Abstract
Background: The development of therapeutic vaccines requires thorough knowledge of potential hazards associated with long-term inactivation of self-proteins. Among potential targets, interleukin 13 (IL-13) merits consideration, as monoclonal antibodies disrupting IL-13 signaling are proving to be exceedingly effective in common conditions such as atopic dermatitis. Objective: Given the mass publication of scientific data, an appraisal of safety aspects is challenging. Methods: We here provide a three-fold approach to survey clinically relevant information on off-target effects, both adverse and beneficial, that may potentially be encountered in patients undergoing long-term IL-13 inactivation. First, we review non-clinical data in vivo and in vitro. Second, we summarize safety data accumulating from patients dosed with anti-IL-13 drugs. Third, we exploit human mutation data as well as emerging large-scale genetic datasets (global exome data from 60,000 patients) to obtain information on any association of IL-13-inactivating genetic variants with disease states. In addition, we: (1) dissect the precise efficacy signals obtained with various drugs targeting IL-13 and/or IL-4, and (2) summarize unintended, but potentially beneficial effects of prolonged IL-13 inactivation on several functional systems. Results: Prolonged repression of IL-13 in several thousand patients so far has not uncovered any non-redundant functions of IL-13 in immune defense. Furthermore, missense mutations in the key genes IL-13, IL-13Rα1, IL-13Rα2, IL-4, IL-4Rα are common, while no case reports have been published on any immune deficiency or increased risk of neoplastic disease associated with such mutations, suggesting that these genes do not harbor non-redundant roles in adult outbred humans. In terms of efficacy, data from clinically used drugs strongly suggest that targeting IL-13 only, as opposed to IL-13 and IL-4, may be effective in eczema while being more selective. Importantly, several lines of evidence suggest that inhibition of IL-13 may in fact harbor potentially beneficial effects on non-targeted systems, including glucose metabolism, hepatic fibrosis, and atherosclerosis, suggesting that respective outcomes should be systematically captured in patients dosed with IL-13 interfering drugs. Collectively, available evidence suggests that IL-13 may fulfill safety requirements required for the target of a therapeutic vaccine.
1. Introduction
The selective inhibition of cytokine function via monoclonal antibodies has revolutionized the treatment of chronic inflammatory conditions. Despite their phenomenal success, there are some limitations to these drugs, including excessive cost for health care providers, emergence of anti-drug antibodies, and the need for relatively frequent dosing, requiring logistics for shipment and storage of sensitive biological products. Therapeutic vaccines eliciting an endogenous antibody response against cytokines provide a potential solution to overcome these shortcomings.
Aside from the question of efficacy, however, the main concern in the use of vaccines, as well as biologic drugs, is the safety of long-term elimination of self-proteins. Thus, blocking of TNF-α may predispose toward opportunistic infections (recently reviewed in [1]). Similarly, elimination of IL-6 requires prior immunization against hepatitis B to avoid infection [2].
The safety of long-term elimination of a given protein is determined by its non-redundant roles. In order to assess these in a systematic fashion for IL-13, the present review will consider mutually complementary data. First, non-clinical data generated in vitro and in animal models will be reviewed. Obviously, an in-depth review of all IL-13-related data is not intended here. Rather, the focus will be on any toxicity/safety-related findings. The importance of non-clinical data is two-fold. On the one hand, they allow prediction of expected safety hazards in clinical settings, thereby informing monitoring. On the other hand, if the clinical data, as is often the case, does not bear out limiting adverse effects that could have been expected based on non-clinical data, this supports the notion that for a given postulated biological function, the target in question (here IL-13) is indeed redundant.
The second, and in many ways most informative, data set consists of safety data accumulating in the clinical use of monoclonal antibodies. In this context, three IL-13-targeting drugs are being developed in addition to the IL-4/IL-13 dual-specific drug dupilumab which has already been approved.
A third data set consists of genetic data. These not only include the traditional reporting of human pedigrees or sporadic mutants associated with clinical phenotypes, but also screens for over-representation of IL-13 blocking mutations in infectious diseases, as well as global databases cataloguing frequent mutations that have failed to emerge as associated with clinical phenotypes. If inactivating mutations are found to be of high frequency, then their non-association with disease states represents an informative negative finding.
Finally, this review will summarize data suggesting that blocking IL-13 may harbor unintended but potentially beneficial effects based on non-clinical data. The purpose here is to highlight that systematic and targeted monitoring of such data in patients undergoing IL-13 blocking antibody treatment is essential and required to gauge the clinical relevance of these data.
2. Non-Clinical Data on IL-13 and Susceptibility to Disease
The present review section is based on a PubMed search using the strategy: (IL-13/ IL-13R/ Interleukin-13/ Interleukin-13 receptor AND ‘infection’) in: abstract/title; cut-off 10 October 2018. By way of limitation, this review is not intended to be exhaustive but rather attempts to focus on data bearing a predictive value on disease susceptibility in humans.
2.1. Schistosoma and Helminth Susceptibility
A wealth of literature is available examining the possibility of increased susceptibility to Schistosoma infection. One group of clinically relevant data are studies of associations of human polymorphisms with disease. Thus, an IL-13 promoter variant (−1111T/C) was found to confer slightly increased susceptibly to Schistosoma infection (T/T genotype protective); however, effects of age, gender, and village (the latter likely reflecting variable genetic background) were in fact found to be many orders of magnitude higher [3]. A study on a Brazilian population found a suggestive protective association of the same promoter allele as well as a non-conserved functional allele (rs20541 → R130Q) against Schistosoma mansoni [4]. A French study found association of the same IL-13 variant (rs1800925) and Schistosoma infection [5]. Another study did not find association of Schistosoma infection and IL-13 but only IL-5 polymorphisms [6]. Studies with genetically altered inbred mice indicate that survival in Schistosoma infection is not impaired in IL-13 deficient mice [7] and that granuloma formation is only impaired in combined IL-4/IL-13 ablation [8]. In fact, ablation of IL-13 ameliorates lung fibrosis in this setting (see below on this aspect). A recent review of intestinal helminth infection also showed redundancy between IL-4 and IL-13, indicating that IL-13 deficiency can be compensated for by IL-4 in murine models of infection [9]. Taken together, despite the available data suggesting a role for IL-13 in the containment of infections such as intestinal nematodes [10], no evidence has been found by genetic association or patients that deficiency in IL-13 affects susceptibility or disease severity or natural course in humans in Schistosoma infection. Nonetheless, mouse data on inbred strains do suggest that an absence of IL-13 might negatively impact the defense against intestinal parasites [11], suggesting that caution may nevertheless be prudent.
2.2. Leishmaniasis
Cutaneous Leishmaniasis (CL) is the most common type of Leishmania infection causing large ulcers on exposed parts of the body. IL-13 is believed to be responsible for non-healing progressing cutaneous disease, previously demonstrated on wild type, IL-13−/−, IL-4Rα−/− and IL-4−/− mice that encountered Leishmania major and Leishmania mexicana [12,13,14]. IL-4Rα−/− mice were fully resistant to L. mexicana, however IL-4−/− mice developed persistent lesions following disease progression [13]. IL-13−/− and IL-4Rα−/− groups developed lesions similar to wild type upon initial infection. However, those cleared, and the mice recovered, indicating that IL-4 is the prevailing cytokine responsible for further susceptibility. The resistant phenotype was highly improved in double knockout mice for IL-13 and IL-4 in L. major infection [14]. A possible explanation for why IL-13−/− mice became resistant to the parasites is that IL-13 is suppressing the functions of interferon-gamma (IFN-γ) and IL-12, which play key roles in clearance as shown in previous investigations, indicating susceptible phenotypes upon their modulation [15,16,17,18]. Thus, an IL-13 vaccine could supress parasite progression to a chronic non-healing state and enhance T helper 1 (Th1) responses, allowing recovery. Certainly, no data exist to suggest that an IL-13 vaccine would exacerbate susceptibility to Leishmania.
2.3. Viral Infections
Regarding a potential effect of blocking IL-13 on viral infections, available evidence from clinical studies with IL-13 blocking antibodies has so far failed to provide any data suggesting clinically relevant impact. Non-clinical studies have shown that Th2 cytokines, including IL-13, may in fact impair immunity to rhinoviruses or respiratory syncytial virus (RSV) either directly [19,20,21] or indirectly via signal transducer and activator of transcription 6 (STAT6) activation [22] and a recent review [19] summarizes evidence suggesting that blocking IL-33/IL-13 may be beneficial in the treatment of virus-associated lung disease. These viral infections in childhood can leave individuals vulnerable to development of chronic inflammatory diseases in adult life and result in impaired antiviral responses [23,24,25]. For instance, a study of influenza A virus shows that transcriptionally active remnants of the virus can remain in lung cells once infection has cleared and can also contribute to chronic lung disease partially accounted for by IL-13 expression [25]. This susceptibility was proposed to be a cause of increased IL-13 signaling via STAT6 [25]. Severe influenza infection can be observed in people suffering from asthma, which leads to further chronic disease exacerbations post viral infection [26]. Furthermore, there is a link between rhinovirus infection, elevated IL-13 levels and contributions of asthma [27]. Asthmatic children had reduced IFN-β and IFN-λ production in comparison to healthy subjects during rhinovirus (RS-16) encounter, leading to disease exacerbations [28]. Similarly, another study of RS-16 illustrated that IL-4 and IL-13 impaired production of IFN-β and IFN-λ and inhibited toll-like receptor 3 (TLR3) and interferon regulatory transcription factor 3 (IRF3), thus allowing the virus to replicate [23]. It has also been suggested that IL-13 is involved in mucus metaplasia that can increase susceptibility to rhinovirus [29] and decreasing IL-13 levels can reduce mucus production, airway hyperresponsiveness and partly reduce airway eosinophilia during rhinoviral infection [30]. Contribution to asthma exacerbations are also the case for RSV as IL-13 is believed to be a mediator of viral pathogenesis driving pulmonary eosinophilia via the IL-33/ST2 pathway [19,31,32]. IL-13-expressing natural killer cells are found to be a source for airway inflammation [33] and were significantly increased during RSV infection [32]. Furthermore, there is a genetic association between a haplotype at the IL-13-IL-4 gene locus and increased IL-13 production with a correlation in elevated risk of RSV bronchiolitis in childhood [34]. RSV can therefore manifest and significantly worsen asthmatic exacerbations by driving airway inflammation and tissue damage during infection [31]. IL-13 overexpression may also be a susceptibility factor for viral respiratory infection, especially in those suffering from an ongoing chronic inflammatory disease. Collectively, these studies suggest that Th2 cytokines can have a negative impact on antiviral responses and can contribute to future health complications. Taken together, therefore, available data suggest that therapeutic targeting of IL-13 can relieve chronic inflammatory disease and possibly provide better protection during respiratory infections.
2.4. Other Infections
High levels of IL-13 (> 10 pg/mL) in respiratory secretions were found to predispose to life-threatening rhinovirus infection in children younger than two years in a large national survey (n = 56,560) from Argentina, suggesting that blocking IL-13 in this setting may in fact be beneficial [27]. Despite a targeted search, a study from China found associations between IL-4 but not IL-13 polymorphisms and coal miners’ pneumoconiosis [35]. Chlamydia commonly cause infections of the respiratory and genital tract. IL-13 increases susceptibility to infection as well as severity by driving increased inflammation and bacterial manifestation [36]. Conversely, respiratory Chlamydia infection in early life was shown to reduce IL-13Rα2 expression and attenuate IL-13 production with potential secondary benefit later in life on asthma susceptibility [37].
2.5. Septic Shock
A Spanish study on 48 children with sepsis found significantly lower IL-13 levels in patients six hours after onset of refractory shock [38], while a British study in 31 adults observed a correlation of TNF-α and IL-13 levels and a high level of each with septic shock [39]. As of the cut-off date of this review, there is no PubMed available evidence indicating that IL-13 deficiency has serious effects on specific susceptibility to infectious agents and/or septic shock.
2.6. Malignancies
While in vitro studies on IL-13 in various cancer model systems are abundant, no clinical or in vivo mouse data link IL-13 directly to pro-tumor or anti-tumor effects to date. Noteworthy are studies linking IL-13 to cancer-associated fibrosis in Hodgkin’s lymphoma and pancreatic cancer [40,41,42,43], tying in with a general pro-fibrotic effect of IL-13 (discussed below). Also, the abundance of IL-13Rα2 on glioblastoma cells was clinically explored by designing an IL-13-linked toxin to kill tumor cells in a phase I clinical trial [44]. There are data on IL-13 promoter polymorphisms as well as IL-13 serum levels in a variety of cancer entities of various stages, but no clear causality patterns have emerged to date. Emerging data from IL-13 blocking clinical trials so far confirm the absence of notable effects on cancer development (see below). However, confidence in the safety of vaccine-based IL-13 blocking will necessarily be increased upon the absence of safety signals in widespread and prolonged clinical dosing with anti-IL-13 drugs in patients.
3. Safety Signals from Clinical Trials and Post-Marketing Surveillance
Dupilumab, a dual-acting drug blocking both IL-4 and IL-13 function (Figure 1b) was approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 2017 for use in atopic dermatitis. Approval for use in asthma is expected for Q4 2018. Recent phase III [45] (n = 210), [46] (n = 1908) studies on asthma showed no significant and/or severe treatment emergent infections. The same was previously found in published studies on atopic dermatitis [47] (n = 325), [48] (n = 1379). No long-term safety concerns have emerged either as part of the Regeneron Open Label Extension (OLE) study, featuring continuous dosing for a total of three years (n = 2000, latest update 2017, REGN668/SAR231893, [49]), nor through post-marketing safety monitoring eighteen months after market introduction in the US. These data are reassuring in terms of safety and are reflected in a recent meta-analysis that identifies conjunctivitis as the only adverse effect emerging from present data [50]. It is worth remembering, however, that patients with a history of helminth infection were excluded from clinical trials.
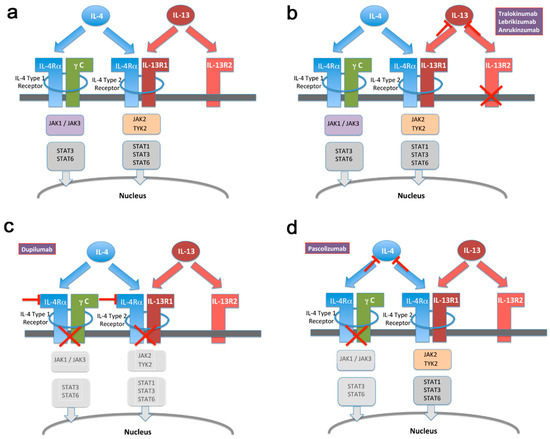
Figure 1.
Schematic of IL-4 and IL-13 mediated signal transduction. (a) Illustration of overlapping as well as non-redundant signaling mechanisms employed by IL-4 and IL-13, respectively. For details, see text. (b–d) Same as in (a) with highlighted drugs targeting specific molecules, respectively. Drug-mediated inactivation of pathway components is illustrated by grey-shading of respective boxes. STAT = signal transducer and activator of transcription. JAK = Janus kinase. TYK = tyrosine kinase.
Currently available safety data for the IL-13 selective monoclonal antibodies confirm the dupilumab data. Thus, for tralokinumab, collectively sizeable phase II populations are available including [51] (n = 79), [52] (n = 409), [53] (n = 452), [54] (n = 194), as well as for lebrikizumab, including [55] (n = 1081), [56] (n = 313), and [57] (n = 209). A further anti-IL-13 monoclonal, anrukinzumab, studied in active ulcerative colitis (n = 84) also found no significant adverse effects [58]. This study also measured and detected feedback elevation of IL-13 in peripheral blood of treated patients. Overall, available clinical data from patients dosed with anti-IL-4/IL-13 drugs have not uncovered safety signals to date that would suggest a vaccine approach to be unsafe, as reflected in a recent independent meta-review of these data [59].
4. Evidence for IL13 Related Phenotypes from Large-Scale Genetic Databases
The advent of high-throughput genome wide sequencing has provided several databases compiling phenotypes linked to genetic polymorphisms and/or mutations. In the case of IL-13, a search in the Single Nucleotide Polymorphism Database (dbSNP) for IL-13, IL-13Rα1 and IL-13Rα2 reveals no clinically-relevant phenotype-associated variants. There are data linking IL-13 polymorphisms with the outcome of children with minimal change nephrotic syndrome [60,61], although the significance of this remains uncertain. Furthermore, there are no published pedigrees and / or single patient case reports involving variants mapping to the above listed genes, nor entries listed on Online Mendelian Inhertience in Man (OMIM).
The completion of the Exome Aggregation Consortium (ExAC) sequencing of 60,000 genomes from a variety of global populations has enabled a quantitative assessment of the impact of genetic variants on overall fitness in terms of evolutionary selection. We extracted these data for key genes related to IL-13 signaling. As shown in Table 1, non-conserved and even loss-of-function mutations are frequent. Except for IL-13Rα1 (numbers set in bold), none of the genes exhibit apparent evolutionary selection against homozygous occurrence of loss-of-function mutations. Although the high z-score could suggest that IL-13Rα1 may be important in pre-natal development, IL-13Rα1-deficient mice in fact exhibit normal fertility rates and display no global development abnormality but only a specific defect in M2-type macrophage development [62]. Furthermore, the Exome Variant Server (EVS) compiles allele frequencies for approximately 6000 genomes from European and African ancestries, respectively. We extracted EVS-listed variants for IL-13 relevant genes with high probability of deleterious function based on: (1) non-conserved amino-acid replacement, (2) high probability (score > 0.8) in PolyPhem2-based prediction, and (3) 100% conservation of the main allele between humans and chimpanzee (Table 2). These data show that deleterious variants in these genes are, in fact, surprisingly common, in some cases amounting to 25% of all minor variants identified (Table 2, printed in bold). Therefore, the absence of clinical case studies reporting health issues in individuals harboring any of these mutations in the public space is significant, suggesting that deficient IL-13 signaling caused by congenital mutations is not associated with severe immunodeficiency or failure to thrive.

Table 1.
Global counter-selection against mutations in key genes affecting interleukin 13 (IL-13) signaling. 1

Table 2.
Frequencies of damaging variants in key genes affecting IL-13 signaling. 1
5. IL-13 on Its Own Represents a More Selective Target Than IL-4/IL-13 in Combination for Treatment of Atopic Conditions
Several drugs targeting IL-13 are undergoing clinical development or already marketed. The precise efficacy, as well as adverse effect profile of each drug is related to its molecular mode of action. As shown in Figure 1a, IL-13 and IL-4 signal through partially overlapping pathways. Both cytokines will activate the so-called type-2 IL-4 receptor, followed by JAK2/Tyk2 phosphorylation and subsequent STAT translocation. By contrast, only IL-4 activates the type-1 IL-4 receptor, whereas IL-13 additionally binds to IL-13α2, commonly regarded as an inhibitory decoy receptor. By binding to IL-4Rα, dupilumab essentially abrogates signaling through both IL-4 and IL-13 (Figure 1b). In contrast, pascolizumab directly binds to IL-4, thereby preserving type-2 receptor activation via IL-13 (Figure 1c). This drug did not show clinical efficacy, thereby suggesting that the effect of this system on asthma and eczema pathogenesis primarily proceeds through the IL-4 type 2 receptor [65]. Finally, IL-13 selective antibodies (Figure 1d) prevent IL-13 binding but preserve the activation of both active receptor systems. The emerging clinical efficacy of these drugs both in asthma and atopic eczema suggests that the more selective inactivation of IL-13 achieves sufficient activity while preserving IL-4 activity, with a potentially important gain in safety profile in terms of immunity to infectious agents [9]. A number of selective as well as global JAK inhibitors are currently also being developed for atopic disease [66], as well as other conditions including cancer. However, the complex nature of precise JAK/STAT coupling of IL-4, as well as other signal transducing pathways [67], prevent an unambiguous mapping of specific adverse effects and/or activity profiles to relative IL-4/IL-13 signaling.
6. Additional Potential Effects of an IL-13 Vaccine
When considering the potential of IL-13 as a vaccine target to improve atopic disease, potential off-target effects in addition to those considered above may occur which, although unintended, may in fact prove of interest for further vaccine applications. Therefore, this final section will summarize data suggesting potential vaccine effects on specific functional systems that warrant dedicated monitoring during clinical applications based on currently available data.
6.1. Improvement of Fibrosis
A substantial amount of data indicates that an anti-IL-13 vaccine may be beneficial for the prevention of the amelioration of hepatic fibrosis in Schistosoma infection [68] as well as non-infectious fibrosis, as reported in many studies (see above) [69,70,71]. A preventative effect in this regard could indeed constitute a major independent indication for anti-IL-13 vaccination.
6.2. Glucose Metabolism
Blocking IL-13 signaling may in fact improve impairment in glucose metabolism [72,73,74] and regulate glucose uptake and pathways involved in skeletal muscle maintenance [75,76]. Conversely, however, IL-13 has also been suggested to fulfill an autocrine anti-inflammatory role in diabetes [77].
6.3. Atherosclerosis
A somewhat related activity of IL-13, also possibly involving macrophage activation, is regulation of atherosclerosis, where the precise role and mechanism and its clinical significance remain to be elucidated [75]. Animal models of IL-13 deficiency suggest IL-13 may act as a contributor in atherosclerosis [78,79,80]. Other studies suggest that IL-13 leads to atherosclerosis by inducing expression of peroxisome proliferator activated receptor δ (PPARδ) or PPARβ in the adipose tissue and by increasing CD36 levels [81,82]. However, obviously atherosclerosis development is multifactorial and complex. Furthermore, the Th1 and Th2 responses contribute differently during various stages of plaque development, and IL-13 alone cannot reverse disease progression [83,84]. Clearly, a more systematic monitoring of glucose metabolism, as well as markers of systemic inflammation in patients currently dosed with anti-IL-13 drugs, would be important to better understand the clinical significance of the available non-clinical data in this regard.
7. Conclusions
The clinical use of dupilumab, as well as selective anti-IL-13 drugs, has shown that blocking IL-13 is effective both for the treatment of asthma and atopic dermatitis and may be of use in other atopic conditions (e.g., severe contact allergy). A precise understanding of the molecular signaling mechanisms suggests that targeting IL-13, while equally effective, preserves IL-4 signaling through the type-2 IL-4 receptor which may constitute an important safety benefit regarding immunity, especially to helminth infections. A large body of genomic data appear to confirm emerging safety data from anti-IL-13/IL-4 clinical use that prolonged blocking of IL-13 is not associated with reduced systemic immunity or opportunistic infections. Notably, it remains to be seen whether the pro-fibrotic activity of IL-13 is of clinically high enough relevance to render a vaccination treatment effective in several conditions involving increased fibrosis (e.g. hepatic fibrosis, pulmonary fibrosis due to various etiologies). Finally, the clinical use of IL-13 drugs should be utilized to specifically monitor the effects of IL-13 blocking on glucose metabolism.
Author Contributions
This manuscript was co-written by J.F. and A.M.
Funding
This research received no external funding.
Acknowledgments
The authors would like to acknowledge the support of Jean-Claude Bourdon in the co-supervision of AM research.
Conflicts of Interest
J.F. is co-owner and CEO of a commercial company (www.healvax.com) that holds licence rights for the clinical development of IL-13 targeting vaccines.
References
- Borman, Z.A.; Côté-Daigneault, J.; Colombel, J.-F. The risk for opportunistic infections in inflammatory bowel disease with biologics: An update. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Mo, Y.Q.; Jing, J.; Ma, J.D.; Zheng, D.H.; Dai, L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int. J. Rheum. Dis. 2017, 20, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Kouriba, B.; Chevillard, C.; Bream, J.H.; Argiro, L.; Dessein, H.; Arnaud, V.; Sangare, L.; Dabo, A.; Beavogui, A.H.; Arama, C.; et al. Analysis of the 5q31-q33 locus shows an association between IL13-1055C/T IL-13-591A/G polymorphisms and Schistosoma haematobium infections. J. Immunol. 2005, 174, 6274–6281. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.V.; Araujo, M.I.; Ponte, E.V.; Oliveira, R.R.; Gao, P.; Cruz, A.A.; Barnes, K.C.; Beaty, T.H. Functional polymorphisms in IL13 are protective against high Schistosoma mansoni infection intensity in a Brazilian population. PLoS ONE 2012, 7, e35863. [Google Scholar] [CrossRef] [PubMed]
- Isnard, A.; Kouriba, B.; Doumbo, O.; Chevillard, C. Association of rs7719175, located in the IL13 gene promoter, with Schistosoma haematobium infection levels and identification of a susceptibility haplotype. Genes Immun. 2011, 12, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.K.; Zhao, Z.Z.; Chen, H.G.; Montgomery, G.W.; Li, Y.S.; McManus, D.P. Analysis of the 5q31 33 locus shows an association between single nucleotide polymorphism variants in the IL-5 gene and symptomatic infection with the human blood fluke, Schistosoma japonicum. J. Immunol. 2007, 179, 8366–8371. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, M.G.; Schopf, L.R.; Neben, T.Y.; Cheever, A.W.; Donaldson, D.D.; Wynn, T.A. IL-13 Is a Key Regulatory Cytokine for Th2 Cell-Mediated Pulmonary Granuloma Formation and IgE Responses Induced by Schistosoma mansoni Eggs. J. Immunol. 1999, 162, 920–930. [Google Scholar]
- Fallon, P.G.; Richardson, E.J.; McKenzie, G.J.; McKenzie, A.N. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 2000, 164, 2585–2591. [Google Scholar] [CrossRef]
- Bao, K.; Reinhardt, R.L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 2015, 75, 25–37. [Google Scholar] [CrossRef]
- Bancroft, A.J.; McKenzie, A.N.; Grencis, R.K. A critical role for IL-13 in resistance to intestinal nematode infection. J. Immunol. 1998, 160, 3453–3461. [Google Scholar]
- Sorobetea, D.; Svensson-Frej, M.; Grencis, R. Immunity to gastrointestinal nematode infections. Mucosal Immunol. 2018, 11, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Hurdayal, R.; Brombacher, F. Interleukin-4 Receptor Alpha: From Innate to Adaptive Immunity in Murine Models of Cutaneous Leishmaniasis. Front. Immunol. 2017, 8, 1354. [Google Scholar] [CrossRef]
- Alexander, J.; Brombacher, F.; McGachy, H.A.; McKenzie, A.N.J.; Walker, W.; Carter, K.C. An essential role for IL-13 in maintaining a non-healing response following Leishmania mexicana infection. Eur. J. Immunol. 2002, 32, 2923–2933. [Google Scholar] [CrossRef]
- Doherty, T.M.; Kastelein, R.; Menon, S.; Andrade, S.; Coffman, R.L.; McKenzie, A.N.J. Modulation of murine macrophage function by IL-13. J. Immunol. 1993, 151, 7151–7160. [Google Scholar] [PubMed]
- Swihart, K.; Fruth, U.; Messmer, N.; Hug, K.; Behin, R.; Huang, S.; Del Giudice, G.; Aguet, M.; Louis, J.A. Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J. Exp. Med. 1995, 181, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.E.; Reiner, S.L.; Zheng, S.; Dalton, D.K.; Locksley, R.M. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J. Exp. Med. 1994, 179, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Mattner, F.; Magram, J.; Ferrante, J.; Launois, P.; Di Padova, K.; Behin, R.; Gately, M.K.; Louis, J.A.; Alber, G. Genetically resistant mice lacking interleukin-12 are susceptible to infection withLeishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 1996, 26, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Scharton-Kersten, T.; Afonso, L.C.; Wysocka, M.; Trinchieri, G.; Scott, P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 1995, 154, 5320–5330. [Google Scholar]
- Donovan, C.; Bourke, J.E.; Vlahos, R. Targeting the IL-33/IL-13 Axis for Respiratory Viral Infections. Trends Pharmacol. Sci. 2016, 37, 252–261. [Google Scholar] [CrossRef]
- Busse, W.W.; Lemanske, R.F. Asthma. N. Engl. J. Med 2001, 344, 350–362. [Google Scholar] [CrossRef]
- Schwarze, J.; Hamelmann, E.; Bradley, K.L.; Takeda, K.; Gelfand, E.W. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J. Clin. Investig. 1997, 100, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.D.; Gao, P.; Kim, B.E.; Lesley, L.J.; Streib, J.E.; Taylor, P.A.; Zaccaro, D.J.; Boguniewicz, M.; Beck, L.A.; Hanifin, J.M.; et al. The signal transducer and activator of transcription 6 gene (STAT6) increases the propensity of patients with atopic dermatitis toward disseminated viral skin infections. J. Allergy Clin. Immunol. 2011, 128, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Contoli, M.; Ito, K.; Padovani, A.; Poletti, D.; Marku, B.; Edwards, M.R.; Stanciu, L.A.; Gnesini, G.; Pastore, A.; Spanevello, A.; et al. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy 2015, 70, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.R.; Bartlett, N.W.; Hussell, T.; Openshaw, P.; Johnston, S.L. The microbiology of asthma. Nat. Rev. Microbiol. 2012, 10, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Letvin, A.N.; Wu, K.; Holtzman, M.J.; Keeler, S.P.; Agapov, E.V.; Hinojosa, M.E. Influenza A Virus Infection Causes Chronic Lung Disease Linked to Sites of Active Viral RNA Remnants. J. Immunol. 2018, 201. [Google Scholar] [CrossRef]
- Nguyen-Van-Tam, J.S.; Openshaw, P.J.M.; Hashim, A.; Gadd, E.M.; Lim, W.S.; Semple, M.G.; Read, R.C.; Taylor, B.L.; Brett, S.J.; McMenamin, J.; et al. Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009). Thorax 2010, 65, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.T.; Hijano, D.R.; Acosta, P.L.; Mateu, C.G.; Marcone, D.N.; Linder, J.E.; Talarico, L.B.; Elder, J.M.; Echavarria, M.; Miller, E.K.; et al. Interleukin-13 associates with life-threatening rhinovirus infections in infants and young children. Pediatr. Pulmonol. 2018, 53, 787–795. [Google Scholar] [CrossRef]
- Baraldo, S.; Contoli, M.; Bazzan, E.; Turato, G.; Padovani, A.; Marku, B.; Calabrese, F.; Caramori, G.; Ballarin, A.; Snijders, D.; et al. Deficient antiviral immune responses in childhood: Distinct roles of atopy and asthma. J. Allergy Clin. Immunol. 2012, 130, 1307–1314. [Google Scholar] [CrossRef]
- Lachowicz-Scroggins, M.E.; Boushey, H.A.; Finkbeiner, W.E.; Widdicombe, J.H. Interleukin-13-induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am. J. Respir. Cell Mol. Biol. 2010, 43, 652–661. [Google Scholar] [CrossRef]
- Dakhama, A.; Park, J.-W.; Taube, C.; Joetham, A.; Balhorn, A.; Miyahara, N.; Takeda, K.; Gelfand, E.W. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J. Immunol. 2005, 175, 1876–1883. [Google Scholar] [CrossRef]
- Castilow, E.M.; Meyerholz, D.K.; Varga, S.M. IL-13 Is Required for Eosinophil Entry into the Lung during Respiratory Syncytial Virus Vaccine-Enhanced Disease. J. Immunol. 2008, 180, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, J.; Qi, F.; Zeng, S.; Xu, L.; Hu, H.; Wang, D.; Liu, B. Natural helper cells contribute to pulmonary eosinophilia by producing IL-13 via IL-33/ST2 pathway in a murine model of respiratory syncytial virus infection. Int. Immunopharmacol. 2015, 28, 337–343. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Krauß, R.H.; Sun, A.C.; Takei, F. Lung Natural Helper Cells Are a Critical Source of Th2 Cell-Type Cytokines in Protease Allergen-Induced Airway Inflammation. Immunity 2012, 36, 451–463. [Google Scholar] [CrossRef]
- Forton, J.T.; Rowlands, K.; Rockett, K.; Hanchard, N.; Herbert, M.; Kwiatkowski, D.P.; Hull, J. Genetic association study for RSV bronchiolitis in infancy at the 5q31 cytokine cluster. Thorax 2009, 64, 345–352. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Song, Z.; Ji, X.; Zhang, Z.; Zhou, J.; Ni, C. Associations of IL-4, IL-4R, and IL-13 gene polymorphisms in coal workers′ pneumoconiosis in China: A case-control study. PLoS ONE 2011, 6, e22624. [Google Scholar] [CrossRef] [PubMed]
- Asquith, K.L.; Horvat, J.C.; Kaiko, G.E.; Carey, A.J.; Beagley, K.W.; Hansbro, P.M.; Foster, P.S. Interleukin-13 promotes susceptibility to chlamydial infection of the respiratory and genital tracts. PLoS Pathog. 2011, 7, e1001339. [Google Scholar] [CrossRef] [PubMed]
- Starkey, M.R.; Essilfie, A.T.; Horvat, J.C.; Kim, R.Y.; Nguyen, D.H.; Beagley, K.W.; Mattes, J.; Foster, P.S.; Hansbro, P.M. Constitutive production of IL-13 promotes early-life Chlamydia respiratory infection and allergic airway disease. Mucosal Immunol. 2013, 6, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Quiros, A.; Casado-Flores, J.; Garrote Adrados, J.A.; Moro, M.N.; Antón, J.A.; Sanz, E.A. Interleukin-13 is involved in the survival of children with sepsis. Acta Paediatr. 2005, 94, 1828–1831. [Google Scholar] [CrossRef]
- Collighan, N.; Giannoudis, P.V.; Kourgeraki, O.; Perry, S.L.; Guillou, P.J.; Bellamy, M.C. Interleukin 13 and inflammatory markers in human sepsis. Br. J. Surg. 2004, 91, 762–768. [Google Scholar] [CrossRef]
- Skinnider, B.F.; Kapp, U.; Mak, T.W. Interleukin 13: A growth factor in hodgkin lymphoma. Int. Arch. Allergy Immunol. 2001, 126, 267–276. [Google Scholar] [CrossRef]
- Nakayama, S.; Yokote, T.; Hiraoka, N.; Nishiwaki, U.; Hanafusa, T.; Nishimura, Y.; Tsuji, M. Role of mast cells in fibrosis of classical Hodgkin lymphoma. Int. J. Immunopathol. Pharmacol. 2016, 29, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Hollins, F.; Woodman, L.; Yang, W.; Monk, P.; May, R.; Bradding, P.; Brightling, C.E. Mast cells express IL-13Rα1: IL-13 promotes human lung mast cell proliferation and FcɛRI expression. Allergy 2006, 61, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hwang, R.F.; Logsdon, C.D.; Ullrich, S.E. Dynamic Mast Cell–Stromal Cell Interactions Promote Growth of Pancreatic Cancer. Cancer Res. 2013, 73, 3927–3937. [Google Scholar] [CrossRef] [PubMed]
- Thaci, B.; Brown, C.E.; Binello, E.; Werbaneth, K.; Sampath, P.; Sengupta, S. Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro. Oncol. 2014, 16, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- De Bruin-Weller, M.; Thaci, D.; Smith, C.H.; Reich, K.; Cork, M.J.; Radin, A.; Zhang, Q.; Akinlade, B.; Gadkari, A.; Eckert, L.; et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br. J. Dermatol. 2018, 178, 1083–1101. [Google Scholar] [CrossRef]
- Simpson, E.L. Dupilumab Improves General Health-Related Quality-of-Life in Patients with Moderate-to-Severe Atopic Dermatitis: Pooled Results from Two Randomized, Controlled Phase 3 Clinical Trials. Dermatol. Ther. (Heidelb) 2017, 7, 243–248. [Google Scholar] [CrossRef]
- Home-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 8 February 2019).
- Wang, F.P.; Tang, X.J.; Wei, C.Q.; Xu, L.R.; Mao, H.; Luo, F.M. Dupilumab treatment in moderate-to-severe atopic dermatitis: A systematic review and meta-analysis. J. Dermatol. Sci. 2018, 90, 190–198. [Google Scholar] [CrossRef]
- Russell, R.J.; Chachi, L.; FitzGerald, J.M.; Russell, R.J.; Chachi, L.; FitzGerald, J.M.; Chaudhuri, R.; Leaker, B.; McGarvey, L.; Siddiqui, S.; et al. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir. Med. 2018, 6, 499–510. [Google Scholar] [CrossRef]
- Parker, J.M.; Glaspole, I.N.; Lancaster, L.H.; Haddad, T.J.; She, D.; Roseti, S.L.; Fiening, J.P.; Grant, E.P.; Kell, C.M.; Flaherty, K.R. A Phase 2 Randomized Controlled Study of Tralokinumab in Subjects with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 197, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Chanez, P.; Leigh, R.; O′Byrne, P.M.; Korn, S.; She, D.; May, R.D.; Streicher, K.; Ranade, K.; Piper, E. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015, 3, 692–701. [Google Scholar] [CrossRef]
- Piper, E.; Brightling, C.; Niven, R.; Oh, C.; Faggioni, R.; Poon, K.; She, D.; Kell, C.; May, R.D.; Geba, G.P.; et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur. Respir. J. 2013, 41, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Korenblat, P.; Chapman, K.R.; Bateman, E.D.; Kopecky, P.; Paggiaro, P.; Yokoyama, A.; Olsson, J.; Gray, S.; Holweg, C.T.; et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): Replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2016, 4, 781–796. [Google Scholar] [CrossRef]
- Korenblat, P.; Kerwin, E.; Leshchenko, I.; Yen, K.; Holweg, C.T.J.; Anzures-Cabrera, J.; Martin, C.; Putnam, W.S.; Governale, L.; Olsson, J.; et al. Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids. Respir. Med. 2018, 134, 143–149. [Google Scholar] [CrossRef]
- Simpson, E.L.; Flohr, C.; Eichenfield, L.F.; Bieber, T.; Sofen, H.; Taïeb, A.; Owen, R.; Putnam, W.; Castro, M.; DeBusk, K.; et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 2018, 78, 863–871. [Google Scholar] [CrossRef]
- Reinisch, W.; Panes, J.; Khurana, S.; Toth, G.; Hua, F.; Comer, G.M.; Hinz, M.; Page, K.; O′Toole, M.; Moorehead, T.M.; et al. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: Efficacy and safety from a phase IIa randomised multicentre study. Gut 2015, 64, 894–900. [Google Scholar] [CrossRef]
- Braddock, M.; Hanania, N.A.; Sharafkhaneh, A.; Colice, G.; Carlsson, M. Potential Risks Related to Modulating Interleukin-13 and Interleukin-4 Signalling: A Systematic Review. Drug Saf. 2018, 41, 489–509. [Google Scholar] [CrossRef]
- Wei, C.L.; Cheung, W.; Heng, C.K.; Arty, N.; Chong, S.S.; Lee, B.W.; Puah, K.L.; Yap, H.K. Interleukin-13 genetic polymorphisms in Singapore Chinese children correlate with long-term outcome of minimal-change disease. Nephrol. Dial. Transplant 2005, 20, 728–734. [Google Scholar] [CrossRef]
- Tenbrock, K.; Schubert, A.; Stapenhorst, L.; Kemper, M.J.; Gellermann, J.; Timmermann, K.; Müller-Wiefel, D.E.; Querfeld, U.; Hoppe, B.; Michalk, D. Type I IgE receptor, interleukin 4 receptor and interleukin 13 polymorphisms in children with nephrotic syndrome. Clin. Sci. (Lond) 2002, 102, 507–512. [Google Scholar] [CrossRef]
- Barik, S.; Miller, M.; Cattin-Roy, A.; Ukah, T.; Zaghouani, H. A distinct dendritic cell population arises in the thymus of IL-13Ralpha1-sufficient but not IL-13Ralpha1-deficient mice. Cell. Immunol. 2018, 331, 130–136. [Google Scholar] [CrossRef] [PubMed]
- ExAC Browser. Available online: http://exac.broadinstitute.org/ (accessed on 8 February 2019).
- NHLBI Grand Opportunity Exome Sequencing Project (ESP). Available online: https://esp.gs.washington.edu/drupal/ (accessed on 8 February 2019).
- Long, A.A. Monoclonal antibodies and other biologic agents in the treatment of asthma. MAbs 2009, 1, 237–246. [Google Scholar] [CrossRef]
- Vale, K. Targeting the JAK-STAT pathway in the treatment of ‘Th2-high’ severe asthma. Future Med. Chem. 2016, 8, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Harris, M.B.; Rothman, P. IL-4/IL-13 signaling beyond JAK/STAT. J. Allergy Clin. Immunol. 2000, 105, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.L.; Domingues, A.L.; Melo, W.G.; Tashiro, T.; de Lorena, V.M.; Montenegro, S.M.; Morais, C.N. Receptor Antagonist of IL-13 Exerts a Potential Negative Regulation During Early Infection of Human Schistosomiasis. Scand. J. Immunol. 2016, 84, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Zhao, Q.; He, C.; Huang, D.; Liu, J.; Chen, F.; Chen, J.; Liao, J.Y.; Cui, X.; Zeng, Y.; et al. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat. Commun. 2015, 6, 8523. [Google Scholar] [CrossRef] [PubMed]
- Hammerich, L.; Tacke, F. Interleukins in chronic liver disease: Lessons learned from experimental mouse models. Clin. Exp. Gastroenterol. 2014, 7, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Gieseck, R.L., 3rd; Ramalingam, T.R.; Hart, K.M.; Vannella, K.M.; Cantu, D.A.; Lu, W.Y.; Ferreira-González, S.; Forbes, S.J.; Vallier, L.; Wynn, T.A. Interleukin-13 Activates Distinct Cellular Pathways Leading to Ductular Reaction, Steatosis, and Fibrosis. Immunity 2016, 45, 145–158. [Google Scholar] [CrossRef]
- Stanya, K.J.; Jacobi, D.; Liu, S.; Bhargava, P.; Dai, L.; Gangl, M.R.; Inouye, K.; Barlow, J.L.; Ji, Y.; Mizgerd, J.P.; et al. Direct control of hepatic glucose production by interleukin-13 in mice. J. Clin. Investig. 2013, 123, 261–271. [Google Scholar] [CrossRef]
- Martinez-Reyes, C.P.; Gomez-Arauz, A.Y.; Torres-Castro, I.; Manjarrez-Reyna, A.N.; Palomera, L.F.; Olivos-García, A.; Mendoza-Tenorio, E.; Sánchez-Medina, G.A.; Islas-Andrade, S.; Melendez-Mier, G.; et al. Serum Levels of Interleukin-13 Increase in Subjects with Insulin Resistance but Do Not Correlate with Markers of Low-Grade Systemic Inflammation. J. Diabetes Res. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Rachmin, I.; O′Meara, C.C.; Ricci-Blair, E.M.; Feng, Y.; Christensen, E.M.; Duffy, J.F.; Zitting, K.M.; Czeisler, C.A.; Pancoast, J.R.; Cannon, C.P.; et al. Soluble interleukin-13ralpha1: A circulating regulator of glucose. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E663–E671. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.-F.; Nie, S.-F.; Chen, Q.-W.; Liao, Y.-H.; Zhang, H.-S.; Dong, J.-T.; Xie, T.; Wang, F.; Tang, T.-T.; Xia, N.; et al. IL-13 may be involved in the development of CAD via different mechanisms under different conditions in a Chinese Han population. Sci. Rep. 2018, 8, 6182. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Q.; Franck, N.; Egan, B.; Sjögren, R.J.O.; Katayama, M.; Duque-Guimaraes, D.; Arner, P.; Zierath, J.R.; Krook, A. Autocrine role of interleukin-13 on skeletal muscle glucose metabolism in type 2 diabetic patients involves microRNA let-7. Am. J. Physiol. Metab. 2013, 305, E1359–E1366. [Google Scholar] [CrossRef] [PubMed]
- Kretowski, A.; Mysliwiec, J.; Kinalska, I. In vitro interleukin-13 production by peripheral blood in patients with newly diagnosed insulin-dependent diabetes mellitus and their first degree relatives. Scand. J. Immunol. 2000, 51, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Cardilo-Reis, L.; Gruber, S.; Schreier, S.M.; Drechsler, M.; Papac-Milicevic, N.; Weber, C.; Wagner, O.; Stangl, H.; Soehnlein, O.; Binder, C.J. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 2012, 4, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Yakubenko, V.P.; Hsi, L.C.; Cathcart, M.K.; Bhattacharjee, A. From macrophage interleukin-13 receptor to foam cell formation: Mechanisms for αMβ2 integrin interference. J. Biol. Chem. 2013, 288, 2778–2788. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008, 79, 360–376. [Google Scholar] [CrossRef]
- Wu, D.; Molofsky, A.B.; Liang, H.-E.; Ricardo-Gonzalez, R.R.; Jouihan, H.A.; Bando, J.K.; Chawla, A.; Locksley, R.M. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011, 332. [Google Scholar] [CrossRef]
- Rahaman, S.O.; Lennon, D.J.; Febbraio, M.; Podrez, E.A.; Hazen, S.L.; Silverstein, R.L. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006, 4, 211–221. [Google Scholar] [CrossRef]
- Mallat, Z.; Ait-Oufella, H.; Tedgui, A. Regulatory T cell responses: Potential role in the control of atherosclerosis. Curr. Opin. Lipidol. 2005, 16, 518–524. [Google Scholar] [CrossRef]
- Foks, A.C.; Lichtman, A.H.; Kuiper, J. Treating Atherosclerosis With Regulatory T Cells. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 280–287. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).