Characteristics of Seasonal Influenza Virus Activity in a Subtropical City in China, 2013–2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Influenza Virus Surveillance
2.2. Data Analysis
3. Results
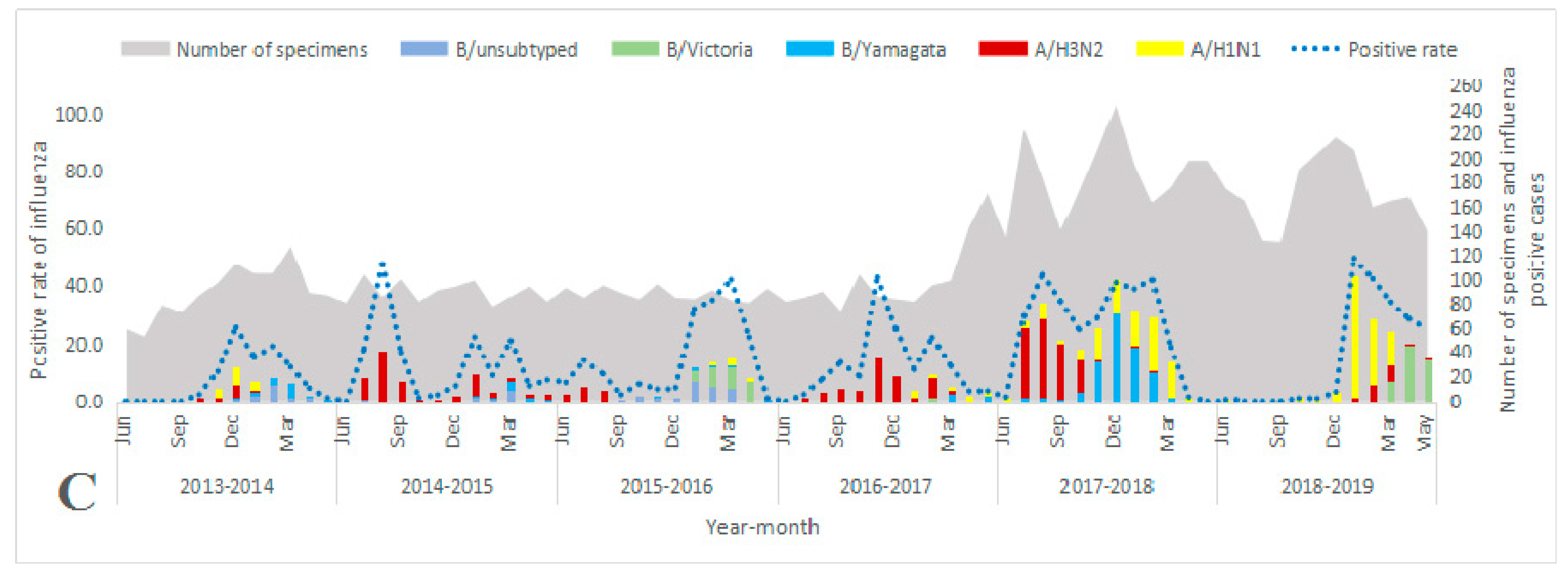
3.1. Overall Characteristics of Influenza Virus Surveillance
3.2. Influenza Virus Activity among Age Groups
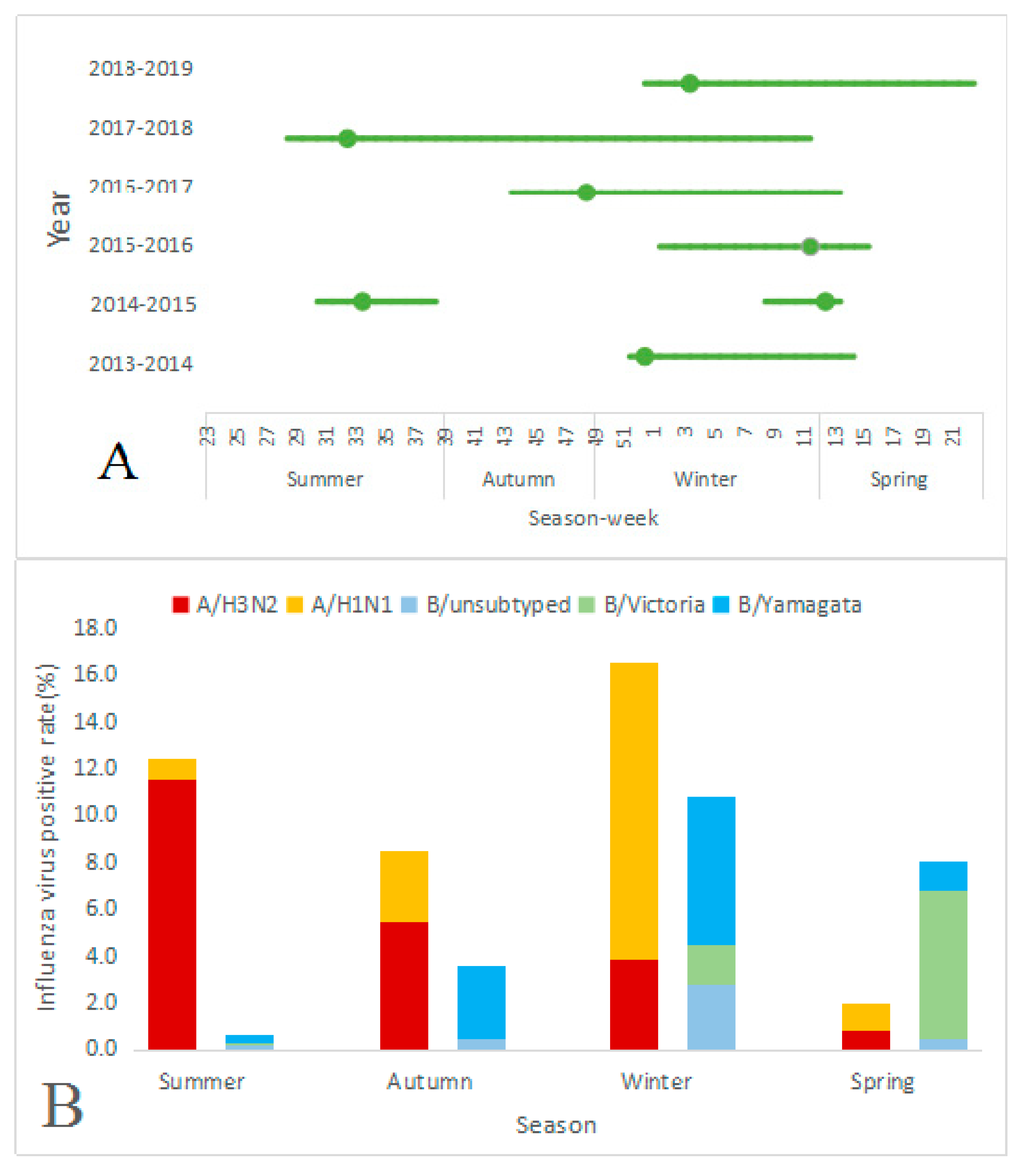
3.3. Seasonality Patterns of Influenza Viruses
3.4. Characteristics of Seasonal Influenza Epidemics by Season
3.5. Matching with the Trivalent Influenza Vaccine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Influenza Strategy 2019–2030; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Feng, L.Z.; Peng, Z.B.; Wang, D.Y.; Yang, P.; Yang, J.; Zhang, Y.Y.; Chen, J.; Jiang, S.Q.; Xu, L.L.; Kang, M.; et al. Technical guidelines for seasonal influenza vaccination in China, 2018–2019. Zhonghua Liu Xing Bing Xue Za Zhi 2018, 39, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef]
- Ma, C.; Pan, Y.; Zhang, L.; Zhang, Y.; Wu, S.; Sun, Y.; Duan, W.; Zhang, M.; Wang, Q.; Yang, P. Influenza vaccine effectiveness against medically attended influenza illness in Beijing, China, 2014/15 season. Hum. Vaccin. Immunother. 2017, 13, 2379–2384. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Mounts, A.W.; Feng, Y.; Luo, Y.; Yang, P.; Feng, Z.; Yang, W.; Yu, H. Seasonal influenza vaccine supply and target vaccinated population in China, 2004–2009. Vaccine 2010, 28, 6778–6782. [Google Scholar] [CrossRef]
- Yu, H.; Alonso, W.J.; Feng, L.; Tan, Y.; Shu, Y.; Yang, W.; Viboud, C. Characterization of regional influenza seasonality patterns in China and implications for vaccination strategies: SPATIO-temporal modeling of surveillance data. PLoS Med. 2013, 10, e1001552. [Google Scholar] [CrossRef]
- Azziz-Baumgartner, E.; Dao, C.N.; Nasreen, S.; Bhuiyan, M.U.; Mah, E.M.S.; Al Mamun, A.; Sharker, M.A.; Zaman, R.U.; Cheng, P.Y.; Klimov, A.I.; et al. Seasonality, timing, and climate drivers of influenza activity worldwide. J. Infect. Dis. 2012, 206, 838–846. [Google Scholar] [CrossRef]
- Alonso, W.J.; Viboud, C.; Simonsen, L.; Hirano, E.W.; Daufenbach, L.Z.; Miller, M.A. Seasonality of influenza in Brazil: A traveling wave from the Amazon to the subtropics. Am. J. Epidemiol. 2007, 165, 1434–1442. [Google Scholar] [CrossRef]
- Tamerius, J.; Nelson, M.I.; Zhou, S.Z.; Viboud, C.; Miller, M.A.; Alonso, W.J. Global influenza seasonality: Reconciling patterns across temperate and tropical regions. Environ. Health Perspect. 2011, 119, 439–445. [Google Scholar] [CrossRef]
- Horwood, P.F.; Karlsson, E.A.; Horm, S.V.; Ly, S.; Heng, S.; Chin, S.; Darapheak, C.; Saunders, D.; Chanthap, L.; Rith, S.; et al. Circulation and characterization of seasonal influenza viruses in Cambodia, 2012–2015. Influenza Other Respir. Viruses 2019, 13, 465–476. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Kuang, Y.; Li, T.; Xu, J.; Li, S.; Huang, T.; Wang, C.; Li, W.; Li, M.; et al. Temporal patterns of influenza A subtypes and B lineages across age in a subtropical city, during pre-pandemic, pandemic, and post-pandemic seasons. BMC Infect. Dis. 2019, 19, 89. [Google Scholar] [CrossRef]
- Hirve, S.; Newman, L.P.; Paget, J.; Azziz-Baumgartner, E.; Fitzner, J.; Bhat, N.; Vandemaele, K.; Zhang, W. Influenza seasonality in the tropics and subtropics—When to vaccinate? PLoS ONE 2016, 11, e0153003. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zhu, W.; Yu, J.; Li, Z.; Zhang, Y.; Wang, Y.; Gu, H.; Zou, W.; Hao, L.; Hu, W. Understanding the complex seasonality of seasonal influenza A and B virus transmission: Evidence from six years of surveillance data in Shanghai, China. Int. J. Infect. Dis. 2019, 81, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xiong, Y.; Xiao, B.; Tang, W.; Ling, H.; Long, J.; Xiao, D.; Zhao, H.; Ye, S.; Chen, S.; et al. Epidemiological and virological characteristics of influenza in Chongqing, China, 2011–2015. PLoS ONE 2016, 11, e0167866. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Li, Y.; Zhu, Y.; Zhang, J.; Li, X.; Zhang, J.; Zhao, K.; Hu, M.; Qin, G.; Wang, X.L. Seasonal pattern of influenza activity in a subtropical city, China, 2010–2015. Sci. Rep. 2017, 7, 17534. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, X.; Chu, Y.; Sun, J.; Qin, G.; Yang, L.; Qin, J.; Xiao, Z.; Ren, J.; Qin, D.; et al. Coherence of influenza surveillance data across different sources and age groups, Beijing, China, 2008–2015. PLoS ONE 2016, 11, e0169199. [Google Scholar] [CrossRef]
- Lin, J.; Kang, M.; Zhong, H.; Zhang, X.; Yang, F.; Ni, H.; Huang, P.; Hong, T.; Ke, C.; He, J. Influenza seasonality and predominant subtypes of influenza virus in Guangdong, China, 2004–2012. J. Thorac. Dis. 2013, 5, S109–S117. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, Z.; Li, X.; Li, Y.; Di, Y.; You, A.; Wang, Z. Analysis of the seasonality characteristics of influenza based on concentration degree and circular distribution methods in Henan. Med. Soc. 2013, 26, 7–9. [Google Scholar]
- Statistical Bureau of Yichang. Statistical Yearbook of Yichang City; Statistical Bureau of Yichang: Yichang, China, 2018. [Google Scholar]
- Chinese Center for Disease Control and Prevention. Influenza Sentinel Surveillance Protocol (2017) [EB/OL]. Available online: http://www.chinacdc.cn/jkzt/crb/xcrxjb/201712/t20171207_156180.html (accessed on 22 August 2019).
- Yang, J.; Lau, Y.C.; Wu, P.; Feng, L.; Wang, X.; Chen, T.; Ali, S.T.; Peng, Z.; Fang, V.J.; Zhang, J.; et al. Variation in influenza B virus epidemiology by lineage, China. Emerg. Infect. Dis. 2018, 24, 1536–1540. [Google Scholar] [CrossRef]
- Li, Z.; Xiaoxu, H.; Min, W.; Yuyin, J. Analysis of seasonal variation characteristics of Yichang city in recent 47 years based on GIS. In Proceedings of the 30th Annual Meeting of the Chinese Meteorological Society, Nan Jing, China, 23–25 October 2013; pp. 1–7. [Google Scholar]
- Moorthy, M.; Castronovo, D.; Abraham, A.; Bhattacharyya, S.; Gradus, S.; Gorski, J.; Naumov, Y.N.; Fefferman, N.H.; Naumova, E.N. Deviations in influenza seasonality: Odd coincidence or obscure consequence? Clin. Microbiol. Infect. 2012, 18, 955–962. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, P.; Feng, L.; Yang, P.; Pan, Y.; Feng, S.; Qin, Y.; Zheng, J.; Puig-Barbera, J.; Muscatello, D.; et al. Influenza vaccine effectiveness against influenza-associated hospitalization in 2015/16 season, Beijing, China. Vaccine 2017, 35, 3129–3134. [Google Scholar] [CrossRef]
- Nguyen, Y.T.; Graitcer, S.B.; Nguyen, T.H.; Tran, D.N.; Pham, T.D.; Le, M.T.; Tran, H.N.; Bui, C.T.; Dang, D.T.; Nguyen, L.T.; et al. National surveillance for influenza and influenza-like illness in Vietnam, 2006–2010. Vaccine 2013, 31, 4368–4374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blair, P.J.; Wierzba, T.F.; Touch, S.; Vonthanak, S.; Xu, X.; Garten, R.J.; Okomo-Adhiambo, M.A.; Klimov, A.I.; Kasper, M.R.; Putnam, S.D. Influenza epidemiology and characterization of influenza viruses in patients seeking treatment for acute fever in Cambodia. Epidemiol. Infect. 2010, 138, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-F.; Tong, Z.-D.; Li, P.; Chen, C.; Dai, Y.-X.; Wang, Y.-C.; Tang, A.; Chen, Y.; Yan, J.-B. Circular distribution analysis on seasonal characteristics of respiratory infectious diseases in Zhoushan. Prev. Med. 2018, 30, 1016–1020. [Google Scholar] [CrossRef]
- Yang, X.T.; Liu, X.F.; He, J.; Yu, D.S.; Liu, D.P.; Li, H.Y.; Li, B.D.; Bai, Y.N. Study on seasonal characteristics and pathogenic distribution of influenza in Gansu province of China. Chin. J. Epidemiol. 2017, 38, 763–766. [Google Scholar] [CrossRef]
- Saha, S.; Chadha, M.; Shu, Y. Divergent seasonal patterns of influenza types A and B across latitude gradient in tropical Asia. Influenza Other Respir. Viruses 2016, 10, 176–184. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: www.who.int/influenza/vaccines/virus/recommendations (accessed on 27 February 2020).
- Caini, S.; Huang, Q.S.; Ciblak, M.A.; Kusznierz, G.; Owen, R.; Wangchuk, S.; Henriques, C.M.; Njouom, R.; Fasce, R.A.; Yu, H.; et al. Epidemiological and virological characteristics of influenza B: RESULTS of the global influenza B study. Influenza Other Respir. Viruses 2015, 9, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, X.; Sun, H.; Xu, Y.; Suo, L.; Yu, X.; Cao, H.; Liu, B. Analysis of clinical features of patients infected with influenza A virus in a district hospital. Chin. J. Exp. Clin. Virol. 2019, 33, 166–170. [Google Scholar]
- Wei, C. A clinical synptoms analysis of elderly influenza and influenza-like patients. Clin. J. Chin. Med. 2016, 7, 122–123. [Google Scholar]
- Pan, Y.; Wang, Q.; Yang, P.; Zhang, L.; Wu, S.; Zhang, Y.; Sun, Y.; Duan, W.; Ma, C.; Zhang, M.; et al. Influenza vaccination in preventing outbreaks in schools: A long-term ecological overview. Vaccine 2017, 35, 7133–7138. [Google Scholar] [CrossRef]
- Yang, J.; Atkins, K.E.; Feng, L.; Pang, M.; Zheng, Y.; Liu, X.; Cowling, B.J.; Yu, H. Seasonal influenza vaccination in China: Landscape of diverse regional reimbursement policy, and budget impact analysis. Vaccine 2016, 34, 5724–5735. [Google Scholar] [CrossRef]
- Peng, Z.B.; Wang, D.Y.; Yang, J.; Yang, P.; Zhang, Y.Y.; Chen, J.; Chen, T.; Zheng, Y.M.; Zheng, J.D.; Jiang, S.Q.; et al. Current situation and related policies on the implementation and promotion of influenza vaccination, in China. Chin. J. Epidemiol. 2018, 39, 1045–1050. [Google Scholar] [CrossRef]
| Characteristics | 2013–2014 | 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | Overall |
|---|---|---|---|---|---|---|---|
| No. of patients | 1071 | 1077 | 1076 | 1187 | 2239 | 2043 | 8693 |
| Sex, n (%) | |||||||
| M | 444 (41.5) | 465 (43.2) | 463 (43.0) | 525 (44.2) | 1016 (45.4) | 923 (45.2) | 3836 (44.1) |
| F | 627 (58.5) | 612 (56.8) | 613 (57.0) | 662 (55.8) | 1223 (54.6) | 1120 (54.8) | 4857 (55.9) |
| Age, y, median (IQR) | 25 (8, 40) | 19 (5, 35) | 20 (5, 34) | 22 (5, 37) | 21 (7, 36) | 23 (7, 34) | 22 (6, 36) |
| Age group (years), n (%) ❉ | |||||||
| 0–4 | 161 (15.0) | 254 (23.6) | 233 (21.7) | 277 (23.3) | 401 (17.9) | 364 (17.8) | 1690 (19.4) |
| 5–14 | 167 (15.6) | 203 (18.9) | 213 (19.8) | 211 (17.8) | 404 (18.0) | 315 (15.4) | 1513 (17.4) |
| 15–24 | 192 (17.9) | 162 (15.0) | 184 (17.1) | 162 (13.7) | 440 (19.7) | 404 (19.8) | 1544 (17.8) |
| 25–59 | 478 (44.6) | 388 (36.0) | 384 (35.7) | 449 (37.8) | 824 (36.8) | 842 (41.2) | 3365 (38.7) |
| 60+ | 73 (6.8) | 70 (6.5) | 62 (5.8) | 88 (7.4) | 170 (7.6) | 118 (5.8) | 581 (6.7) |
| Weekly number of positive cases, median (IQR) | 4 (1, 6) | 2 (1, 5.5) | 3 (1, 7) | 3 (1, 5) | 13 (7, 21) | 11 (6, 15) | 4 (2, 10) |
| Weekly number of samples tested, median(IQR) | 20.5 (16, 24) | 20.5 (17, 24.5) | 21 (18, 24) | 21.5 (17, 26.5) | 43.5 (37, 48.5) | 38 (32.5, 42.5) | 24 (19, 36) |
| Age group (years) ❉, n1 *, n2 # (%) | 2013–2014 | 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | Overall |
|---|---|---|---|---|---|---|---|
| 0–4 | 161, 12 (7.5) | 254, 28 (11.0) | 233, 31 (13.3) | 277, 18 (6.5) | 401, 82 (20.4) | 364, 33 (9.1) | 1690, 204 (12.1) |
| 5–14 | 167, 47 (28.1) | 203, 49 (24.1) | 213, 56 (26.3) | 211, 40 (19.0) | 404, 137 (33.9) | 315, 71 (22.5) | 1513, 400 (26.4) |
| 15–24 | 192, 8 (4.2) | 162, 11 (6.8) | 184, 20 (10.9) | 162, 20 (12.3) | 440, 90 (20.5) | 404, 53 (13.1) | 1544, 202 (13.1) |
| 25–59 | 478, 25 (5.2) | 388, 45 (11.6) | 384, 45 (11.7) | 449, 50 (11.1) | 824, 213 (25.8) | 842, 157 (18.6) | 3365, 535 (15.9) |
| 60+ | 73, 5 (6.8) | 70, 10 (14.3) | 62, 3 (4.8) | 88, 11 (12.5) | 170, 60 (35.3) | 118, 9 (7.6) | 581, 98 (16.9) |
| Total | 1071, 97 (9.1) | 1077, 143 (13.3) | 1076, 155 (14.4) | 1187, 139 (11.7) | 2239, 582 (26.0) | 2043, 323 (15.8) | 8693, 1439 (16.6) |
| Characteristics | 2013–2014 | 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | |
|---|---|---|---|---|---|---|---|
| Epidemic periodicity | Annual | Semiannual | Annual | Year-round | Year-round | Annual | |
| Epidemic period | 2013.12.16– 2014.4.7 | 2014.7.21– 2014.9.22 | 2015.2.16– 2015.3.30 | 2016.1.4– 2016.4.18 | 2016.10.24– 2017.4.3 | 2017.7.10– 2018.3.19 | 2018.12.24– 2019.6.2 |
| Epidemic season | Winter, Spring | Summer | Winter, Spring | Winter, Spring | Autumn, Winter Spring | Summer, Autumn Winter | Winter, Spring |
| Start time (week number) | 51 | 30 | 8 | 1 | 43 | 28 | 52 |
| End time (week number) | 15 | 39 | 14 | 16 | 14 | 12 | 22 |
| Duration * (number of weeks) | 16 | 9 | 6 | 15 | 23 | 36 | 23 |
| Peak time (week number) | 52 | 33 | 12 | 11 | 48 | 32 | 4 |
| Predominant subtypes/ lineages | A/H1N1, B/Yamagata | A/H3N2 | B/Yamagata | B/Victoria | A/H3N2 | A/H3N2, B/Yamagata, A/H1N1 | A/H1N1, B/Victoria |
| Recommended B lineage component in trivalent influenza vaccine | B/Yamagata | B/Yamagata | B/Yamagata | B/Victoria | B/Victoria | B/Victoria | |
| Matching # | Yes | Yes | No | - | No | Yes | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, A.; Liu, J.; Ye, C.; Yu, J.; Peng, Z.; Feng, L.; Wang, L.; Qin, Y.; Zheng, Y.; Li, Z. Characteristics of Seasonal Influenza Virus Activity in a Subtropical City in China, 2013–2019. Vaccines 2020, 8, 108. https://doi.org/10.3390/vaccines8010108
Zhu A, Liu J, Ye C, Yu J, Peng Z, Feng L, Wang L, Qin Y, Zheng Y, Li Z. Characteristics of Seasonal Influenza Virus Activity in a Subtropical City in China, 2013–2019. Vaccines. 2020; 8(1):108. https://doi.org/10.3390/vaccines8010108
Chicago/Turabian StyleZhu, Aiqin, Jianhua Liu, Chuchu Ye, Jianxing Yu, Zhibing Peng, Luzhao Feng, Liping Wang, Ying Qin, Yaming Zheng, and Zhongjie Li. 2020. "Characteristics of Seasonal Influenza Virus Activity in a Subtropical City in China, 2013–2019" Vaccines 8, no. 1: 108. https://doi.org/10.3390/vaccines8010108
APA StyleZhu, A., Liu, J., Ye, C., Yu, J., Peng, Z., Feng, L., Wang, L., Qin, Y., Zheng, Y., & Li, Z. (2020). Characteristics of Seasonal Influenza Virus Activity in a Subtropical City in China, 2013–2019. Vaccines, 8(1), 108. https://doi.org/10.3390/vaccines8010108





