1. Introduction
Vaccination is a cost-efficient strategy to control various diseases such as autoimmunity, anaphylaxis, cancer, and especially infections. This is exemplified by the remarkable reduction in the mortality of epidemic outbreaks in recent years and the prolonged life-span of people as a result of prevalent vaccination [
1].
The conventional vaccines are made of the inactivated or live attenuated whole microbes and can elicit strong immunoresponses, but are also argued to be associated with certain safety concerns. These concerns on whole microbe-based vaccines arise from their potential of reversion of virulence or induction of the off-target immunity to cause unwanted side effects while weakening vaccination effects [
2,
3]. Therefore, subunit vaccines, which contain only the purified antigens (Ags), were timely developed at the beginning of 1970s when the genetic reassortment of virus strains rapidly matured and was innovatively introduced into the production of vaccines [
4]. Nowadays, subunit vaccines have received more and more research interests owing to their good reputation in inducing the accurate immunoresponses while causing minimal safety concerns. In addition, subunit vaccines are sharing the advantage of flexibility in formulation with other bioactive ingredients or combination with a carrier to boost immunostimulatory activities [
5]. However, subunit vaccines often show a crucial drawback of poor immunogenicity owing to lack of other microbial components, including especially the ones bearing the intrinsic adjuvanticity. To compensate for this defect, subunit vaccines are often combined with an adjuvant or engineered with proper excipients to form a carrier engendering vaccine adjuvant-delivery system (VADS) to enhance efficacy [
6,
7].
Alums (insoluble aluminum salts) are a conventional adjuvant or VADS that has been widely used for nearly a century to enhance the efficacy of numerous inactivated or subunit vaccines with unknown mechanisms [
2]. As a classical vaccine adjuvant, micron-sized alums, such as Al(OH)
3, AlPO
4, and (Al)
2(SO
4)
3, usually exist in the form of gel-like aggregates constituted of the sub-10-nm-sized crystals of aluminum (Al) salts and thus, vary remarkably in shape and size. Alums are known to induce potent humoral immunity with high levels of the anti-Ag Abs (antibodies). But alums are also known of the impotency in triggering cellular immunoresponses and can rarely induce effective anti-Ag CTLs (cytotoxic T lymphocytes), which, however, are one of the necessary elements for erasing certain pathogens, such as the intracellular microbes and cancer cells [
8]. In addition, alums in the form of the micron-sized coarse aggregates show relatively high reactogenicity and sometimes cause local inflammatory effects and even severe reactions in recipients [
9]. Therefore, in the past years alums have been reformulated to convert the coarse gel-like forms into certain new modalities, such as nanoparticles (NPs) [
10], nanorods [
11], and the complex of alum-TLR4a (Toll-like receptor 4 agonist) MPLA (monophosphoryl lipid A) [
12]. Surprisingly, it was recently reported that injection of alum with a carefully optimized protocol and a precise vaccination time schedule, induced effective anti-tumor CTL responses showing non-specific tumor suppression [
13]. These outcomes indicate that innovative strategies may remarkably improve adjuvanticity of alums and even enable the alum-adjuvanted vaccines to elicit both humoral and cellular immunity, suggesting the Al-based adjuvants of more potentials in boosting immunoresponse than those in practical use [
14].
Recently, based on the phosphophilicity of aluminum [
15], we have successfully engineered the phospholipid bilayer-coated aluminum NPs (PLANs) as a novel type of VADS that proves able to stimulate strong humoral and even cellular immunoresponses against Ags [
16]. However, in that study, PLANs were made with the unstable aluminum hydroxide (Al(OH)
3) NPs obtained by neutralizing in a solution of aluminum chloride (AlCl
3) with alkaline sodium hydroxide (NaOH). The prepared Al(OH)
3) NPs demonstrated a high heterogenicity in size and morphology, significantly lowering the batch-to-batch consistency and thus reproducibility of the final product PLANs [
9]. Although the instability and heterogenicity of the Al(OH)
3-based PLANs were improved through using a procedure of reverse ethanol injection-lyophilization (REIL), the engagement of organic solvent in the preparation introduced unbeneficial factors with the potential to compromise the activity of Ags of interest.
In this report, the Ag-loaded PLANs and PEGylated-PLANs (PEG-PLANs) as a novel VADS were engineered using the stable aluminum oxide (Al2O3) NPs (ANs) instead of the unstable Al(OH)3 NPs while employing the thin film-rehydration procedure in preparation. This classical procedure is of no novelty but allows the labile Ags to avoid the contact with the organic solvent, thus maintaining well their immunostimulatory activity. In addition, the comparative studies were performed on AMs (aluminum microparticles), ANs, PLANs, and PEG-PLANs to investigate their special abilities in triggering the anti-Ag immunoresponses through in vitro and in vivo experiments.
2. Materials and Methods
2.1. Materials
SPC (soy phosphatidylcholine), DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine), and DSPE-PEG2000 (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(methoxy(polyethylene glycol)2000) (ammonium salt)) were products by Avanti Polar Lipids (Alabaster, Alabama, USA). Aluminum oxide (Al2O3) nanoparticles with a size of 40 nm, ovalbumin (OVA), bovine serum albumin (BSA), calcein, 4′,6-diamidino-2-phenylindole (DAPI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), CFSE (5(6)-carboxyfluorescein diacetate N-succinimidyl ester), and 3,3,5-tetramethylbenzidine (TMB) were products by Sigma-Aldrich (Merck, Darmstadt, Germany). Goat anti-mouse IgG-horse radish peroxidase (HRP), IgG1-HRP, IgG2a-HRP, and mouse cytokines IFN-γ and IL-4 ELISA assay kits, fluorochrome-labeled anti-mouse Abs against different cell surface antigens such as CD40, CD80, and CD86 for APC activation assay, and CD4 and CD8 for T lymphocytes activation assay were purchased from either eBioscience (San Diego, USA) or BioLegend (San Diego, USA). LysoTracker®-red, HyClone RPMI 1640, DMEM/F12 cell culture medium were purchased from Thermo Fisher Scientific Inc. (Waltham, AMs, USA). Chromatographic grade solvents, analytic grade agents, and other chemicals were provided by Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Pure water was produced using Milli-Q® IQ 7000 Ultrapure Water System (Merck, Germany).
2.2. Preparation of Al-Based Carriers
The blank or cargo-loaded Al-based nanocarriers including PLANs and PEG-PLANs were prepared by thin film-rehydration procedure. Briefly, in a pear-shaped flask, SPC was dissolved in chloroform, which was evaporated at room temperature under a reduced pressure using a rotary evaporator and completely removed in a vacuum chamber overnight to form a thin lipid film. Then an aqueous suspension of (OVA- or calcein-associated) ANs was added into the flask for rehydration of the lipid film at an electromagnetic stir of 360 rpm (round per min) at 45 °C for 2 h under N
2 protection. Then, the rehydrated samples were washed thrice with saline to remove the excess empty liposomes and free cargos by centrifugation at 2000 rpm for 15 min at 4 °C. The proper ratio of phospholipid to ANs was estimated according to our previous report [
16], while phospholipids of empty liposomes were quantified by the high performance liquid chromatography (HPLC) method described in the Characterization section.
For preparation of the PEG-PLANs, a procedure of post insertion was used [
17]. Briefly, an appropriate amount of DSPE-PEG
2000 (with the mole ratio of SPC/DSPE-PEG
2000 = 20:1) was dissolved in chloroform in a 5-mL ampoule. Then the samples were removed of organic solvent by nitrogen gas flow to form a thin film, which was subsequently dried in a vacuum chamber overnight. Finally, the thin film of DSPE-PEG
2000 was rehydrated with an aqueous dispersion of PLANs at a stir of 120 rpm at 45 °C for 1 h under N
2 protection, thus forming PEG-PLANs.
Calcein, which at a neutral pH condition is a membrane impermeable fluorescent agent with a self-quenched concentration of >4 mM [
18], was complexed (at 0.01 mM) with Al particles before lipid coating to label the prepared carriers. The labeled carriers were used for inspecting APC uptake, intracellular location, and in vivo traffic of Al-based VADSs by either a LSCM (laser scanning confocal microscopy) (Leica TCS SP5, Wetzlar, Germany) or flow cytometry (BD FACSVerse™, San Jose, CA, USA).
Aluminum phosphate (AlPO4) crystal salt was smashed into AlPO4 microparticles (AMs) with a size of around 2 μm and was used as a control, i.e., as the conventional adjuvant alum.
2.3. Characterization of the Al-Based Nanocarriers
The nanoparticulate samples were characterized of size and zeta potential by DLS (dynamic light scattering) and ELS (electrophoretic light scattering), respectively. A Malvern ZS90 Zetasizer (Malvern, Worcestershire, UK) with a 633 nm-wavelength He–Ne laser was used to collect the optics data with the detector at 90° to incident light at 25 °C.
FTIR (Fourier transform infrared spectroscopy) spectra of the lyophilized PLANs, anhydrous AlPO4, and SPC were obtained for dissection of the bond interaction between aluminum and phosphate group of SPC. FTIR detection was carried out by transmission method, using the 2 mg sample dispersed in a 200 mg KBr disk, in a FTIR spectrophotometer (IR Prestige-21, Shimadzu, Japan).
The Al-based nanocarriers were imaged using a TEM (transmission electron microscope) system (HF-3300 TEM System, Hitachi Ltd., Tokyo, Japan), and the samples were negatively stained with uranyl, according to previous reports [
16,
19].
Phospholipids were quantitated with a HPLC (high performance liquid chromatography) system (LC-6AD liquid chromatograph, SPD-20A UV detector, SIL-10AF autosampler, Shimadzu) equipped with ODS C18 column (5 µm, 4.6 × 150 mm). The SPC in empty liposomes was isolated from PLANs or PEG-PLANs by centrifugation at 5000×
g for 10 min at 4 °C and was quantified by HPLC method according to a previous report but with a little modification [
20]. The UV detection wavelength was 205 nm and a mobile phase of methanol-isopropanol-water-trifluoroacetic acid (95:5:100:0.05,
v/
v) with flow rate of 1 mL/min at 30 °C.
The Ag association efficiency of samples was calculated according to the ratio of the carrier-associated Ag to total Ag. The carrier-associated Ag was deduced from subtraction of the total Ag with free Ag, which was isolated from sample by centrifugation at 10,000× g for 10 min at 4 °C and quantitated by Bradford assay.
For Ag release test, in triplicate, 0.5 mL of samples that had been removed of free Ag was added into a 10-mL conical flask with a ground stopper and diluted with the buffered solutions (pH 7.4 or 5.5) to 5 mL and incubated at 37 °C under stirring of 100 rpm. At different time intervals (0.5, 1, 2, 4, 8, 16, 24, 48 h), 30 μL of release medium was accurately taken out of the flask, which was immediately supplemented with an identical amount of blank medium. The sample-containing medium was centrifuged at 10,000×
g for 10 min at 4 °C for isolation of the released Ags in the supernatant, which was transferred into a hole of a 96-well plate. Then the plate was stored at 4 °C in a refrigerator until the Ag assay, which was carried out with the micro-Bradford protocol, whereby the plate was read at 595 nm in a microplate reader (µQuant, BioTek Instruments, Inc., Vermont, USA) [
21,
22].
2.4. APC Uptake and Safety Evaluation of the Al-Based Carriers
The prepared Al-based carriers, including AMs, ANs, PLANs, and PEG-PLANs, were evaluated of APC uptake using mouse BMDCs (mouse bone marrow-derived dendritic cells), which were derived from mouse bone marrow precursors according to previous reports [
23,
24]. For APC uptake assay, the BMDCs were first transferred onto a 24-well plate with 1 mL of 10
5 cells per well and incubated for 24 h in a cell culture chamber at 37 °C at an atmosphere of 5% CO
2. Then, each well containing BMDCs was supplemented with 100 µL of free calcein or calcein-loaded carriers (AMs, ANs, PLANs and PEG-PLANs, at 10 µg/mL Al final concentration), and mixed homogeneously for another 4 h incubation in the cell culture chamber. After the incubation, the co-cultured cells were removed of the un-associated agents or nanocarriers through centrifugation at 800×
g at 4 °C and thrice PBS washing. Finally, the cells were tested by flow cytometry (BD FACSVerse™, San Jose, CA, USA) and assayed with FlowJo software (Tree Star, Ashland, OR, USA) to evaluate cellular uptake of carriers. Also, a small fraction of cells was imaged by LSCM (with a laser scanning confocal microscope, Leica TCS SP5, Wetzlar, Germany).
The safety of the blank Al-based carriers was evaluated by testing their cytotoxicity with the classical MTT method. Briefly, 100 μL of 10
5 mouse macrophages (RAW264.7) was seeded in each well of a 96-well plate and incubated in a complete growth medium DMEM for a 24-h culture in a cell chamber. Then, in triplicate, the plate wells were individually added with one type of the Al-carrier samples at a certain concentration (each sample with a series of concentration ranging from 0 to 200 μg/mL Al), followed by another 24 h incubation at 37 °C. After the incubation and removal of supernatant, each well of the plate was added with 200 μL of DMEM containing 1 mg/mL MTT and incubated at 37 °C for 4 h. Thereafter, the plate was again removed of the supernatant and supplemented with 200 μL of DMSO in each well for another incubation at 37 °C for 30 min under shaking. After that, the optical absorbance (OA) of each well of the plate was measured using μQuant microplate reader at 540 nm wavelength. The viability index (%) was used to evaluate the safety of the Al-based VADS and was calculated according to the following Equation (1):
2.5. APC Activation and Intracellular Localization of Al-Based Nanocarriers
For tracking the intracellular agents, a fraction of the BMDCs co-cultured with Al particles were transferred onto a 12-well plate (10
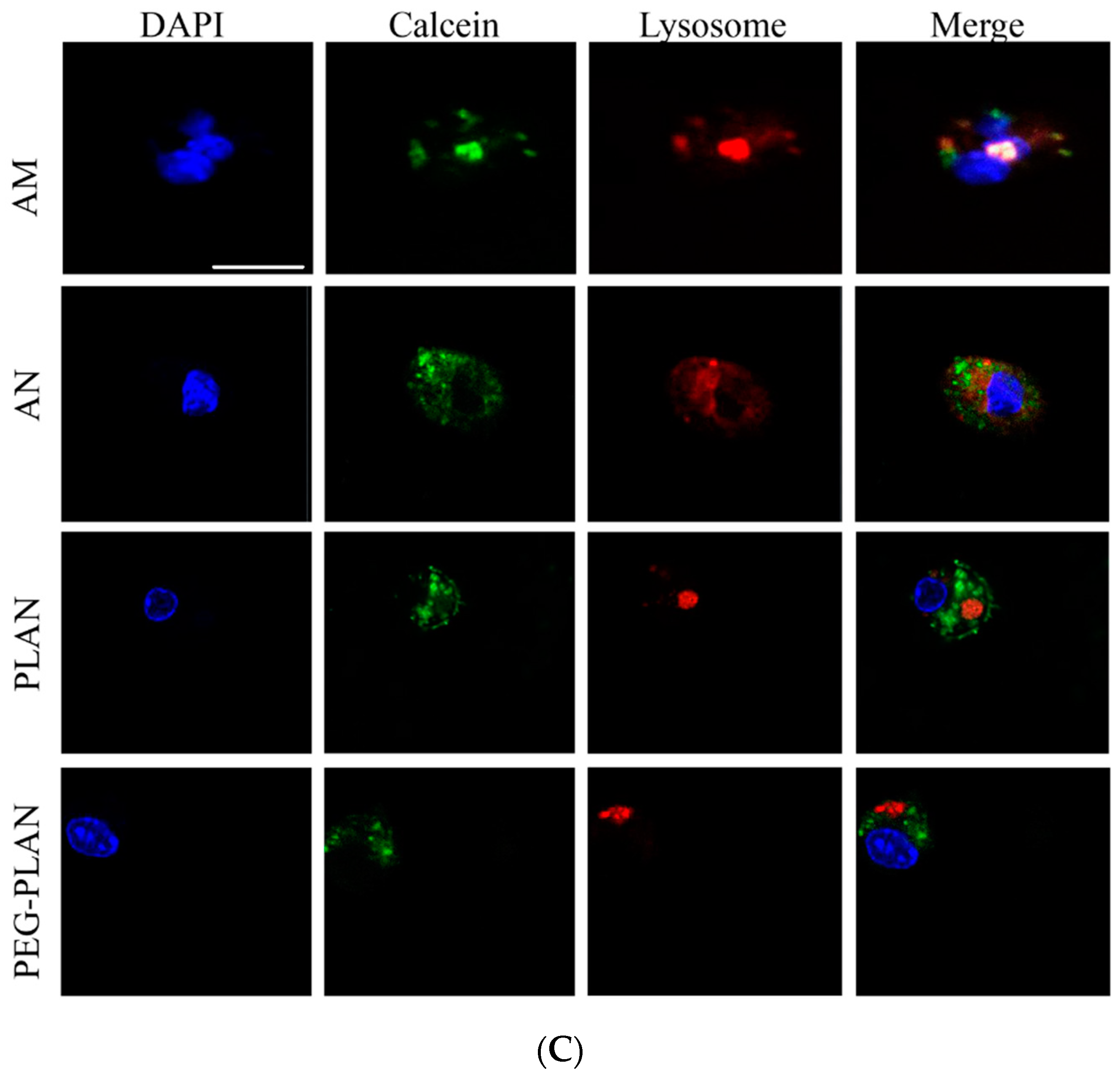
4 cells/well) and incubated in pre-warmed RPMI 1640 medium (37 °C) containing 50 nM LysoTracker-red and DAPI to localize cellular lysosomes and nuclei, respectively. After washing thrice, BMDCs were observed in a LSCM to distinguish in cells the DAPI-stained blue nuclei, LysoTracker-red-labelled lysosomes, and the green domains (occupied by calcein-carriers). The separation of green-occupied domains from the red dots (LysoTracker-red-labelled lysosomes) is thought the evidence indicating that the carrier has engendered lysosome escape to the cargos [
25].
The ability of OVA-loaded Al-based carriers (AMs, ANs, PLANs, and PEG-PLANs) to activate APCs for maturation was tested with BMDCs [
26]. Briefly, the cells cultured in a 24-well plate (0.5 × 10
5 cells/well) with 0.5 mL RPMI 1640 medium were fed with 50 µL of 20 µg soluble OVA or carrier-loaded OVA for 30 h stimulation. After refreshment of culture RPMI 1640 medium, BMDCs were exposed to the fluorochrome-conjugated Abs against CD40, CD80, and CD86, which are markers of maturation of the activated APCs.
2.6. Mouse Vaccination
Female Kunming mice, aged 8 weeks, were provided from the Experimental Animal Center of Anhui Medical University. Animal experiments were approved by the Animal Ethic Committee at Anhui Medical University and were performed in compliance with the Declaration of Helsinki for Care and Use of Laboratory Animals (approval number LLSC20170284).
Mice were divided into different groups (6 mice per group) and subcutaneously immunized only once for each group with free OVA, the OVA-loaded AM, ANs, PLANs, or PEG-PLANs at a dose of 10 µg OVA, regardless of their bodyweight. The subcutaneous injection site was kept at the lateral aspect of left thigh near the knee.
2.7. In Vivo Tracking of Nanocarriers
To track in vivo Al-based nanocarriers, the calcein-loaded samples (ANs, PLANs, PEG-PLANs) were subcutaneously injected into mice at the left thigh lateral aspect near the knee (n = 3). Next, 4, 8, and 16 h after vaccination, the inguinal LNs responsible for draining the hindlimb region were isolated from the vaccinated mice for further investigation. To observe regional distribution of fluorescent nanocarriers, mouse inguinal LNs (lymph nodes) were harvested for making the OCT (optimum cutting temperature) compound-embedded cryosections to be viewed using a fluorescence microscope (IX83, Olympus, Japan). For quantitative analysis of the uptake of Al-based nanocarriers by immune cells within LNs, inguinal LNs were harvested to isolate immunocytes, which were assayed by flow cytometry.
2.8. Assay of the Ag-Specific Immunity Elicited by Al-Based Carriers
Three weeks after immunization, blood samples were collected from the mice via the retro-orbital sinus [
27], which were let to stand for 30 min for clotting and then serum was collected through centrifugation at 3000×
g for 10 min at 4 °C. The serum samples were assayed of the Ag-specific Abs by the indirect ELISA protocol [
28]. Briefly, a 96-well plate was coated with 100 µL of OVA (10 µg/mL) in carbonate buffer (15 mM Na
2CO
3, 35 mM NaHCO
3, pH 9.6) per well and incubated at 4 °C overnight. Then, after washing thrice with 200 µL PBST (PBS containing 0.05%
v/
v Tween-20), each well of the plate was blocked with 200 µL of 1%
w/
v BSA-containing PBST and incubated at 37 °C for 1 h for covering non-specific binding sites. After washing thrice with PBST, each well of the plate was loaded with 100 μL serum sample and then, after serial dilution with PBST-BSA (PBST containing 0.1%
w/
v BSA), it was incubated at 37 °C for 1 h. After washing thrice with PBST, each well was added with 100 μL of the HRP-conjugated secondary antibodies diluted with PBST-BSA (1:5000) and then incubated at 37 °C for 1 h. After washing five times with PBST, each well was supplemented with 100 μL of TMB substrate solution (containing 1 mM TMB, 3 mM H
2O
2, and 0.2 mM TBABH (tetramethylammonium borohydride)) for 20 min color development. Finally, the reaction was terminated by 100 μL of 1 M H
2SO
4 solution. Then, immediately, the optical absorbance (OA) at 450 nm, with a reference wavelength at 570 nm, of each well was measured using an automated microplate reader (µQuant, BioTek Instruments, Inc., Vermont, USA).
The serum samples were also tested of cytokines including IL-4 and IFN-γ by sandwich enzyme immunoassay using ELISA assay kits.
2.9. Differentiation of T Lymphocytes in Spleen
Three weeks after immunization, under an aseptic operation, the mice were excised of spleens for separation of splenocytes, which were isolated according to a previous report [
28]. A total of 500 µL of 5 × 10
5 cells were added to each well of a 24-well plate and incubated in the presence of 2.5 µg/mL OVA for 72 h at 37 °C in a cell chamber containing 5% CO
2. Thereafter, the plate was centrifuged at 1000×
g for 10 min and the supernatants were carefully collected and stored at −20 °C for detection of cytokines IL-4 and IFN-γ using the sandwich enzyme immunoassay. Then, 500 µL of PRMI 1640 was added to each well and the plate was vortexed to resuspend cells, which were stained with anti-CD4-Ab-PE and anti-CD8-FITC at 4 °C for 2 h. Finally, the cells were assayed by flow cytometry for evaluation of CD4+ T lymphocytes and CD8+ CTLs generated by vaccination with different formulations.
2.10. Statistical Analysis
All results were given as mean ± SD (standard deviation). Statistical differences among multiple groups were analyzed with ANOVA, and Dunnett’s post hoc
t-test was employed to analyze the differences between groups. And the analyses were conducted using SPSS software, and a
p < 0.05 was considered of significance, though this value is argued more than often leading to “hyped claims” [
29].
4. Discussion
Previously, we made PLANs using Al(OH)
3 NPs, which, however, were unstable polymorphic nanoaggregates and difficult to control in the size and shape, both of which are the important factor to influence the immunostimulatory functions [
16]. Therefore, in this investigation, the very stable Al
2O
3 NPs with a size of 40 nm were used instead of Al(OH)
3 NPs to prepare a novel version of PLANs and the PEGylated PLANs (PEG-PLANs) as a VADS. PEGylation of nanoparticles proves beneficial for the delivery of vaccines in several aspects, such as targeting dLNs, avoiding phagocytosis by non-professional APCs, and especially, enhancing product stability [
26]. Enhancement of the stability of nanoparticles by PEGylation is argued to be caused by the steric stabilization effects of a highly hydrophilic polymer and has already been successfully employed for making the stealth liposome products [
34], such as the marketed Doxil
®, Onivyde
®, and Onpattro
® [
35]. Thus in this investigation, the PEG-PLANs were also engineered as a VADS for evaluation of their vaccine delivery effects. These Al-based nanocarriers, as well as the micro-sized alum (used as a control), were comparatively studied of the immunostimulatory activities through in vitro and in vivo experiments.
In comparison to alum, whilst ANs (Al
2O
3 NPs) showed only a few benefits in triggering immune system such as high safety, enhancing cellular uptake, and engendering lysosome escape, both PLANs and PEG-PLANs manifested many additional superiorities, including efficiently targeting dLNs, inducing Th1-biased immunoresponses, eliciting stronger humoral as well as cellular immunity. Notably, unlike alum with little ability to activate APC phagocytic response, perhaps due to its firm binding and disturbance of cell membranes [
32], ANs can remarkably facilitate cellular uptake of their delivered cargos. A possible explanation for this is that the small size of ANs may engender the binding of ANs to APCs to exert only moderate membrane disturbance, which is insufficient to cause abortive phagocytic response but able to induce cellular internalization (
Figure 2). In the case of PLANs, while the phospholipid coating has a high affinity to cell membranes, it also conceals the phosphophilicity of Al to nullify its membrane-binding/-sorting functions, thus facilitating cell internalization [
16]. For PEG-PLANs, the enhanced cellular phagocytosis is argued to be attributed to the selective adsorption by PEG of interstitial proteins, rather than clusterin. The reason is that clusterin has recently proven able to form the specific protein corona surrounding NPs that accounts for the generation of stealth effects to abolish monocyte phagocytosis [
36].
The intracellular investigations showed that the Al-nanocarriers engineered in this study after cellular internalization may manage lysosome escape, allowing Ags to avoid the destructive degradation by lysosomal enzymes. Lysosome escape may also render the delivered Ags the opportunity for cross-presentation and the downstream display of MHC-1-Ag-epitopes, favoring the Th1-biased responses and cellular immunity [
37]. By comparison, micro-sized alum can hardly be internalized by APCs and thus has no chance to affect organelles and intracellular pathways, e.g., to engender lysosome escape and cross-presentation for Ags. As a result, a majority of Ags delivered by alum is subjected to lysosomal degradation and even extracellular destruction, thus compromising immunostimulatory effects while missing Th1 responses. This is in consistence with the well-known fact that the alum-adjuvanted vaccines generally induce recipients to produce predominantly the anti-Ag Abs over CTLs [
9]. Although recently it was reported that injection of alum alone with a specific protocol stimulated non-specific anti-tumor CTL responses [
13], the results needs further verification in both wide utility and practical feasibility.
This study also confirmed that small size and coating allow both PLANs and PEG-PLANs after topical administration to smoothly traffic to the dLNs, wherein the Al-based nanocarriers efficiently target and activate different populations of the immunocytes that are densely compartmentalized within dLNs. These immunocytes include DCs, murine macrophages (MPs), as well as follicular B cells and, upon activation, will immediately trigger T lymphocytes to orchestrate together for sponsoring the anti-Ag responses [
38]. By comparison, the micron-sized alum after topical administration are trapped in the vaccination site, due to binding to cells as well as due to their large size, and therefore, will mainly be picked up by sentinel APCs, including skin-resident MPs, DCs, and Langerhans cells (LCs). However, only DCs and LCs can manage to migrate via the afferent lymphatics to the dLNs [
39], wherein they mature and trigger T lymphocytes, thus lowering vaccination efficiency as a result of MP consumption of Ags without participation in the following reactions. Moreover, owing to being stranded in local site, the alum-carried Ags were mostly denied of the opportunity to access follicular B cells, whose activation, notably, is necessary for production of anti-Ag Abs, thus leading to inefficient vaccination [
40]. By contrast, the Al-based nanocarriers, especially, PLANs and PEG-PLANs, as a VADS are capable of targeting and activating multiple populations of the dLN immune cells, which orchestrate together to efficiently initiate the Ag-specific immunoresponses for establishing immunity against pathogens bearing identical Ags. PEG-liposomes have also been employed by many researchers for delivery of vaccines for targeting dLNs [
26,
35]. For example, Perrie’s group prepared the PEGylated cationic nanoliposomes as a VADS and observed that the PEGylation engendered the cationic nanocarriers to target mouse dLNs as a result of the passive drainage. However, compared to the un-PEGylated counterparts, the PEGylated liposomes produced deceased depot effects while inducing lowered production of IgG2b and IFN-γ but elevated IL-5, suggesting the PEGylated cationic liposomes may favor triggering the Th2-biased immunoresponses [
41]. By contrast, PEG-PLANs showed a great promise in stimulating the Th1/Th2 balanced reactions, as indicated by the high levels of IFN-γ and IgG2a and the high fraction of CD8+ T lymphocytes produced in mice immunized with PEG-PLANs versus PLANs.