A Live-Attenuated Prime, Inactivated Boost Vaccination Strategy with Chimeric Hemagglutinin-Based Universal Influenza Virus Vaccines Provides Protection in Ferrets: A Confirmatory Study
Abstract
1. Introduction
2. Results
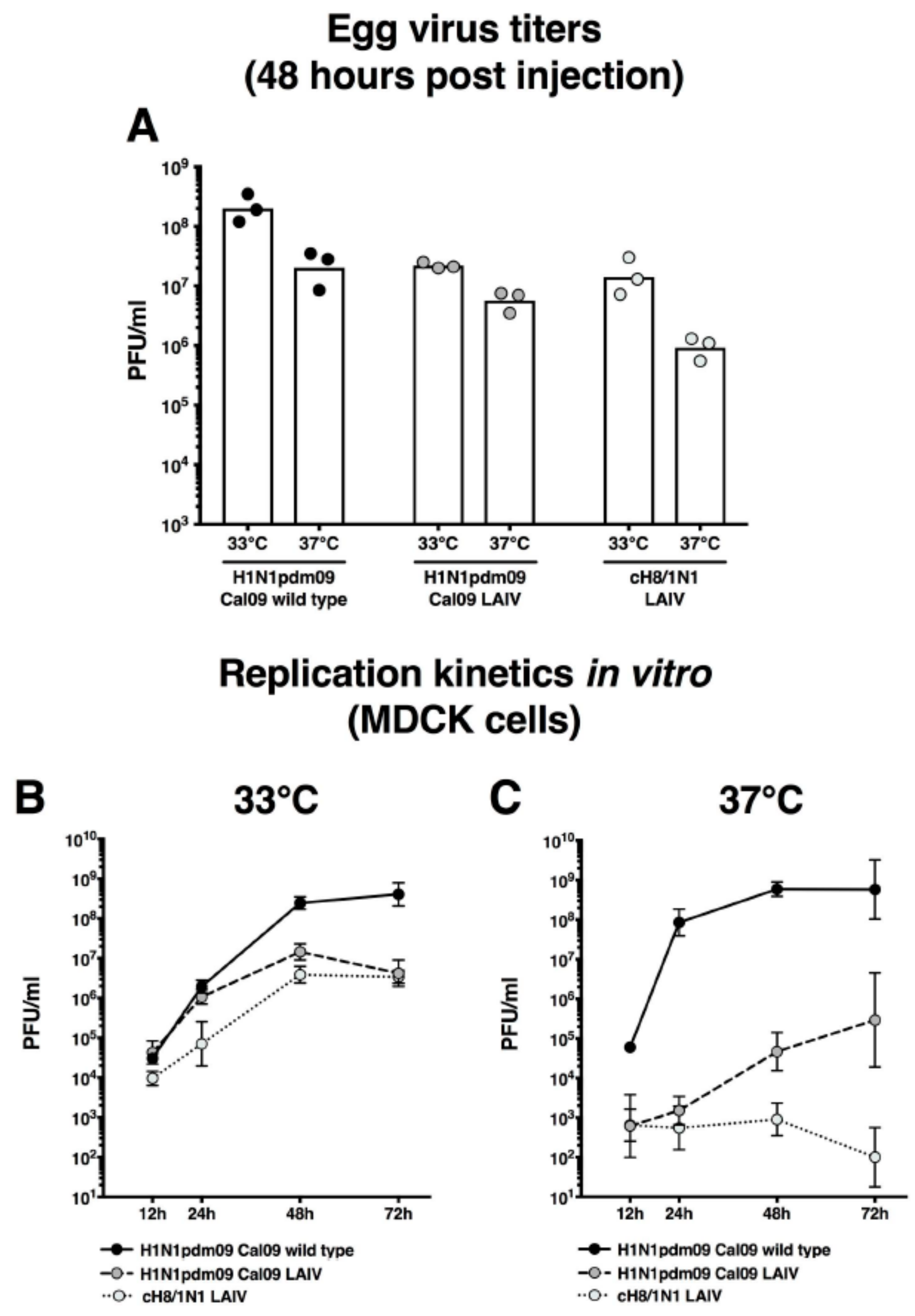
2.1. cH8/1N1 Live-Attenuated Vaccine Virus has a Temperature Sensitive Phenotype
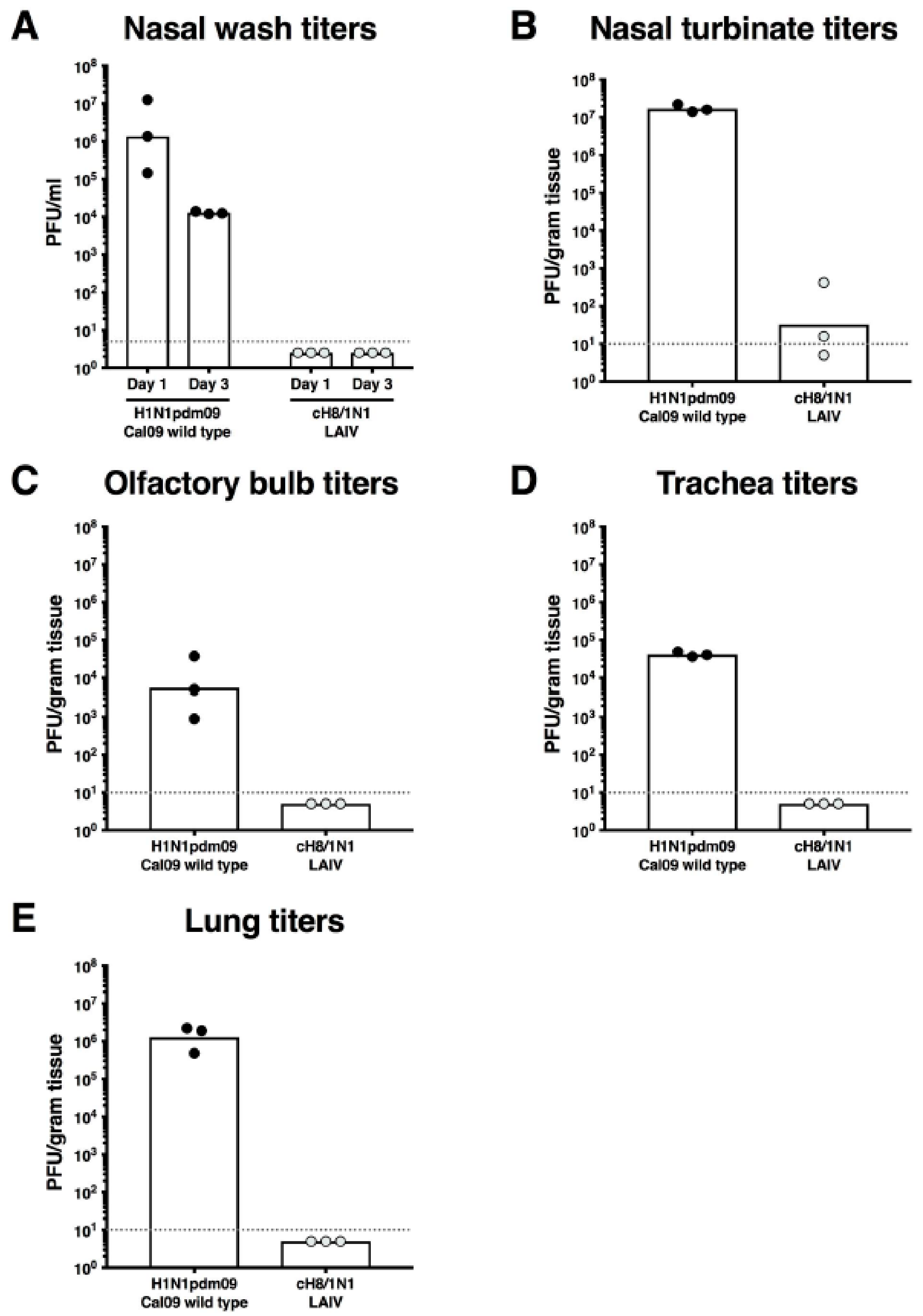
2.2. cH8/1N1 LAIV Is Attenuated In Vivo
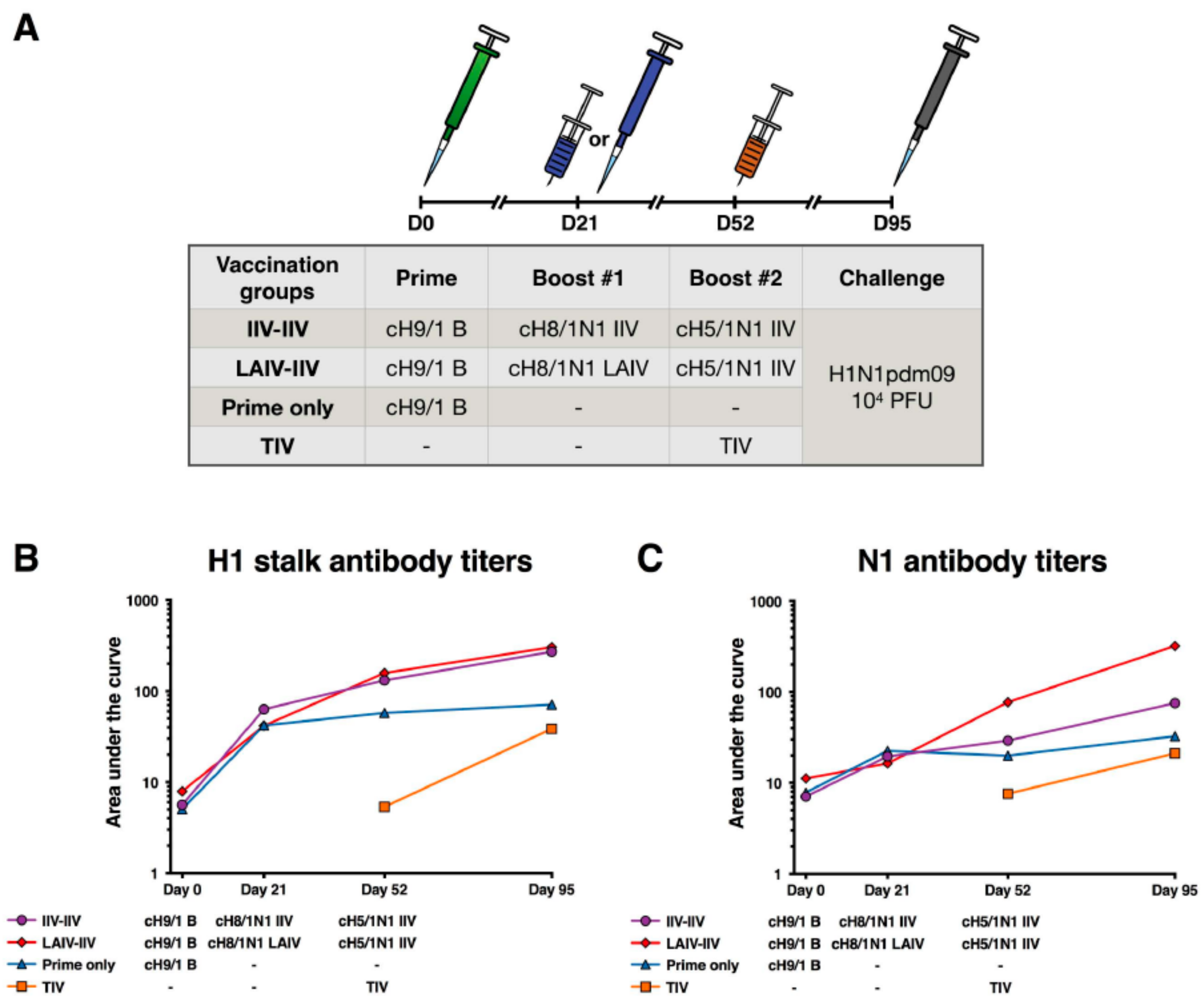
2.3. cHA-Based Vaccination with an LAIV Prime Followed by an IIV Boost Regimen or IIV Prime-Boost Regimen Elicit Equivalent Stalk Antibody Responses
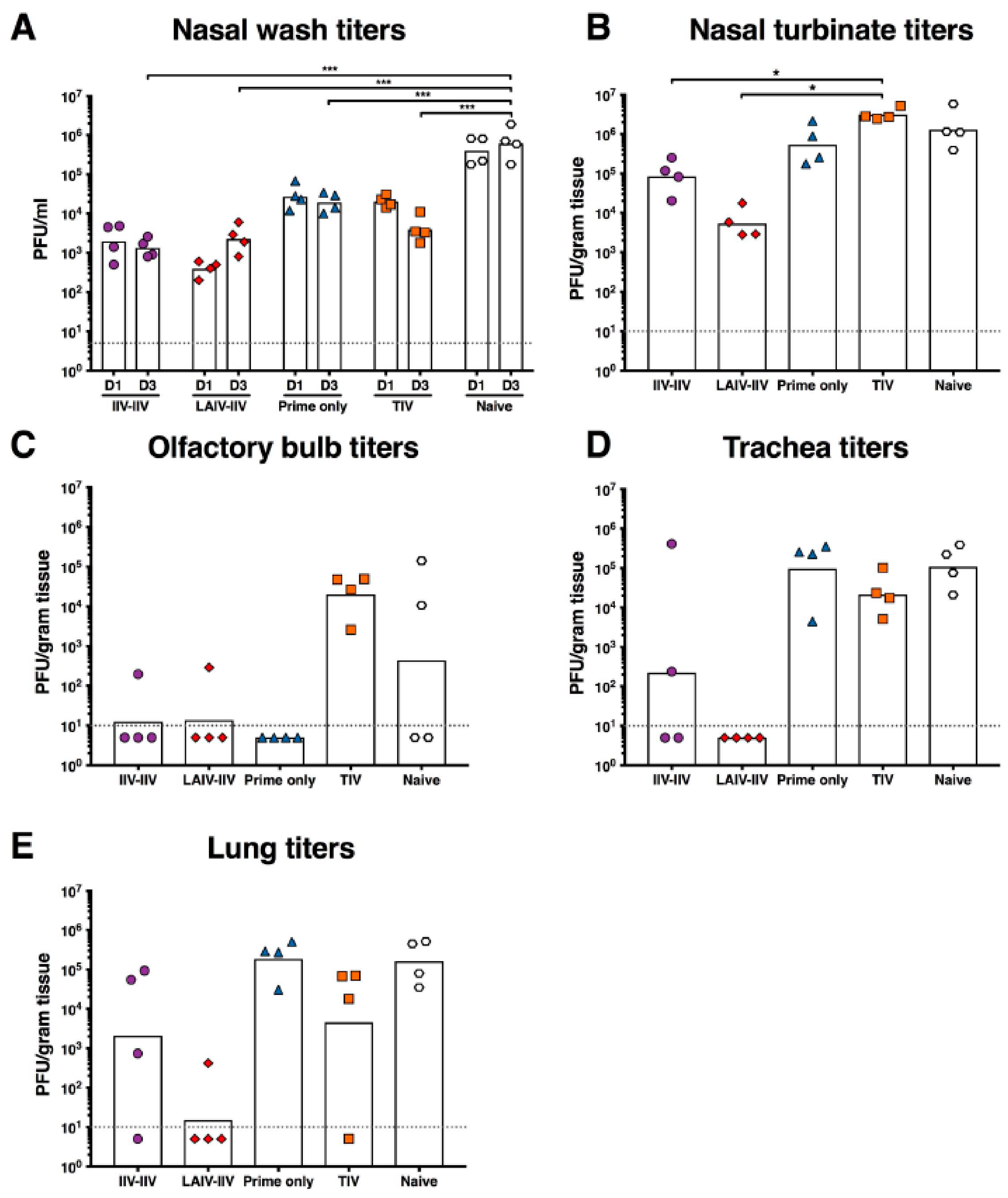
2.4. LAIV-IIV Vaccination Was Most Effective in Reducing Viral Loads after the Pandemic H1N1 Virus Challenge
3. Discussion
4. Materials and Methods
4.1. Viruses and Vaccines
4.2. Animals
4.3. LAIV Phenotypic Analysis and In Vivo Pathogenicity Testing
4.4. Ferret Vaccination and Challenge
4.5. ELISAs
4.6. Plaque Assays
4.7. Ethics Statement
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Influenza (Seasonal) Fact Sheet 211. Available online: http://www.who.int/mediacentre/factsheets/fs211/en/ (accessed on 1 April 2018).
- Gerdil, C. The annual production cycle for influenza vaccine. Vaccine 2003, 21, 1776–1779. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Krammer, F. Universal influenza virus vaccines and therapeutic antibodies. Clin. Microbiol. Infect. 2017, 23, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Hai, R.; Krammer, F.; Tan, G.S.; Pica, N.; Eggink, D.; Maamary, J.; Margine, I.; Albrecht, R.A.; Palese, P. Influenza viruses expressing chimeric hemagglutinins: Globular head and stalk domains derived from different subtypes. J. Virol. 2012, 86, 5774–5781. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Palm, A.E.; Krammer, F.; Wilson, P.C. From Original Antigenic Sin to the Universal Influenza Virus Vaccine. Trends Immunol. 2018, 39, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Kinzler, D.; Choi, A.; Hirsh, A.; Beaulieu, E.; Lecrenier, N.; Innis, B.L.; Palese, P.; Mallett, C.P.; Krammer, F. A chimeric haemagglutinin-based influenza split virion vaccine adjuvanted with AS03 induces protective stalk-reactive antibodies in mice. NPJ Vaccines 2016, 1, 16015. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Margine, I.; Hai, R.; Flood, A.; Hirsh, A.; Tsvetnitsky, V.; Chen, D.; Palese, P. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J. Virol. 2014, 88, 2340–2343. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Pica, N.; Hai, R.; Margine, I.; Palese, P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 2013, 87, 6542–6550. [Google Scholar] [CrossRef] [PubMed]
- Ryder, A.B.; Nachbagauer, R.; Buonocore, L.; Palese, P.; Krammer, F.; Rose, J.K. Vaccination with Vesicular Stomatitis Virus-Vectored Chimeric Hemagglutinins Protects Mice against Divergent Influenza Virus Challenge Strains. J. Virol. 2015, 90, 2544–2550. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Liu, W.C.; Choi, A.; Wohlbold, T.J.; Atlas, T.; Rajendran, M.; Solórzano, A.; Berlanda-Scorza, F.; García-Sastre, A.; Palese, P.; et al. A universal influenza virus vaccine candidate confers protection against pandemic H1N1 infection in preclinical ferret studies. NPJ Vaccines 2017, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Isakova-Sivak, I.; Rudenko, L. Safety, immunogenicity and infectivity of new live attenuated influenza vaccines. Expert Rev. Vaccines 2015, 14, 1313–1329. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Holmes, S.J.; Mendelman, P.M.; Huggins, L.; Cho, I.; Rhorer, J. Safety of a trivalent live attenuated intranasal influenza vaccine, FluMist, administered in addition to parenteral trivalent inactivated influenza vaccine to seniors with chronic medical conditions. Vaccine 1999, 17, 1905–1909. [Google Scholar] [CrossRef]
- Klein, S.L.; Marriott, I.; Fish, E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Pekosz, A. Sex-based biology and the rational design of influenza vaccination strategies. J. Infect. Dis. 2014, 209 (Suppl. 3), S114–S119. [Google Scholar] [CrossRef]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Cotter, C.R.; Jin, H.; Chen, Z. A single amino acid in the stalk region of the H1N1pdm influenza virus HA protein affects viral fusion, stability and infectivity. PLoS Pathog. 2014, 10, e1003831. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Miller, M.S.; Hai, R.; Ryder, A.B.; Rose, J.K.; Palese, P.; García-Sastre, A.; Krammer, F.; Albrecht, R.A. Hemagglutinin Stalk Immunity Reduces Influenza Virus Replication and Transmission in Ferrets. J. Virol. 2015, 90, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Tan, G.S.; Mullarkey, C.E.; Lee, A.J.; Lam, M.M.W.; Krammer, F.; Henry, C.; Wilson, P.C.; Ashkar, A.A.; Palese, P.; et al. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc. Natl. Acad. Sci. USA 2016, 113, 11931–11936. [Google Scholar] [CrossRef] [PubMed]
- Leon, P.E.; He, W.; Mullarkey, C.E.; Bailey, M.J.; Miller, M.S.; Krammer, F.; Palese, P.; Tan, G.S. Optimal activation of Fc-mediated effector functions by influenza virus hemagglutinin antibodies requires two points of contact. Proc. Natl. Acad. Sci. USA 2016, 113, E5944–E5951. [Google Scholar] [CrossRef] [PubMed]
- Mullarkey, C.E.; Bailey, M.J.; Golubeva, D.A.; Tan, G.S.; Nachbagauer, R.; He, W.; Novakowski, K.E.; Bowdish, D.M.; Miller, M.S.; Palese, P. Broadly Neutralizing Hemagglutinin Stalk-Specific Antibodies Induce Potent Phagocytosis of Immune Complexes by Neutrophils in an Fc-Dependent Manner. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Polezhaev, F.I.; Garmashova, L.M.; Koval, T.A.; Taranova, G.P.; Topuriya, N.V.; Aleksandrova, G.I. Attenuated ts-recombinants of influenza A/USSR/77 (H1N1) virus obtained by crossing with the cold-adapted donor A/Leningrad/134/57 (H2N2) virus. Acta Virol. 1982, 26, 221–226. [Google Scholar] [PubMed]
- Ambrose, C.S.; Yi, T.; Falloon, J. An integrated, multistudy analysis of the safety of Ann Arbor strain live attenuated influenza vaccine in children aged 2–17 years. Influenza Other Respir. Viruses 2011, 5, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Maassab, H.F.; Cox, N.J.; Murphy, B.R.; Kendal, A.P. Biological, genetic and biochemical characterization of a cold-adapted recombinant A/Victoria/3/75 virus and its evaluation in volunteers. Dev. Biol. Stand. 1977, 39, 25–31. [Google Scholar] [PubMed]
- Paules, C.I.; Marston, H.D.; Eisinger, R.W.; Baltimore, D.; Fauci, A.S. The Pathway to a Universal Influenza Vaccine. Immunity 2017, 47, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sastre, A.; Palese, P. Genetic manipulation of negative-strand RNA virus genomes. Annu. Rev. Microbiol. 1993, 47, 765–790. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M.; Sun, W.; Comella, P.; Nachbagauer, R.; Wohlbold, T.J.; Amanat, F.; Kirkpatrick, E.; Palese, P.; Krammer, F. An immuno-assay to quantify influenza virus hemagglutinin with correctly folded stalk domains in vaccine preparations. PLoS ONE 2018, 13, e0194830. [Google Scholar] [CrossRef] [PubMed]
- Pica, N.; Hai, R.; Krammer, F.; Wang, T.T.; Maamary, J.; Eggink, D.; Tan, G.S.; Krause, J.C.; Moran, T.; Stein, C.R.; et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc. Natl. Acad. Sci. USA 2012, 109, 2573–2578. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Margine, I.; Tan, G.S.; Pica, N.; Krause, J.C.; Palese, P. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS ONE 2012, 7, e43603. [Google Scholar] [CrossRef] [PubMed]
- Margine, I.; Palese, P.; Krammer, F. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J. Vis. Exp. 2013, e51112. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nachbagauer, R.; Krammer, F.; Albrecht, R.A. A Live-Attenuated Prime, Inactivated Boost Vaccination Strategy with Chimeric Hemagglutinin-Based Universal Influenza Virus Vaccines Provides Protection in Ferrets: A Confirmatory Study. Vaccines 2018, 6, 47. https://doi.org/10.3390/vaccines6030047
Nachbagauer R, Krammer F, Albrecht RA. A Live-Attenuated Prime, Inactivated Boost Vaccination Strategy with Chimeric Hemagglutinin-Based Universal Influenza Virus Vaccines Provides Protection in Ferrets: A Confirmatory Study. Vaccines. 2018; 6(3):47. https://doi.org/10.3390/vaccines6030047
Chicago/Turabian StyleNachbagauer, Raffael, Florian Krammer, and Randy A. Albrecht. 2018. "A Live-Attenuated Prime, Inactivated Boost Vaccination Strategy with Chimeric Hemagglutinin-Based Universal Influenza Virus Vaccines Provides Protection in Ferrets: A Confirmatory Study" Vaccines 6, no. 3: 47. https://doi.org/10.3390/vaccines6030047
APA StyleNachbagauer, R., Krammer, F., & Albrecht, R. A. (2018). A Live-Attenuated Prime, Inactivated Boost Vaccination Strategy with Chimeric Hemagglutinin-Based Universal Influenza Virus Vaccines Provides Protection in Ferrets: A Confirmatory Study. Vaccines, 6(3), 47. https://doi.org/10.3390/vaccines6030047





