Cytolytic Perforin as an Adjuvant to Enhance the Immunogenicity of DNA Vaccines
Abstract
1. Introduction
2. DNA Vaccine Adjuvants
2.1. Cytokines
2.2. Heat Shock Proteins
2.3. Chicken Complement Inhibitor
2.4. Viral Fusion Protein
2.5. Cytolytic Protein
3. Cytolytic DNA Vaccines
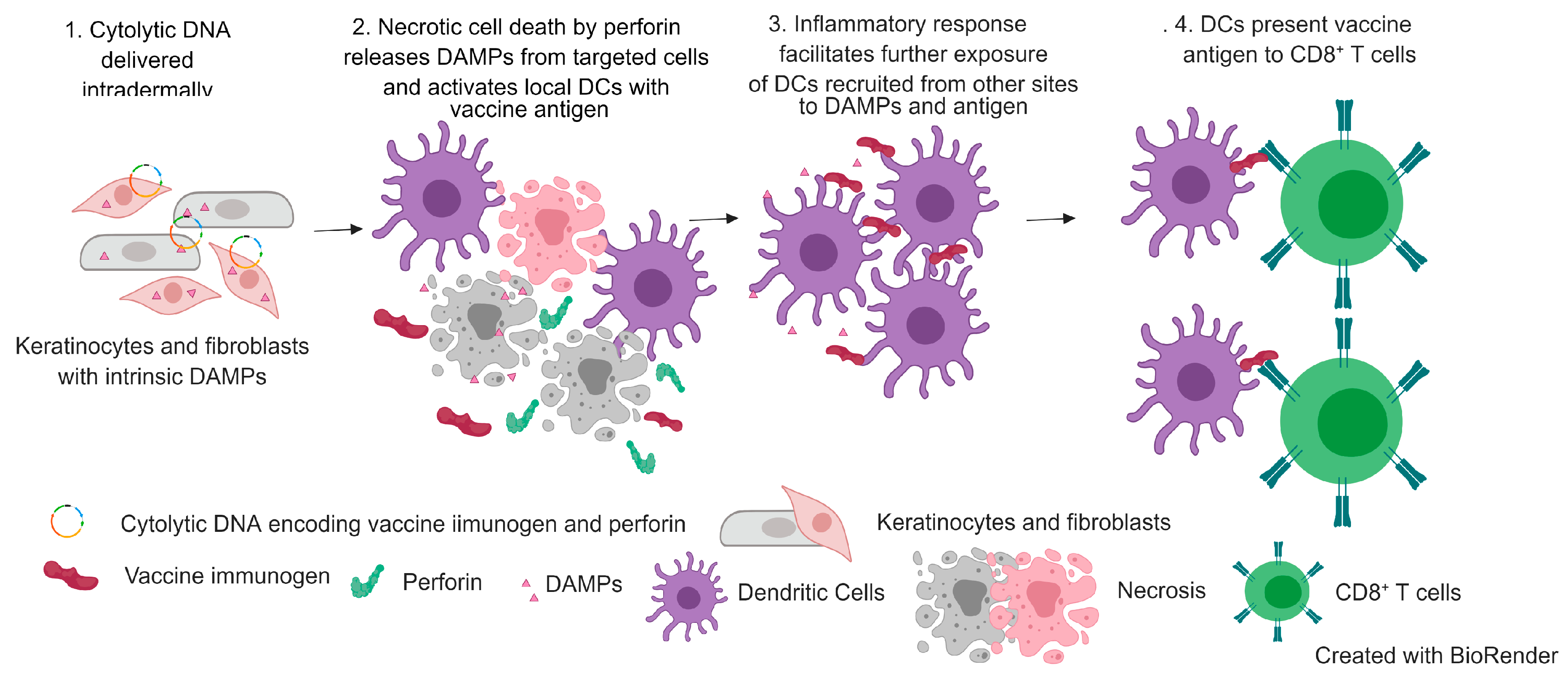
3.1. Mechanism
3.2. Cytolytic HIV and HCV DNA Vaccines
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. 10 Facts on Immunization. Available online: https://www.who.int/features/factfiles/immunization/en/ (accessed on 20 December 2018).
- Plotkin, S.; Robinson, J.M.; Cunningham, G.; Iqbal, R.; Larsen, S. The complexity and cost of vaccine manufacturing––An overview. Vaccine 2017, 35, 4064–4071. [Google Scholar] [CrossRef]
- Li, S.; Plebanski, M.; Smooker, P.; Gowans, E.J. Editorial: Why Vaccines to HIV, HCV, and Malaria Have So Far Failed-Challenges to Developing Vaccines Against Immunoregulating Pathogens. Front. Microbiol. 2015, 6, 1318. [Google Scholar] [CrossRef]
- Wilhelm, J. HIV, Tuberculosis, and Malaria. Available online: https://www.sabin.org/sites/sabin.org/files/wilhelm_v2.pdf (accessed on 19 February 2019).
- Rappuoli, R.; Aderem, A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature 2011, 473, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Whalen, R.G. DNA vaccines for emerging infectious diseases: What if? Emerg. Infect. Dis. 1996, 2, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Suschak, J.J.; Williams, J.A.; Schmaljohn, C.S. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum. Vaccin. Immunother. 2017, 13, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Noh, J.Y.; Lee, D.Y.; Kim, S.J.; Song, C.S.; Kim, Y.C. Effective humoral immune response from a H1N1 DNA vaccine delivered to the skin by microneedles coated with PLGA-based cationic nanoparticles. J. Controlled Release 2017, 265, 66–74. [Google Scholar] [CrossRef]
- Hurtado-Melgoza, M.L.; Ramos-Ligonio, A.; Álvarez-Rodríguez, L.M.; Meza-Menchaca, T.; López-Monteon, A. Differential humoral and cellular immunity induced by vaccination using plasmid DNA and protein recombinant expressing the NS3 protein of dengue virus type 3. J. Biomed. Sci. 2016, 23, 85. [Google Scholar] [CrossRef]
- Maslow, J.N. Vaccines for emerging infectious diseases: Lessons from MERS coronavirus and Zika virus. Hum. Vaccin. Immunother. 2017, 13, 2918–2930. [Google Scholar] [CrossRef]
- Ferraro, B.; Morrow, M.P.; Hutnick, N.A.; Shin, T.H.; Lucke, C.E.; Weiner, D.B. Clinical applications of DNA vaccines: Current progress. Clin. Infect. Dis. 2011, 53, 296–302. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines—How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Wahren, B.; Liu, M.A. DNA Vaccines: Recent Developments and the Future. Vaccines 2014, 2, 785–796. [Google Scholar] [CrossRef]
- Kenney, R.T.; Frech, S.A.; Muenz, L.R.; Villar, C.P.; Glenn, G.M. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 2004, 351, 2295–2301. [Google Scholar] [CrossRef]
- Fehres, C.; Garcia-Vallejo, J.J.; Unger, W.; Van Kooyk, Y. Skin-Resident Antigen-Presenting Cells: Instruction Manual for Vaccine Development. Front. Immunol. 2013, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- Gothelf, A.; Gehl, J. What you always needed to know about electroporation based DNA vaccines. Hum. Vaccin. Immunother. 2012, 8, 1694–1702. [Google Scholar] [CrossRef]
- Aihara, H.; Miyazaki, J. Gene transfer into muscle by electroporation in vivo. Nat. biotechnol. 1998, 16, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Dean, D.A. Electroporation-mediated gene delivery. Adv. Genet. 2015, 89, 49–88. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Saffari, M.; Moghimi, H.R.; Dass, C.R. Barriers to Liposomal Gene Delivery: from Application Site to the Target. Iran. J. Pharm. Res. 2016, 15, 3–17. [Google Scholar]
- Gvili, J.; Machluf, M. 544. PLGA Nanoparticles for DNA Vaccination–Waiving Complexity and Increasing Efficiency. Mol. Ther. 2006, 13, S209. [Google Scholar] [CrossRef]
- Penumarthi, A.; Parashar, D.; Abraham, A.N.; Dekiwadia, C.; Macreadie, I.; Shukla, R.; Smooker, P.M. Solid lipid nanoparticles mediate non-viral delivery of plasmid DNA to dendritic cells. J. Nanoparticle Res. 2017, 19, 210. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and clinical development of nanoparticles for gene delivery. Mol. Ther. Methods. Clin. Dev. 2016, 3, 16023. [Google Scholar] [CrossRef]
- Seternes, T.; Tonheim, T.C.; Løvoll, M.; Bøgwald, J.; Dalmo, R.A. Specific endocytosis and degradation of naked DNA in the endocardial cells of cod (Gadus morhua L.). J. Exp. Biol. 2007, 210, 2091. [Google Scholar] [CrossRef]
- Elnekave, M.; Furmanov, K.; Nudel, I.; Arizon, M.; Clausen, B.E.; Hovav, A.-H. Directly Transfected Langerin+ Dermal Dendritic Cells Potentiate CD8+; T Cell Responses following Intradermal Plasmid DNA Immunization. J. Immunol. 2010, 185, 3463. [Google Scholar] [CrossRef]
- Greenland, J.R.; Letvin, N.L. Chemical adjuvants for plasmid DNA vaccines. Vaccine 2007, 25, 3731–3741. [Google Scholar] [CrossRef]
- Coban, C.; Kobiyama, K.; Jounai, N.; Tozuka, M.; Ishii, K.J. DNA vaccines. Hum. Vaccin. Immunother. 2013, 9, 2216–2221. [Google Scholar] [CrossRef]
- Badovinac, V.P.; Harty, J.T. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol. Rev. 2006, 211, 67–80. [Google Scholar] [CrossRef]
- Hovav, A.-H.; Panas, M.W.; Rahman, S.; Sircar, P.; Gillard, G.; Cayabyab, M.J.; Letvin, N.L. Duration of Antigen Expression In Vivo following DNA Immunization Modifies the Magnitude, Contraction, and Secondary Responses of CD8+ T Lymphocytes. J. Immunol. 2007, 179, 6725. [Google Scholar] [CrossRef]
- Jabbari, A.; Harty, J.T. Secondary memory CD8+ T cells are more protective but slower to acquire a central–memory phenotype. J. Exp. Med. 2006, 203, 919. [Google Scholar] [CrossRef]
- Mahanty, S.; Prigent, A.; Garraud, O. Immunogenicity of infectious pathogens and vaccine antigens. BMC Immunol. 2015, 16, 31. [Google Scholar] [CrossRef]
- Ahmad-Nejad, P.; Häcker, H.; Rutz, M.; Bauer, S.; Vabulas, R.M.; Wagner, H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 2002, 32, 1958–1968. [Google Scholar] [CrossRef]
- Krieg, A.M.; Yi, A.-K.; Matson, S.; Waldschmidt, T.J.; Bishop, G.A.; Teasdale, R.; Koretzky, G.A.; Klinman, D.M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995, 374, 546. [Google Scholar] [CrossRef]
- Dalpke, A.; Zimmermann, S.; Heeg, K. CpG-Oligonucleotides in Vaccination: Signaling and Mechanisms of Action. Immunobiology 2001, 204, 667–676. [Google Scholar] [CrossRef]
- Li, Y.; Leneghan, D.B.; Miura, K.; Nikolaeva, D.; Brian, I.J.; Dicks, M.D.; Fyfe, A.J.; Zakutansky, S.E.; de Cassan, S.; Long, C.A.; et al. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci. Rep. 2016, 6, 18848. [Google Scholar] [CrossRef]
- Tomusange, K.; Wijesundara, D.; Gummow, J.; Garrod, T.; Li, Y.; Gray, L.; Churchill, M.; Grubor-Bauk, B.; Gowans, E.J. A HIV-Tat/C4-binding protein chimera encoded by a DNA vaccine is highly immunogenic and contains acute EcoHIV infection in mice. Sci. Rep. 2016, 6, 29131. [Google Scholar] [CrossRef]
- Marsac, D.; Loirat, D.; Petit, C.; Schwartz, O.; Michel, M.L. Enhanced Presentation of Major Histocompatibility Complex Class I-Restricted Human Immunodeficiency Virus Type 1 (HIV-1) Gag-Specific Epitopes after DNA Immunization with Vectors Coding for Vesicular Stomatitis Virus Glycoprotein-Pseudotyped HIV-1 Gag Particles. J. Virol. 2002, 76, 7544. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Kim, J.J.; Nottingham, L.K.; Wilson, D.M.; Bagarazzi, M.L.; Tsai, A.; Morrison, L.D.; Javadian, A.; Chalian, A.A.; Agadjanyan, M.G.; Weiner, D.B. Engineering DNA vaccines via co-delivery of co-stimulatory molecule genes. Vaccine 1998, 16, 1828–1835. [Google Scholar] [CrossRef]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.F. DNA vaccine for cancer immunotherapy. Hum. Vaccin. Immunother. 2014, 10, 3153–3164. [Google Scholar] [CrossRef]
- Kim, J.J.; Tsai, A.; Nottingham, L.K.; Morrison, L.; Cunning, D.M.; Oh, J.; Lee, D.J.; Dang, K.; Dentchev, T.; Chalian, A.A.; et al. Intracellular adhesion molecule-1 modulates beta-chemokines and directly costimulates T cells in vivo. J. Clin. Invest. 1999, 103, 869–877. [Google Scholar] [CrossRef]
- Kim, J.J.; Yang, J.S.; Montaner, L.; Lee, D.J.; Chalian, A.A.; Weiner, D.B. Coimmunization with IFN-gamma or IL-2, but not IL-13 or IL-4 cDNA can enhance Th1-type DNA vaccine-induced immune responses in vivo. J. Interferon Cytokine Res. 2000, 20, 311–319. [Google Scholar] [CrossRef]
- Larsen, D.L.; Dybdahl-Sissoko, N.; McGregor, M.W.; Drape, R.; Neumann, V.; Swain, W.F.; Lunn, D.P.; Olsen, C.W. Coadministration of DNA encoding interleukin-6 and hemagglutinin confers protection from influenza virus challenge in mice. J. Virol. 1998, 72, 1704–1708. [Google Scholar]
- Chow, Y.H.; Chiang, B.L.; Lee, Y.L.; Chi, W.K.; Lin, W.C.; Chen, Y.T.; Tao, M.H. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J. Immunol. 1998, 160, 1320–1329. [Google Scholar]
- Kim, J.J.; Trivedi, N.N.; Nottingham, L.K.; Morrison, L.; Tsai, A.; Hu, Y.; Mahalingam, S.; Dang, K.; Ahn, L.; Doyle, N.K.; et al. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur. J. Immunol. 1998, 28, 1089–1103. [Google Scholar] [CrossRef]
- Takeshita, F.; Tanaka, T.; Matsuda, T.; Tozuka, M.; Kobiyama, K.; Saha, S.; Matsui, K.; Ishii, K.J.; Coban, C.; Akira, S.; et al. Toll-like receptor adaptor molecules enhance DNA-raised adaptive immune responses against influenza and tumors through activation of innate immunity. J. Virol. 2006, 80, 6218–6224. [Google Scholar] [CrossRef]
- Applequist, S.E.; Rollman, E.; Wareing, M.D.; Liden, M.; Rozell, B.; Hinkula, J.; Ljunggren, H.G. Activation of innate immunity, inflammation, and potentiation of DNA vaccination through mammalian expression of the TLR5 agonist flagellin. J. Immunol. 2005, 175, 3882–3891. [Google Scholar] [CrossRef]
- Sasaki, S.; Amara, R.R.; Yeow, W.-S.; Pitha, P.M.; Robinson, H.L. Regulation of DNA-raised immune responses by cotransfected interferon regulatory factors. J. Virol. 2002, 76, 6652–6659. [Google Scholar] [CrossRef]
- Coban, C.; Kobiyama, K.; Aoshi, T.; Takeshita, F.; Horii, T.; Akira, S.; Ishii, K.J. Novel strategies to improve DNA vaccine immunogenicity. Curr. Gene Ther. 2011, 11, 479–484. [Google Scholar] [CrossRef]
- Muthumani, G.; Laddy, D.J.; Sundaram, S.G.; Fagone, P.; Shedlock, D.J.; Kannan, S.; Wu, L.; Chung, C.W.; Lankaraman, K.M.; Burns, J.; et al. Co-immunization with an optimized plasmid-encoded immune stimulatory interleukin, high-mobility group box 1 protein, results in enhanced interferon-gamma secretion by antigen-specific CD8 T cells. Immunology 2009, 128, e612–e620. [Google Scholar] [CrossRef]
- Lladser, A.; Mougiakakos, D.; Tufvesson, H.; Ligtenberg, M.A.; Quest, A.F.; Kiessling, R.; Ljungberg, K. DAI (DLM-1/ZBP1) as a genetic adjuvant for DNA vaccines that promotes effective antitumor CTL immunity. Mol. Ther. 2011, 19, 594–601. [Google Scholar] [CrossRef]
- Liniger, M.; Summerfield, A.; Ruggli, N. MDA5 can be exploited as efficacious genetic adjuvant for DNA vaccination against lethal H5N1 influenza virus infection in chickens. PLoS ONE 2012, 7, e49952. [Google Scholar] [CrossRef]
- Halbroth, B.R.; Sebastian, S.; Poyntz, H.C.; Bregu, M.; Cottingham, M.G.; Hill, A.V.S.; Spencer, A.J. Development of a Molecular Adjuvant to Enhance Antigen-Specific CD8(+) T Cell Responses. Sci. Rep. 2018, 8, 15020. [Google Scholar] [CrossRef]
- King, C.A.; Spellerberg, M.B.; Zhu, D.; Rice, J.; Sahota, S.S.; Thompsett, A.R.; Hamblin, T.J.; Radl, J.; Stevenson, F.K. DNA vaccines with single-chain Fv fused to fragment C of tetanus toxin induce protective immunity against lymphoma and myeloma. Nat. Med. 1998, 4, 1281–1286. [Google Scholar] [CrossRef]
- Gargett, T.; Grubor-Bauk, B.; Garrod, T.; Yu, W.; Miller, D.; Major, L.; Wesselingh, S.; Suhrbier, A.; Gowans, E. Induction of antigen-positive cell death by the expression of Perforin, but not DTa, from a DNA vaccine enhances the immune respons. Immunol. Cell Biol. 2014, 92, 359–367. [Google Scholar] [CrossRef]
- Grubor-Bauk, B.; Yu, W.; Wijesundara, D.; Gummow, J.; Garrod, T.; Brennan, A.J.; Voskoboinik, I.; Gowans, E.J. Intradermal delivery of DNA encoding HCV NS3 and perforin elicits robust cell-mediated immunity in mice and pigs. Gene Ther. 2016, 23, 26–37. [Google Scholar] [CrossRef]
- Gummow, J.; Li, Y.; Yu, W.; Garrod, T.; Wijesundara, D.; Brennan, A.J.; Mullick, R.; Voskoboinik, I.; Grubor-Bauk, B.; Gowans, E.J. A Multiantigenic DNA Vaccine That Induces Broad Hepatitis C Virus-Specific T-Cell Responses in Mice. J. Viro.l 2015, 89, 7991–8002. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Z.P.; Qin, S.; Liu, C.B.; Zou, L.L. Calreticulin is an effective immunologic adjuvant to tumor-associated antigens. Exp. Ther. Med. 2017, 14, 3399–3406. [Google Scholar] [CrossRef][Green Version]
- Peng, S.; Ji, H.; Trimble, C.; He, L.; Tsai, Y.-C.; Yeatermeyer, J.; Boyd, D.A.K.; Hung, C.-F.; Wu, T.C. Development of a DNA Vaccine Targeting Human Papillomavirus Type 16 Oncoprotein E6. J. Virol. 2004, 78, 8468. [Google Scholar] [CrossRef]
- Garrod, T.J.; Grubor-Bauk, B.; Gargett, T.; Li, Y.; Miller, D.S.; Yu, W.; Major, L.; Burrell, C.J.; Wesselingh, S.; Suhrbier, A.; et al. DNA vaccines encoding membrane-bound or secreted forms of heat shock protein 70 exhibit improved potency. Eur. J. Immunol. 2014, 44, 1992–2002. [Google Scholar] [CrossRef]
- Kalams, S.A.; Parker, S.; Jin, X.; Elizaga, M.; Metch, B.; Wang, M.; Hural, J.; Lubeck, M.; Eldridge, J.; Cardinali, M.; et al. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS ONE 2012, 7, e29231. [Google Scholar] [CrossRef]
- Norell, H.; Poschke, I.; Charo, J.; Wei, W.Z.; Erskine, C.; Piechocki, M.P.; Knutson, K.L.; Bergh, J.; Lidbrink, E.; Kiessling, R. Vaccination with a plasmid DNA encoding HER-2/neu together with low doses of GM-CSF and IL-2 in patients with metastatic breast carcinoma: a pilot clinical trial. J. Transl. Med. 2010, 8, 53. [Google Scholar] [CrossRef]
- Staff, C.; Mozaffari, F.; Haller, B.K.; Wahren, B.; Liljefors, M. A Phase I safety study of plasmid DNA immunization targeting carcinoembryonic antigen in colorectal cancer patients. Vaccine 2011, 29, 6817–6822. [Google Scholar] [CrossRef]
- Baden, L.R.; Blattner, W.A.; Morgan, C.; Huang, Y.; Defawe, O.D.; Sobieszczyk, M.E.; Kochar, N.; Tomaras, G.D.; McElrath, M.J.; Russell, N.; et al. Timing of plasmid cytokine (IL-2/Ig) administration affects HIV-1 vaccine immunogenicity in HIV-seronegative subjects. J. Infect. Dis. 2011, 204, 1541–1549. [Google Scholar] [CrossRef]
- Elizaga, M.L.; Li, S.S.; Kochar, N.K.; Wilson, G.J.; Allen, M.A.; Tieu, H.V.N.; Frank, I.; Sobieszczyk, M.E.; Cohen, K.W.; Sanchez, B.; et al. Safety and tolerability of HIV-1 multiantigen pDNA vaccine given with IL-12 plasmid DNA via electroporation, boosted with a recombinant vesicular stomatitis virus HIV Gag vaccine in healthy volunteers in a randomized, controlled clinical trial. PLoS ONE 2018, 13, e0202753. [Google Scholar] [CrossRef]
- Li, S.S.; Kochar, N.K.; Elizaga, M.; Hay, C.M.; Wilson, G.J.; Cohen, K.W.; De Rosa, S.C.; Xu, R.; Ota-Setlik, A.; Morris, D.; et al. DNA Priming Increases Frequency of T-Cell Responses to a Vesicular Stomatitis Virus HIV Vaccine with Specific Enhancement of CD8+ T-Cell Responses by Interleukin-12 Plasmid DNA. Clin. Vaccin. Immunol. 2017, 24. [Google Scholar] [CrossRef]
- Kalams, S.A.; Parker, S.D.; Elizaga, M.; Metch, B.; Edupuganti, S.; Hural, J.; De Rosa, S.; Carter, D.K.; Rybczyk, K.; Frank, I.; et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J. Infect. Dis. 2013, 208, 818–829. [Google Scholar] [CrossRef]
- McNeel, D.G.; Dunphy, E.J.; Davies, J.G.; Frye, T.P.; Johnson, L.E.; Staab, M.J.; Horvath, D.L.; Straus, J.; Alberti, D.; Marnocha, R.; et al. Safety and Immunological Efficacy of a DNA Vaccine Encoding Prostatic Acid Phosphatase in Patients With Stage D0 Prostate Cancer. J. Clin. Oncol. 2009, 27, 4047–4054. [Google Scholar] [CrossRef]
- Trimble, C.L.; Peng, S.; Kos, F.; Gravitt, P.; Viscidi, R.; Sugar, E.; Pardoll, D.; Wu, T.C. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin. Cancer Res. 2009, 15, 361–367. [Google Scholar] [CrossRef]
- Kim, J.J.; Nottingham, L.K.; Tsai, A.; Lee, D.J.; Maguire, H.C.; Oh, J.; Dentchev, T.; Manson, K.H.; Wyand, M.S.; Agadjanyan, M.G.; et al. Antigen-specific humoral and cellular immune responses can be modulated in rhesus macaques through the use of IFN-gamma, IL-12, or IL-18 gene adjuvants. J. Med. Primatol. 1999, 28, 214–223. [Google Scholar] [CrossRef]
- Scheerlinck, J.-P.Y. Genetic adjuvants for DNA vaccines. Vaccine 2001, 19, 2647–2656. [Google Scholar] [CrossRef]
- Okada, E.; Sasaki, S.; Ishii, N.; Aoki, I.; Yasuda, T.; Nishioka, K.; Fukushima, J.; Miyazaki, J.; Wahren, B.; Okuda, K. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J. immunol. 1997, 159, 3638–3647. [Google Scholar]
- Kim, J.J.; Simbiri, K.A.; Sin, J.I.; Dang, K.; Oh, J.; Dentchev, T.; Lee, D.; Nottingham, L.K.; Chalian, A.A.; McCallus, D.; et al. Cytokine molecular adjuvants modulate immune responses induced by DNA vaccine constructs for HIV-1 and SIV. J. Interferon Cytokine Res. 1999, 19, 77–84. [Google Scholar] [CrossRef]
- Xin, K.Q.; Hamajima, K.; Sasaki, S.; Honsho, A.; Tsuji, T.; Ishii, N.; Cao, X.R.; Lu, Y.; Fukushima, J.; Shapshak, P.; et al. Intranasal administration of human immunodeficiency virus type-1 (HIV-1) DNA vaccine with interleukin-2 expression plasmid enhances cell-mediated immunity against HIV-1. Immunology 1998, 94, 438–444. [Google Scholar] [CrossRef]
- Tsuji, T.; Hamajima, K.; Fukushima, J.; Xin, K.Q.; Ishii, N.; Aoki, I.; Ishigatsubo, Y.; Tani, K.; Kawamoto, S.; Nitta, Y.; et al. Enhancement of cell-mediated immunity against HIV-1 induced by coinnoculation of plasmid-encoded HIV-1 antigen with plasmid expressing IL-12. J. Immunol. 1997, 158, 4008–4013. [Google Scholar]
- Barouch, D.H.; Santra, S.; Steenbeke, T.D.; Zheng, X.X.; Perry, H.C.; Davies, M.E.; Freed, D.C.; Craiu, A.; Strom, T.B.; Shiver, J.W.; et al. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J. Immunol. 1998, 161, 1875–1882. [Google Scholar]
- Xin, K.Q.; Hamajima, K.; Sasaki, S.; Tsuji, T.; Watabe, S.; Okada, E.; Okuda, K. IL-15 expression plasmid enhances cell-mediated immunity induced by an HIV-1 DNA vaccine. Vaccine 1999, 17, 858–866. [Google Scholar] [CrossRef]
- Wang, L.; Rollins, L.; Gu, Q.; Chen, S.Y.; Huang, X.F. A Mage3/Heat Shock Protein70 DNA vaccine induces both innate and adaptive immune responses for the antitumor activity. Vaccine 2009, 28, 561–570. [Google Scholar] [CrossRef][Green Version]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Liu, T.-T.; Wu, Y.; Niu, T. Human DKK1 and human HSP70 fusion DNA vaccine induces an effective anti-tumor efficacy in murine multiple myeloma. Oncotarget 2017, 9, 178–191. [Google Scholar] [CrossRef]
- Potash, M.J.; Chao, W.; Bentsman, G.; Paris, N.; Saini, M.; Nitkiewicz, J.; Belem, P.; Sharer, L.; Brooks, A.I.; Volsky, D.J. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc. Natl. Acad. Sci. USA 2005, 102, 3760–3765. [Google Scholar] [CrossRef]
- Garrod, T.; Grubor-Bauk, B.; Yu, S.; Gargett, T.; Gowans, E.J. Encoded novel forms of HSP70 or a cytolytic protein increase DNA vaccine potency. Hum. Vaccin. Immunother. 2014, 10, 2679–2683. [Google Scholar] [CrossRef]
- Spencer, A.J.; Hill, F.; Honeycutt, J.D.; Cottingham, M.G.; Bregu, M.; Rollier, C.S.; Furze, J.; Draper, S.J.; Sogaard, K.C.; Gilbert, S.C.; et al. Fusion of the Mycobacterium tuberculosis antigen 85A to an oligomerization domain enhances its immunogenicity in both mice and non-human primates. PLoS ONE 2012, 7, e33555. [Google Scholar] [CrossRef]
- Ogun, S.A.; Dumon-Seignovert, L.; Marchand, J.B.; Holder, A.A.; Hill, F. The oligomerization domain of C4-binding protein (C4bp) acts as an adjuvant, and the fusion protein comprised of the 19-kilodalton merozoite surface protein 1 fused with the murine C4bp domain protects mice against malaria. Infect. Immun. 2008, 76, 3817–3823. [Google Scholar] [CrossRef]
- Minhinnick, A.; Satti, I.; Harris, S.; Wilkie, M.; Sheehan, S.; Stockdale, L.; Manjaly Thomas, Z.R.; Lopez-Ramon, R.; Poulton, I.; Lawrie, A.; et al. A first-in-human phase 1 trial to evaluate the safety and immunogenicity of the candidate tuberculosis vaccine MVA85A-IMX313, administered to BCG-vaccinated adults. Vaccine 2016, 34, 1412–1421. [Google Scholar] [CrossRef]
- Ci, Y.; Yang, Y.; Xu, C.; Shi, L. Vesicular stomatitis virus G protein transmembrane region is crucial for the hemi-fusion to full fusion transition. Sci Rep 2018, 8, 10669. [Google Scholar] [CrossRef]
- Mao, C.-P.; Hung, C.-F.; Kang, T.H.; He, L.; Tsai, Y.-C.; Wu, C.-Y.; Wu, T.C. Combined administration with DNA encoding vesicular stomatitis virus G protein enhances DNA vaccine potency. J. Virol. 2010, 84, 2331–2339. [Google Scholar] [CrossRef]
- Freer, G.; Burkhart, C.; Ciernik, I.; Bachmann, M.F.; Hengartner, H.; Zinkernagel, R.M. Vesicular stomatitis virus Indiana glycoprotein as a T-cell-dependent and -independent antigen. J. Virol. 1994, 68, 3650. [Google Scholar]
- Bateman, A.; Bullough, F.; Murphy, S.; Emiliusen, L.; Lavillette, D.; Cosset, F.-L.; Cattaneo, R.; Russell, S.J.; Vile, R.G. Fusogenic Membrane Glycoproteins As a Novel Class of Genes for the Local and Immune-mediated Control of Tumor Growth. Cancer Res. 2000, 60, 1492. [Google Scholar]
- Bateman, A.R.; Harrington, K.J.; Kottke, T.; Ahmed, A.; Melcher, A.A.; Gough, M.J.; Linardakis, E.; Riddle, D.; Dietz, A.; Lohse, C.M.; et al. Viral Fusogenic Membrane Glycoproteins Kill Solid Tumor Cells by Nonapoptotic Mechanisms That Promote Cross Presentation of Tumor Antigens by Dendritic Cells. Cancer Res. 2002, 62, 6566. [Google Scholar]
- Chiang, S.C.C.; Theorell, J.; Entesarian, M.; Meeths, M.; Mastafa, M.; Al-Herz, W.; Frisk, P.; Gilmour, K.C.; Ifversen, M.; Langenskiöld, C.; et al. Comparison of primary human cytotoxic T-cell and natural killer cell responses reveal similar molecular requirements for lytic granule exocytosis but differences in cytokine production. Blood 2013, 121, 1345. [Google Scholar] [CrossRef]
- Law, R.H.; Lukoyanova, N.; Voskoboinik, I.; Caradoc-Davies, T.T.; Baran, K.; Dunstone, M.A.; D’Angelo, M.E.; Orlova, E.V.; Coulibaly, F.; Verschoor, S.; et al. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature 2010, 468, 447–451. [Google Scholar] [CrossRef]
- Leitner, W.W.; Restifo, N.P. DNA vaccines and apoptosis: to kill or not to kill? J. Clin. Investig. 2003, 112, 22–24. [Google Scholar] [CrossRef]
- Wijesundara, D.K.; Yu, W.; Quah, B.J.C.; Eldi, P.; Hayball, J.D.; Diener, K.R.; Voskoboinik, I.; Gowans, E.J.; Grubor-Bauk, B. Cytolytic DNA vaccine encoding lytic perforin augments the maturation of- and antigen presentation by- dendritic cells in a time-dependent manner. Sci. Rep. 2017, 7, 8530. [Google Scholar] [CrossRef]
- Qin, J.Y.; Zhang, L.; Clift, K.L.; Hulur, I.; Xiang, A.P.; Ren, B.-Z.; Lahn, B.T. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS ONE 2010, 5, e10611. [Google Scholar] [CrossRef]
- Brennan, A.J.; Chia, J.; Browne, K.A.; Ciccone, A.; Ellis, S.; Lopez, J.A.; Susanto, O.; Verschoor, S.; Yagita, H.; Whisstock, J.C.; et al. Protection from endogenous perforin: Glycans and the C terminus regulate exocytic trafficking in cytotoxic lymphocytes. Immunity 2011, 34, 879–892. [Google Scholar] [CrossRef]
- Lopez, J.A.; Susanto, O.; Jenkins, M.R.; Lukoyanova, N.; Sutton, V.R.; Law, R.H.; Johnston, A.; Bird, C.H.; Bird, P.I.; Whisstock, J.C.; et al. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Blood 2013, 121, 2659–2668. [Google Scholar] [CrossRef]
- Zhan, Y.; van de Water, B.; Wang, Y.; Stevens, J.L. The roles of caspase-3 and bcl-2 in chemically-induced apoptosis but not necrosis of renal epithelial cells. Oncogene 1999, 18, 6505. [Google Scholar] [CrossRef][Green Version]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef]
- Melcher, A.; Todryk, S.; Hardwick, N.; Ford, M.; Jacobson, M.; Vile, R.G. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat. Med. 1998, 4, 581–587. [Google Scholar] [CrossRef]
- Gallucci, S.; Lolkema, M.; Matzinger, P. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 1999, 5, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Sauter, B.; Albert, M.L.; Francisco, L.; Larsson, M.; Somersan, S.; Bhardwaj, N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 2000, 191, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef]
- Wijesundara, D.K.; Gummow, J.; Li, Y.; Yu, W.; Quah, B.J.; Ranasinghe, C.; Torresi, J.; Gowans, E.J.; Grubor-Bauk, B. Induction of Genotype Cross-Reactive, Hepatitis C Virus-Specific, Cell-Mediated Immunity in DNA-Vaccinated Mice. J. Virol. 2018, 92, e02133-17. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, R.; Halder, U.C.; Chattopadhyay, S.; Chanda, S.; Nandi, S.; Bagchi, P.; Nayak, M.K.; Chakrabarti, O.; Kobayashi, N.; Chawla-Sarkar, M. Rotaviral enterotoxin nonstructural protein 4 targets mitochondria for activation of apoptosis during infection. J. Biol. Chem. 2012, 287, 35004–35020. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Zhao, H.; Xu, A.T.; Wang, Y.; Tang, J.; Feng, W.H. Porcine reproductive and respiratory syndrome virus nonstructural protein 4 induces apoptosis dependent on its 3C-like serine protease activity. PLoS ONE 2013, 8, e69387. [Google Scholar] [CrossRef]

| Adjuvants | Antigens | Delivery | Host | Responses | Ref. |
|---|---|---|---|---|---|
| Costimulatory molecules | |||||
| CD80, CD86 | HIV-1 (Env, Gag, Pol) | DC, IM | Mouse Chimpanzee | +CMI | [39] |
| CD40 LT | beta-gal | DC, SC | Mouse | +Ab, +CMI | [40] |
| ICAM-1 | HIV-1 (Env) | DC, IM | Mouse | +CMI | [41] |
| Cytokines | |||||
| IL-2, IFN-γ | HIV-1 (Env, Gag, Pol) | DC, IM | Mouse | +Ab, +CMI | [42] |
| IL-6 | Influenza (HA) | DC, GG | Mouse | +Ab | [43] |
| IL-2,12, IFN-γ | HBV | DC, IM | Mouse | +CMI | [44] |
| TNF-α, IL-15 | HIV (Env, Gag, Pol) | DC, IM | Mouse | +CMI | [45] |
| Toll like receptor adaptor/signaling molecules | |||||
| TRIF | Influenza (HA), tumor E7 | BC, IM/EP | Mouse | +CMI | [46] |
| MyD88 | Influenza (HA), tumor E7 | BC, IM/EP | Mouse | +Ab | [46] |
| FliC | Influenza A (Np) | DC, ID | Mouse | +Ab, +CMI | [47] |
| IRF 1,3, 7 | Influenza virus (HA, Np) | DC/BC, IM | Mouse | +Ab, +CMI | [48] |
| TBK-1 | P. f (SE36) | DC, IM | Mouse | +Ab, +CMI | [49] |
| HMGB1 | HIV-1 (Gag, Env) | DC, IM/EP | Mouse | +Ab, +CMI | [50] |
| DAI | Survivin | DC, ID | Mouse | +CMI | [51] |
| chMDA5 | Influenza (HA) | DC, IM | Chicken | +Ab | [52] |
| Ii | P. f (ME) | FC, IM | Mouse | +CMI | [53] |
| Toxins/Viral proteins | |||||
| FrC | Sc-fv | FC, IM | Mouse | +Ab | [54] |
| DTa | HIV (Gag) | BC, ID | Mouse | −CMI | [55] |
| NSP4 | HCV NS3 | BC, ID | Mouse | +/−CMI | [56] |
| VSVG | HIV (Gag) NS3 | DC, ID BC, ID | Mouse Mouse | +CMI +/−CMI | [37] [57] |
| Heat shock proteins | |||||
| Calreticulin | mucin 1 HPV-16 E7 | DC FC, GG | Mouse Mouse | +CMI +CMI | [58] [59] |
| HSP70 | HIV (Gag) | BC, ID | Mouse | +CMI | [60] |
| Complement inhibitor | |||||
| IMX313 | HIV (Tat) | FC, ID | Mouse | +Ab, +CMI | [36] |
| Cytolytic protein | |||||
| PRF | HIV (Gag) HCV (NS3) HCV (NS345B) | BC, ID BC, ID BC, ID | Mouse Mouse, Pig Mouse | +CMI +CMI +CMI | [55] [26] [57] |
| Adjuvants | Antigens | Delivery | Responses | Trial Phase | Ref. |
|---|---|---|---|---|---|
| IL-12, IL-15 | HIV-1 (Gag) | DC, IM | +/−Ab, +/−CMI | I | [61] |
| GM-CSF, IL-2 | Her2 | RP, IM | +Ab, +CMI | I | [62] |
| GM-CSF | CEA | RP, ID | +Ab, +CMI | I | [63] |
| IL-2/Ig | HIV-1 Gag/Pol/Nef/Env | BC, IM | +Ab, +CMI | I | [64] |
| IL-12 | HIV (MAG-Gag, Pol, Env, Nef, Tat, Vif) | DC, IM/EP | −Ab, +CMI | I | [65,66] |
| IL-12 | HIV-1 (Env, Gag, Pol) | DC, IM/EP | +CMI | I | [67] |
| GM-CSF | PAP | RPID | −Ab, +CMI | I/IIa | [68] |
| HSP70 | HPV16 (E7) | FC, IM | −Ab, +/−CMI | I | [69] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, A.C.; Wijesundara, D.K.; Masavuli, M.G.; Mekonnen, Z.A.; Gowans, E.J.; Grubor-Bauk, B. Cytolytic Perforin as an Adjuvant to Enhance the Immunogenicity of DNA Vaccines. Vaccines 2019, 7, 38. https://doi.org/10.3390/vaccines7020038
Shrestha AC, Wijesundara DK, Masavuli MG, Mekonnen ZA, Gowans EJ, Grubor-Bauk B. Cytolytic Perforin as an Adjuvant to Enhance the Immunogenicity of DNA Vaccines. Vaccines. 2019; 7(2):38. https://doi.org/10.3390/vaccines7020038
Chicago/Turabian StyleShrestha, Ashish C., Danushka K. Wijesundara, Makutiro G. Masavuli, Zelalem A. Mekonnen, Eric J. Gowans, and Branka Grubor-Bauk. 2019. "Cytolytic Perforin as an Adjuvant to Enhance the Immunogenicity of DNA Vaccines" Vaccines 7, no. 2: 38. https://doi.org/10.3390/vaccines7020038
APA StyleShrestha, A. C., Wijesundara, D. K., Masavuli, M. G., Mekonnen, Z. A., Gowans, E. J., & Grubor-Bauk, B. (2019). Cytolytic Perforin as an Adjuvant to Enhance the Immunogenicity of DNA Vaccines. Vaccines, 7(2), 38. https://doi.org/10.3390/vaccines7020038





