar]

| Inclusion | Exclusion |
|---|---|
| 1. Scholarly peer-reviewed articles, conference papers, government reports, media reports. 2. Papers focused on governance of vaccination and public trust. | 1. Publications that only mentioned vaccine safety in the conclusions. 2. Papers focused on preclinical medicine and veterinary medicine research. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Penders, B.; Horstman, K. Addressing Vaccine Hesitancy in China: A Scoping Review of Chinese Scholarship. Vaccines 2020, 8, 2. https://doi.org/10.3390/vaccines8010002
Yang R, Penders B, Horstman K. Addressing Vaccine Hesitancy in China: A Scoping Review of Chinese Scholarship. Vaccines. 2020; 8(1):2. https://doi.org/10.3390/vaccines8010002
Chicago/Turabian StyleYang, Ronghui, Bart Penders, and Klasien Horstman. 2020. "Addressing Vaccine Hesitancy in China: A Scoping Review of Chinese Scholarship" Vaccines 8, no. 1: 2. https://doi.org/10.3390/vaccines8010002
APA StyleYang, R., Penders, B., & Horstman, K. (2020). Addressing Vaccine Hesitancy in China: A Scoping Review of Chinese Scholarship. Vaccines, 8(1), 2. https://doi.org/10.3390/vaccines8010002






