An Influenza Virus Hemagglutinin-Based Vaccine Platform Enables the Generation of Epitope Specific Human Cytomegalovirus Antibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study Approval
2.2. Cell Lines, Antibodies, and Viruses
2.3. Chimeric Influenza Virus Model
2.4. Chimeric Influenza Virus Construction and Amplification
2.5. Immunofluorescence Staining
2.6. Influenza Virus Hemagglutination Inhibition Activity and Neutralization Assay
2.7. Animal Vaccinations
2.8. CMV Serum Inhibition Assay
2.9. Flow Cytometry gH Binding Analysis
3. Results
3.1. Generation of Chimeric Influenza/CMV
3.2. A gH MAB Inhibits Hemagglutination and Neutralizes Chimeric Influenza Viruses
3.3. Vaccination with Chimeric Influenza Viruses Elicits gH-Specific Antibodies
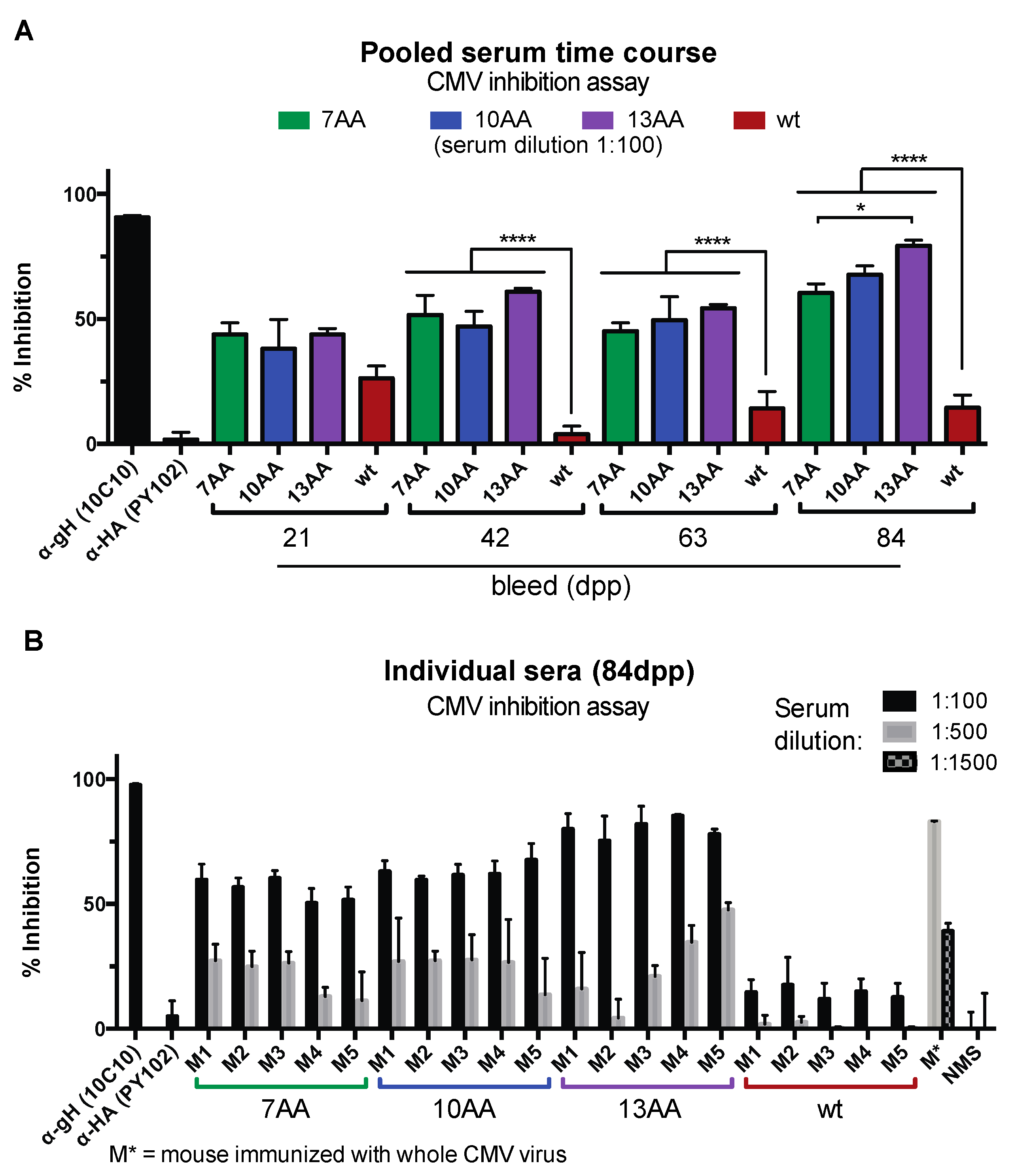
3.4. Chimeric HA/gH Influenza Viruses Generate CMV Inhibitory Antibodies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mocarski, E.S. Betaherpes viral genes and their functions. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Damato, E.G.; Winnen, C.W. Cytomegalovirus Infection: Perinatal Implications. J. Obstet. Gynecol. Neonatal Nurs. 2002, 31, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.J.; Pellett, P.E. Risk of Congenital Cytomegalovirus Infection. Clin. Infect. Dis. 2005, 40, 1701–1702. [Google Scholar] [CrossRef]
- Cheeran, M.C.J.; Lokensgard, J.R.; Schleiss, M.R. Neuropathogenesis of congenital cytomegalovirus infection: Disease mechanisms and prospects for intervention. Clin. Microbiol. Rev. 2009, 22, 99–126. [Google Scholar] [CrossRef]
- Dove, A. A Long Shot on Cytomegalovirus. Available online: https://www.the-scientist.com/uncategorized/a-long-shot-on-cytomegalovirus-47008 (accessed on 14 June 2019).
- Goldner, T.; Zimmermann, H.; Lischka, P. Phenotypic characterization of two naturally occurring human Cytomegalovirus sequence polymorphisms located in a distinct region of ORF UL56 known to be involved in in vitro resistance to letermovir. Antivir. Res. 2015, 116, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Chou, S. Approach to Drug-Resistant Cytomegalovirus in Transplant Recipients. Curr. Opin. Infect. Dis. 2015, 28, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, L.; Nasar, A.; Goodlet, K.J.; Nailor, M.D.; Tokman, S.; Chou, S. Emergence of letermovir resistance in a lung transplant recipient with ganciclovir-resistant cytomegalovirus infection. Am. J. Transplant. 2018, 18, 3060–3064. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.J.; Tortorella, D. Virion Glycoprotein-Mediated Immune Evasion by Human Cytomegalovirus: A Sticky Virus Makes a Slick Getaway. Microbiol. Mol. Boil. Rev. 2016, 80, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.T.; Yurochko, A.D. Analysis of Cytomegalovirus Binding/Entry-Mediated Events. Methods Mol. Biol. 2014, 1119, 113–121. [Google Scholar]
- Anderholm, K.M.; Bierle, C.J.; Schleiss, M.R. Cytomegalovirus Vaccines: Current Status and Future Prospects. Drugs 2016, 76, 1625–1645. [Google Scholar] [CrossRef]
- Diamond, D.J.; La Rosa, C.; Chiuppesi, F.; Contreras, H.; Dadwal, S.; Wussow, F.; Bautista, S.; Nakamura, R.; Zaia, J.A. A fifty-year odyssey: Prospects for a cytomegalovirus vaccine in transplant and congenital infection. Expert Rev. Vaccines 2018, 17, 889–911. [Google Scholar] [CrossRef]
- Inoue, N.; Abe, M.; Kobayashi, R.; Yamada, S. Vaccine development for cytomegalovirus. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; pp. 271–296. [Google Scholar]
- Pass, R.F.; Zhang, C.; Evans, A.; Simpson, T.; Andrews, W.; Huang, M.-L.; Corey, L.; Hill, J.; Davis, E.; Flanigan, C.; et al. Vaccine Prevention of Maternal Cytomegalovirus Infection. N. Engl. J. Med. 2009, 360, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Munoz, F.M.; Callahan, S.T.; Rupp, R.; Wootton, S.H.; Edwards, K.M.; Turley, C.B.; Stanberry, L.R.; Patel, S.M.; Mcneal, M.M.; et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial. Vaccine 2016, 34, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.D.; Stanton, A.; McCarrell, E.; Smith, C.; Osman, M.; Harber, M.; Davenport, A.; Jones, G.; Wheeler, D.C.; O’Beirne, J.; et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: A phase 2 randomised placebo-controlled trial. Lancet 2011, 377, 1256–1263. [Google Scholar] [CrossRef]
- Vincenti, F.; Budde, K.; Merville, P.; Shihab, F.; Ram Peddi, V.; Shah, M.; Wyburn, K.; Cassuto-Viguier, E.; Weidemann, A.; Lee, M.; et al. A randomized, phase 2 study of ASP0113, a DNA-based vaccine, for the prevention of CMV in CMV-seronegative kidney transplant recipients receiving a kidney from a CMV-seropositive donor. Am. J. Transplant. 2018, 18, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; La Rosa, C.; Longmate, J.; Drake, J.; Slape, C.; Zhou, Q.; Lampa, M.G.; O’Donnell, M.; Cai, J.-L.; Farol, L.; et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: Randomised phase 1b trial. Lancet Haematol. 2016, 3, e87–e98. [Google Scholar] [CrossRef]
- La Rosa, C.; Longmate, J.; Martinez, J.; Zhou, Q.; Kaltcheva, T.I.; Tsai, W.; Drake, J.; Carroll, M.; Wussow, F.; Chiuppesi, F.; et al. MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults. Blood 2017, 129, 114–125. [Google Scholar] [CrossRef]
- Zhou, W.; Longmate, J.; Lacey, S.F.; Palmer, J.M.; Gallez-Hawkins, G.; Thao, L.; Spielberger, R.; Nakamura, R.; Forman, S.J.; Zaia, J.A.; et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood 2009, 113, 6465–6476. [Google Scholar] [CrossRef]
- Fu, T.-M.; An, Z.; Wang, D. Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease. Vaccine 2014, 32, 2525–2533. [Google Scholar] [CrossRef]
- Banaszynski, L.A.; Chen, L.-C.; Maynard-Smith, L.A.; Ooi, A.G.L.; Wandless, T.J. A Rapid, Reversible, and Tunable Method to Regulate Protein Function in Living Cells Using Synthetic Small Molecules. Cell 2006, 126, 995–1004. [Google Scholar] [CrossRef]
- Glaß, M.; Busche, A.; Wagner, K.; Messerle, M.; Borst, E.M.; Glass, M. Conditional and reversible disruption of essential herpesvirus proteins. Nat. Methods 2009, 6, 577–579. [Google Scholar] [CrossRef]
- Wussow, F.; Chiuppesi, F.; Martinez, J.; Campo, J.; Johnson, E.; Flechsig, C.; Newell, M.; Tran, E.; Ortiz, J.; La Rosa, C.; et al. Human Cytomegalovirus Vaccine Based on the Envelope gH/gL Pentamer Complex. PLoS Pathog. 2014, 10, e1004524. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.J.; Stein, K.R.; Duty, J.A.; Schwarz, T.M.; Noriega, V.M.; Kraus, T.; Moran, T.M.; Tortorella, D. Functional screening for anti-CMV biologics identifies a broadly neutralizing epitope of an essential envelope protein. Nat. Commun. 2016, 7, 13627. [Google Scholar] [CrossRef] [PubMed]
- Macagno, A.; Bernasconi, N.L.; Vanzetta, F.; Dander, E.; Sarasini, A.; Revello, M.G.; Gerna, G.; Sallusto, F.; Lanzavecchia, A. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J. Virol. 2010, 84, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Comps-Agrar, L.; Ellerman, D.; Warming, S.; Feierbach, B.; Fouts, A.E.; Stengel, K.F.; Schoeffler, A.J.; Eaton, D.L. Mechanism for neutralizing activity by the anti-CMV gH/gL monoclonal antibody MSL-109. Proc. Natl. Acad. Sci. USA 2014, 111, 8209–8214. [Google Scholar]
- Ciferri, C.; Chandramouli, S.; Leitner, A.; Donnarumma, D.; Cianfrocco, M.A.; Gerrein, R.; Friedrich, K.; Aggarwal, Y.; Palladino, G.; Aebersold, R.; et al. Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies. PLOS Pathog. 2015, 11, e1005230. [Google Scholar] [CrossRef]
- Gerlach, T.; Elbahesh, H.; Saletti, G.; Rimmelzwaan, G.F. Recombinant influenza A viruses as vaccine vectors. Expert Rev. Vaccines 2019, 18, 379–392. [Google Scholar] [CrossRef]
- Penghui, Y.; Fang, S.; Ruilan, W.; Guanglin, L.; Hongjing, G.; Yueqiang, D.; Zhongpeng, Z.; Xiaolan, Y.; Zhaohai, W.; Shaogeng, Z.; et al. Oncolytic Activity of a Novel Influenza A Virus Carrying Granulocyte-Macrophage Colony-Stimulating Factor in Hepatocellular Carcinoma. Hum. Gene Ther. 2019, 30, 330–338. [Google Scholar] [CrossRef]
- Hamilton, J.R.; Vijayakumar, G.; Palese, P. A Recombinant Antibody-Expressing Influenza Virus Delays Tumor Growth in a Mouse Model. Cell Rep. 2018, 22, 1–7. [Google Scholar] [CrossRef]
- Reale, M.A.; Manheimer, A.J.; Moran, T.M.; Norton, G.; Bona, C.A.; Schulman, J.L. Characterization of monoclonal antibodies specific for sequential influenza A/PR/8/34 virus variants. J. Immunol. 1986, 137, 1352–1358. [Google Scholar]
- Gardner, T.J.; Bolovan-Fritts, C.; Teng, M.W.; Redmann, V.; Kraus, T.A.; Sperling, R.; Moran, T.; Britt, W.; Weinberger, L.S.; Tortorella, D. Development of a High-Throughput Assay To Measure the Neutralization Capability of Anti-Cytomegalovirus Antibodies. Clin. Vaccine Immunol. 2013, 20, 540–550. [Google Scholar] [CrossRef]
- Fulton, B.O.; Sun, W.; Heaton, N.S.; Palese, P. The Influenza B Virus Hemagglutinin Head Domain Is Less Tolerant to Transposon Mutagenesis than That of the Influenza A Virus. J. Virol. 2018, 92, e00754-18. [Google Scholar] [CrossRef] [PubMed]
- Racaniello, V.R.; Palese, P. Isolation of influenza C virus recombinants. J. Virol. 1979, 32, 1006–1014. [Google Scholar] [PubMed]
- Lowen, A.C.; Mubareka, S.; Tumpey, T.M.; Garcia-Sastre, A.; Palese, P. The guinea pig as a transmission model for human influenza viruses. Proc. Natl. Acad. Sci. USA 2006, 103, 9988–9992. [Google Scholar] [CrossRef] [PubMed]
- Hai, R.; Krammer, F.; Tan, G.S.; Pica, N.; Eggink, D.; Maamary, J.; Margine, I.; Albrecht, R.A.; Palese, P. Influenza Viruses Expressing Chimeric Hemagglutinins: Globular Head and Stalk Domains Derived from Different Subtypes. J. Virol. 2012, 86, 5774–5781. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Kirkpatrick, E.; Ermler, M.; Nachbagauer, R.; Broecker, F.; Krammer, F.; Palese, P. Development of Influenza B Universal Vaccine Candidates Using the “Mosaic” Hemagglutinin Approach. J. Virol. 2019, 93, e00333-19. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.S.; Leon, P.E.; Albrecht, R.A.; Margine, I.; Hirsh, A.; Bahl, J.; Krammer, F. Broadly-Reactive Neutralizing and Non-neutralizing Antibodies Directed against the H7 Influenza Virus Hemagglutinin Reveal Divergent Mechanisms of Protection. PLoS Pathog. 2016, 12, e1005578. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, D.; Amanat, F.; Strohmeier, S.; Nachbagauer, R.; Krammer, F. Cross-reactive mouse monoclonal antibodies raised against the hemagglutinin of A/Shanghai/1/2013 (H7N9) protect against novel H7 virus isolates in the mouse model. Emerg. Microbes Infect. 2018, 7, 1–12. [Google Scholar] [CrossRef]
- Xu, R.; Ekiert, D.C.; Krause, J.C.; Hai, R.; Crowe, J.E.; Wilson, I.A. Structural basis of pre-existing immunity to the 2009 H1N1 pandemic influenza virus. Science 2010, 328, 357–360. [Google Scholar] [CrossRef]
- Muster, T.; Guinea, R.; Trkola, A.; Purtscher, M.; Klima, A.; Steindl, F.; Palese, P.; Katinger, H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J. Virol. 1994, 68, 4031–4034. [Google Scholar]
- Burke, D.F.; Smith, D.J. A Recommended Numbering Scheme for Influenza A HA Subtypes. PLoS ONE 2014, 9, e112302. [Google Scholar] [CrossRef]
- Schwede, T. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef] [PubMed]
- Heaton, N.S.; Sachs, D.; Chen, C.-J.; Hai, R.; Palese, P. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 20248–20253. [Google Scholar] [CrossRef] [PubMed]
- Whittle, J.R.R.; Zhang, R.; Khurana, S.; King, L.R.; Manischewitz, J.; Golding, H.; Dormitzer, P.R.; Haynes, B.F.; Walter, E.B.; Moody, M.A.; et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc. Natl. Acad. Sci. USA 2011, 108, 14216–14221. [Google Scholar] [CrossRef] [PubMed]
- Zacour, M.; Ward, B.J.; Brewer, A.; Tang, P.; Boivin, G.; Li, Y.; Warhuus, M.; McNeil, S.A.; LeBlanc, J.J.; Hatchette, T.F.; et al. Standardization of Hemagglutination Inhibition Assay for Influenza Serology Allows for High Reproducibility between Laboratories. Clin. Vaccine Immunol. 2016, 23, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.J.; Hernandez, R.E.; Noriega, V.M.; Tortorella, D. Human cytomegalovirus gH stability and trafficking are regulated by ER-associated degradation and transmembrane architecture. Sci. Rep. 2016, 6, 23692. [Google Scholar] [CrossRef] [PubMed]
- Ohlin, M.; Söderberg-Naucler, C. Human antibody technology and the development of antibodies against cytomegalovirus. Mol. Immunol. 2015, 67, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.S.; Huffman, T.; Jenks, J.A.; De La Rosa, E.C.; Xie, G.; Vandergrift, N.; Pass, R.F.; Pollara, J.; Permar, S.R. HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc. Natl. Acad. Sci. USA 2018, 115, 6267–6272. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Freed, D.C.; He, X.; Li, F.; Tang, A.; Cox, K.S.; Dubey, S.A.; Cole, S.; Medi, M.B.; Liu, Y.; et al. A replication-defective human cytomegalovirus vaccine for prevention of congenital infection. Sci. Transl. Med. 2016, 8, 362ra145. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Fowler, K.B.; Britt, W.J.; Stagno, S.; Pass, R.F. Symptomatic Congenital Cytomegalovirus Infection in Infants Born to Mothers With Preexisting Immunity to Cytomegalovirus. Pediatrics 1999, 104, 55–60. [Google Scholar] [CrossRef]
- Soderberg-Naucler, C.; Söderberg-Nauclér, C.; Söderberg-Naucler, C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J. Intern. Med. 2006, 259, 219–246. [Google Scholar] [CrossRef]
- Schleiss, M.R.; Berka, U.; Watson, E.; Aistleithner, M.; Kiefmann, B.; Mangeat, B.; Swanson, E.C.; Gillis, P.A.; Hernandez-Alvarado, N.; Fernández-Alarcón, C.; et al. Additive Protection against Congenital Cytomegalovirus Conferred by Combined Glycoprotein B/pp65 Vaccination Using a Lymphocytic Choriomeningitis Virus Vector. Clin. Vaccine Immunol. 2017, 24, e00300-16. [Google Scholar] [CrossRef]
- Swanson, E.C.; Gillis, P.; Hernandez-Alvarado, N.; Fernández-Alarcón, C.; Schmit, M.; Zabeli, J.C.; Wussow, F.; Diamond, D.J.; Schleiss, M.R. Comparison of monovalent glycoprotein B with bivalent gB/pp65 (GP83) vaccine for congenital cytomegalovirus infection in a guinea pig model: Inclusion of GP83 reduces gB antibody response but both vaccine approaches provide equivalent protection against pup mortality. Vaccine 2015, 33, 4013–4018. [Google Scholar] [PubMed]
- Choi, K.Y.; Root, M.; McGregor, A. A Novel Non-Replication-Competent Cytomegalovirus Capsid Mutant Vaccine Strategy Is Effective in Reducing Congenital Infection. J. Virol. 2016, 90, 7902–7919. [Google Scholar] [CrossRef] [PubMed]
- Cardin, R.D.; Bravo, F.J.; Pullum, D.A.; Orlinger, K.; Watson, E.M.; Aspoeck, A.; Fuhrmann, G.; Guirakhoo, F.; Monath, T.; Bernstein, D.I. Replication-defective lymphocytic choriomeningitis virus vectors expressing guinea pig cytomegalovirus gB and pp65 homologs are protective against congenital guinea pig cytomegalovirus infection. Vaccine 2016, 34, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, Y.; García-Sastre, A.; Rodriguez, D.; Rodriguez, J.R.; Murata, K.; Tsuji, M.; Palese, P.; Esteban, M.; Zavala, F.; Nussenzweig, R.S. Recombinant viruses expressing a human malaria antigen can elicit potentially protective immune CD8+ responses in mice. Proc. Natl. Acad. Sci. USA 1998, 95, 3954–3959. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Polonis, V.; Isobe, H.; Zaghouani, H.; Guinea, R.; Moran, T.; Bona, C.; Palese, P. Chimeric influenza virus induces neutralizing antibodies and cytotoxic T cells against human immunodeficiency virus type 1. J. Virol. 1998, 67, 6659–6666. [Google Scholar]
- Saccoccio, F.M.; Sauer, A.L.; Cui, X.; Armstrong, A.E.; Habib, E.-S.E.; Johnson, D.C.; Ryckman, B.J.; Klingelhutz, A.J.; Adler, S.P.; McVoy, M.A. Peptides from cytomegalovirus UL130 and UL131 proteins induce high titer antibodies that block viral entry into mucosal epithelial cells. Vaccine 2011, 29, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Chiuppesi, F.; Kaltcheva, T.; Meng, Z.; Barry, P.A.; Diamond, D.J.; Wussow, F. Identification of a Continuous Neutralizing Epitope within UL128 of Human Cytomegalovirus. J. Virol. 2017, 91, e01857-16. [Google Scholar] [CrossRef]
- Wong, S.-S.; Webby, R.J. Traditional and New Influenza Vaccines. Clin. Microbiol. Rev. 2013, 26, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Mohn, K.G.-I.; Smith, I.; Sjursen, H.; Cox, R.J. Immune responses after live attenuated influenza vaccination. Hum. Vaccines Immunother. 2018, 14, 571–578. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Palese, P. Development of next generation hemagglutinin-based broadly protective influenza virus vaccines. Curr. Opin. Immunol. 2018, 53, 51–57. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behzadi, M.A.; Stein, K.R.; Bermúdez-González, M.C.; Simon, V.; Nachbagauer, R.; Tortorella, D. An Influenza Virus Hemagglutinin-Based Vaccine Platform Enables the Generation of Epitope Specific Human Cytomegalovirus Antibodies. Vaccines 2019, 7, 51. https://doi.org/10.3390/vaccines7020051
Behzadi MA, Stein KR, Bermúdez-González MC, Simon V, Nachbagauer R, Tortorella D. An Influenza Virus Hemagglutinin-Based Vaccine Platform Enables the Generation of Epitope Specific Human Cytomegalovirus Antibodies. Vaccines. 2019; 7(2):51. https://doi.org/10.3390/vaccines7020051
Chicago/Turabian StyleBehzadi, Mohammad Amin, Kathryn R. Stein, Maria Carolina Bermúdez-González, Viviana Simon, Raffael Nachbagauer, and Domenico Tortorella. 2019. "An Influenza Virus Hemagglutinin-Based Vaccine Platform Enables the Generation of Epitope Specific Human Cytomegalovirus Antibodies" Vaccines 7, no. 2: 51. https://doi.org/10.3390/vaccines7020051
APA StyleBehzadi, M. A., Stein, K. R., Bermúdez-González, M. C., Simon, V., Nachbagauer, R., & Tortorella, D. (2019). An Influenza Virus Hemagglutinin-Based Vaccine Platform Enables the Generation of Epitope Specific Human Cytomegalovirus Antibodies. Vaccines, 7(2), 51. https://doi.org/10.3390/vaccines7020051




