Postgenomic Approaches and Bioinformatics Tools to Advance the Development of Vaccines against Bacteria of the Burkholderia cepacia Complex
Abstract
1. Introduction
2. Which Is the Best Type of Vaccine for Bcc?
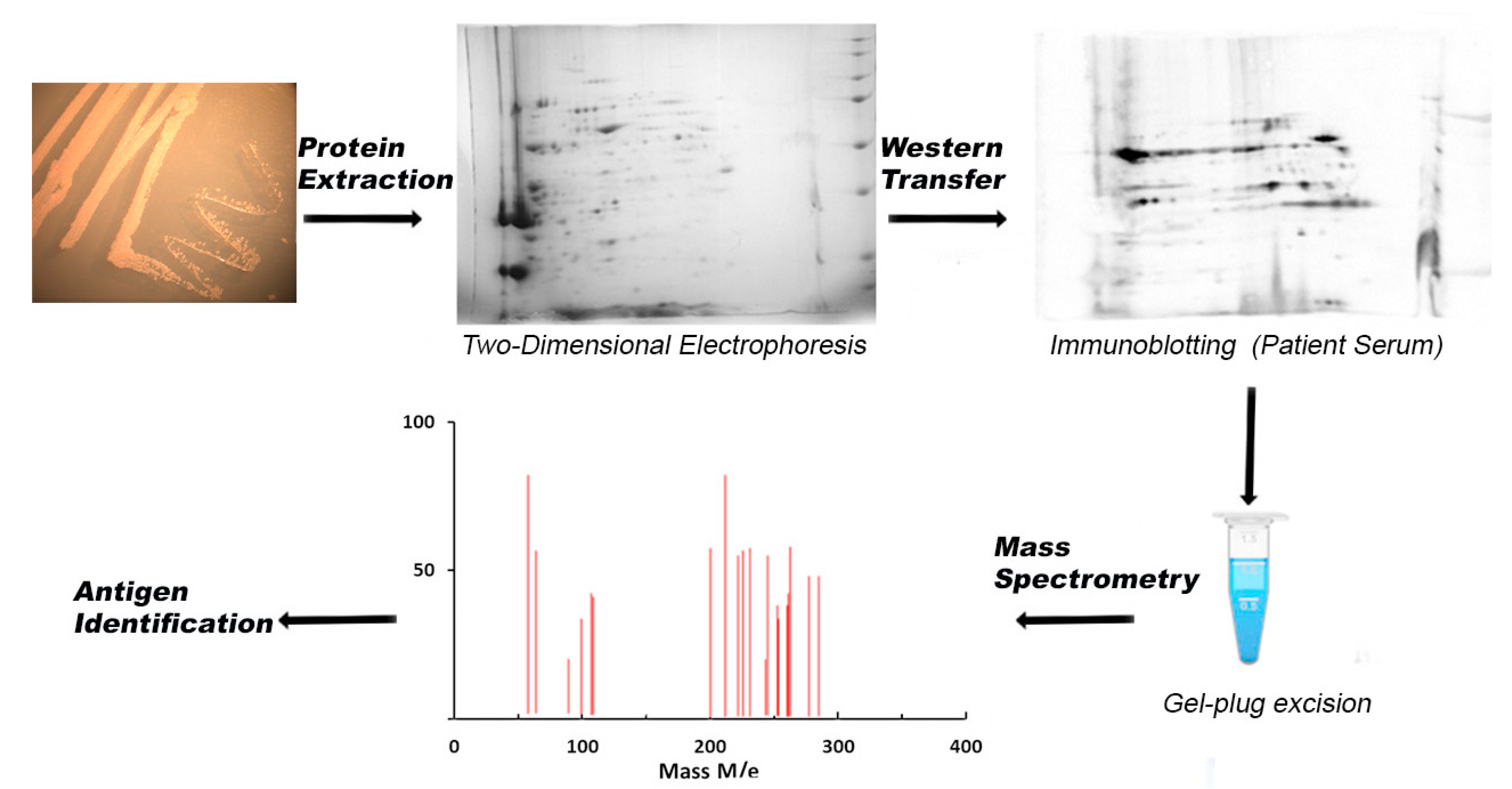
3. Experimental Techniques to Identify Immunogenic Proteins
4. Bioinformatics Tools to Predict Immunogenic Epitopes
5. Final Remarks and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burkholder, W.H. Sour skin, a bacterial rot of Onion bulbs. Phytopathology 1950, 40, 115–117. [Google Scholar]
- Govan, J.R.; Deretic, V. Microbial pathogenesis in cystic fibrosis: Mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 1996, 60, 539–574. [Google Scholar] [PubMed]
- Martina, P.; Leguizamon, M.; Prieto, C.I.; Sousa, S.A.; Montanaro, P.; Draghi, W.O.; Stämmler, M.; Bettiol, M.; de Carvalho, C.C.C.R.; Palau, J.; et al. Burkholderia puraquae sp. nov., a novel species of the Burkholderia cepacia complex isolated from hospital settings and agricultural soils. Int. J. Syst. Evol. Microbiol. 2018, 68, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.F.; King, G.M. Volcanic soils as sources of novel CO-oxidizing Paraburkholderia and Burkholderia: Paraburkholderia hiiakae sp. nov., Paraburkholderia metrosideri sp. nov., Paraburkholderia paradisi sp. nov., Paraburkholderia peleae sp. nov., and Burkholderia alpina sp. Front. Microbiol. 2017, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Bach, E.; Sant’Anna, F.H.; Magrich dos Passos, J.F.; Balsanelli, E.; de Baura, V.A.; Pedrosa, F.O.; de Souza, E.M.; Passaglia, L.M.P. Detection of misidentifications of species from the Burkholderia cepacia complex and description of a new member, the soil bacterium Burkholderia catarinensis sp. nov. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.S.; Aw, Y.K.; Lee, L.H.; Yule, C.M.; Cheow, Y.L.; Lee, S.M. Burkholderia paludis sp. nov., an Antibiotic-Siderophore Producing Novel Burkholderia cepacia Complex Species, Isolated from Malaysian Tropical Peat Swamp Soil. Front. Microbiol. 2016, 7, 2046. [Google Scholar] [CrossRef] [PubMed]
- Loutet, S.A.; Valvano, M.A. A decade of Burkholderia cenocepacia virulence determinant research. Infect. Immun. 2010, 78, 4088–4100. [Google Scholar] [CrossRef] [PubMed]
- Scoffone, V.C.; Chiarelli, L.R.; Trespidi, G.; Mentasti, M.; Riccardi, G.; Buroni, S. Burkholderia cenocepacia infections in cystic fibrosis patients: Drug resistance and therapeutic approaches. Front. Microbiol. 2017, 8, 1592. [Google Scholar] [CrossRef] [PubMed]
- Salsgiver, E.L.; Fink, A.K.; Knapp, E.A.; LiPuma, J.J.; Olivier, K.N.; Marshall, B.C.; Saiman, L. Changing Epidemiology of the Respiratory Bacteriology of Patients With Cystic Fibrosis. Chest 2016, 149, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Cystic Fibrosis Foundation Patient Registry 2016. Available online: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2016-Patient-Registry-Annual-Data-Report.pdf (accessed on 10 May 2018).
- ECFS Patient Registry Annual Data Report 2015. Available online: https://www.ecfs.eu/sites/default/files/general-content-images/working-groups/ecfs-patient-registry/ECFSPR_Report2015_Nov2017.pdf (accessed on 10 May 2018).
- Mangram, A.; Jarvis, W.R. Nosocomial Burkholderia cepacia Outbreaks and Pseudo-Outbreaks. Infect. Control Hosp. Epidemiol. 1996, 17, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Isles, A.; Maclusky, I.; Corey, M.; Gold, R.; Prober, C.; Fleming, P.; Levison, H. Pseudomonas cepacia infection in cystic fibrosis: An emerging problem. J. Pediatr. 1984, 104, 206–210. [Google Scholar] [CrossRef]
- Lipuma, J.J. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010, 23, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Saiman, L.; Siegel, J.D.; LiPuma, J.J.; Brown, R.F.; Bryson, E.A.; Chambers, M.J.; Downer, V.S.; Fliege, J.; Hazle, L.A.; Jain, M.; et al. Infection Prevention and Control Guideline for Cystic Fibrosis: 2013 Update. Infect. Control Hosp. Epidemiol. 2014, 35, S1–S67. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.A.; Ramos, C.G.; Leitão, J.H. Burkholderia cepacia complex: Emerging multihost pathogens equipped with a wide range of virulence factors and determinants. Int. J. Microbiol. 2011, 2011, 607575. [Google Scholar] [CrossRef] [PubMed]
- Festini, F.; Buzzetti, R.; Bassi, C.; Braggion, C.; Salvatore, D.; Taccetti, G.; Mastella, G. Isolation measures for prevention of infection with respiratory pathogens in cystic fibrosis: A systematic review. J. Hosp. Infect. 2006, 64, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ada, G. Overview of Vaccines and Vaccination. Mol. Biotechnol. 2005, 29, 255–272. [Google Scholar] [CrossRef]
- Ada, G. Vaccines and Vaccination. N. Engl. J. Med. 2001, 345, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Choh, L.-C.; Ong, G.-H.; Vellasamy, K.M.; Kalaiselvam, K.; Kang, W.-T.; Al-Maleki, A.R.; Mariappan, V.; Vadivelu, J.; Estes, M.; Picking, W.; et al. Burkholderia vaccines: Are we moving forward? Front Cell Infect Microbiol. 2013, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Pradenas, G.; Myers, J.; Torres, A. Characterization of the Burkholderia cenocepacia TonB Mutant as a Potential Live Attenuated Vaccine. Vaccines 2017, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Shinoy, M.; Dennehy, R.; Coleman, L.; Carberry, S.; Schaffer, K.; Callaghan, M.; Doyle, S.; McClean, S. Immunoproteomic analysis of proteins expressed by two related pathogens, Burkholderia multivorans and Burkholderia cenocepacia, during human infection. PLoS ONE 2013, 8, e80796. [Google Scholar] [CrossRef] [PubMed]
- McClean, S.; Healy, M.E.; Collins, C.; Carberry, S.; O’Shaughnessy, L.; Dennehy, R.; Adams, Á.; Kennelly, H.; Corbett, J.M.; Carty, F.; et al. Linocin and OmpW Are Involved in Attachment of the Cystic Fibrosis-Associated Pathogen Burkholderia cepacia Complex to Lung Epithelial Cells and Protect Mice against Infection. Infect. Immun. 2016, 84, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.A.; Morad, M.; Feliciano, J.R.; Pita, T.; Nady, S.; El-hennamy, R.E.; Abdel-rahman, M.; Cavaco, J.; Pereira, L.; Barreto, C.; et al. The Burkholderia cenocepacia OmpA-like protein BCAL2958: Identification, characterization, and detection of anti-BCAL2958 antibodies in serum from B. cepacia complex-infected Cystic Fibrosis patients. AMB Express 2016, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Makidon, P.E.; Knowlton, J.; Groom, J.V.; Blanco, L.P.; LiPuma, J.J.; Bielinska, A.U.; Baker, J.R. Induction of immune response to the 17 kDa OMPA Burkholderia cenocepacia polypeptide and protection against pulmonary infection in mice after nasal vaccination with an OMP nanoemulsion-based vaccine. Med. Microbiol. Immunol. 2010, 199, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, V.; Vellasamy, K.M.; Thimma, J.S.; Hashim, O.H.; Vadivelu, J. Identification of immunogenic proteins from Burkholderia cepacia secretome using proteomic analysis. Vaccine 2010, 28, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Bertot, G.M.; Restelli, M.A.; Galanternik, L.; Aranibar Urey, R.C.; Valvano, M.A.; Grinstein, S. Nasal Immunization with Burkholderia multivorans Outer Membrane Proteins and the Mucosal Adjuvant Adamantylamide Dipeptide Confers Efficient Protection against Experimental Lung Infections with B. multivorans and B. cenocepacia. Infect. Immun. 2007, 75, 2740–2752. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.V.; Sousa, S.A.; Leitão, J.H.; Moreira, L.M.; Videira, P.A.; Sá-Correia, I. Studies on the involvement of the exopolysaccharide produced by cystic fibrosis-associated isolates of the Burkholderia cepacia complex in biofilm formation and in persistence of respiratory infections. J. Clin. Microbiol. 2004, 42, 3052–3058. [Google Scholar] [CrossRef] [PubMed]
- Richau, J.A.; Leitão, J.H.; Correia, M.; Lito, L.; Salgado, M.J.; Barreto, C.; Cescutti, P.; Sá-Correia, I. Molecular typing and exopolysaccharide biosynthesis of Burkholderia cepacia isolates from a Portuguese cystic fibrosis center. J. Clin. Microbiol. 2000, 38, 1651–1655. [Google Scholar] [PubMed]
- Bylund, J.; Burgess, L.-A.; Cescutti, P.; Ernst, R.K.; Speert, D.P. Exopolysaccharides from Burkholderia cenocepacia inhibit neutrophil chemotaxis and scavenge reactive oxygen species. J. Biol. Chem. 2006, 281, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Cuzzi, B.; Herasimenka, Y.; Silipo, A.; Lanzetta, R.; Liut, G.; Rizzo, R.; Cescutti, P. Versatility of the Burkholderia cepacia complex for the biosynthesis of exopolysaccharides: A comparative structural investigation. PLoS ONE 2014, 9, e94372. [Google Scholar] [CrossRef] [PubMed]
- Pradenas, G.; Ross, B.; Torres, A. Burkholderia cepacia Complex Vaccines: Where Do We Go from here? Vaccines 2016, 4, E10. [Google Scholar] [CrossRef] [PubMed]
- Grandi, G. Bacterial surface proteins and vaccines. F1000 Biol. Rep. 2010, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Martin, A. Bacterial virulence in the moonlight: Multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect. Immun. 2011, 79, 3476–3491. [Google Scholar] [CrossRef] [PubMed]
- Ling, E.; Feldman, G.; Portnoi, M.; Dagan, R.; Overweg, K.; Mulholland, F.; Chalifa-Caspi, V.; Wells, J.; Mizrachi-Nebenzahl, Y. Glycolytic enzymes associated with the cell surface of Streptococcus pneumoniae are antigenic in humans and elicit protective immune responses in the mouse. Clin. Exp. Immunol. 2004, 138, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Olaya-Abril, A.; Jiménez-Munguía, I.; Gómez-Gascón, L.; Rodríguez-Ortega, M.J. Surfomics: Shaving live organisms for a fast proteomic identification of surface proteins. J. Proteom. 2014, 97, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, G.; Tang, F.; Shao, J.; Lu, Y.; Bao, Y.; Yao, H.; Lu, C. Pre-Absorbed Immunoproteomics: A Novel Method for the Detection of Streptococcus suis Surface Proteins. PLoS ONE 2011, 6, e21234. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.V.; Sarkar-Tyson, M.; Smither, S.J.; Atkins, T.P.; Oyston, P.C.F.; Brown, K.A.; Liu, Y.; Wait, R.; Titball, R.W. The identification of surface proteins of Burkholderia pseudomallei. Vaccine 2007, 25, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Sabarth, N.; Lamer, S.; Zimny-Arndt, U.; Jungblut, P.R.; Meyer, T.F.; Bumann, D. Identification of Surface Proteins of Helicobacter pylori by Selective Biotinylation, Affinity Purification, and Two-dimensional Gel Electrophoresis. J. Biol. Chem. 2002, 277, 27896–27902. [Google Scholar] [CrossRef] [PubMed]
- Fulton, K.M.; Twine, S.M. Immunoproteomics: Current Technology and Applications. In Immunoproteomics. Methods in Molecular Biology (Methods and Protocols), vol 1061; Humana Press: Totowa, NJ, USA, 2013; pp. 21–57. [Google Scholar]
- Suwannasaen, D.; Mahawantung, J.; Chaowagul, W.; Limmathurotsakul, D.; Felgner, P.L.; Davies, H.; Bancroft, G.J.; Titball, R.W.; Lertmemongkolchai, G. Human Immune Responses to Burkholderia pseudomallei Characterized by Protein Microarray Analysis. J. Infect. Dis. 2011, 203, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.R.; Higgins, R.; Lariviere, S. Evaluation of slide agglutination and ring precipitation tests for capsular serotyping of Haemophilus pleuropneumoniae. J. Clin. Microbiol. 1982, 15, 1019–1023. [Google Scholar] [PubMed]
- Elia, G. Biotinylation reagents for the study of cell surface proteins. Proteomics 2008, 8, 4012–4024. [Google Scholar] [CrossRef] [PubMed]
- Gatlin, C.L.; Pieper, R.; Huang, S.-T.; Mongodin, E.; Gebregeorgis, E.; Parmar, P.P.; Clark, D.J.; Alami, H.; Papazisi, L.; Fleischmann, R.D.; et al. Proteomic profiling of cell envelope-associated proteins from Staphylococcus aureus. Proteomics 2006, 6, 1530–1549. [Google Scholar] [CrossRef] [PubMed]
- Hardouin, J.; Lasserre, J.-P.; Sylvius, L.; Joubert-Caron, R.; Caron, M. Cancer Immunomics: From Serological Proteome Analysis to Multiple Affinity Protein Profiling. Ann. N. Y. Acad. Sci. 2007, 1107, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Bottomley, M.J.; D’Oro, U.; Finco, O.; De Gregorio, E. Reverse vaccinology 2.0: Human immunology instructs vaccine antigen design. J. Exp. Med. 2016, 213, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Dormitzer, P.R.; Ulmer, J.B.; Rappuoli, R. Structure-based antigen design: A strategy for next generation vaccines. Trends Biotechnol. 2008, 26, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Ferlenghi, I.; Cozzi, R.; Scarselli, M. Structural Vaccinology: A Three-dimensional View for Vaccine Development. Curr. Top. Med. Chem. 2013, 13, 2629–2637. [Google Scholar] [CrossRef]
- Sousa, S.A.; Feliciano, J.R.; Pita, T.; Guerreiro, S.I.; Leitão, J.H. Burkholderia cepacia Complex Regulation of Virulence Gene Expression: A Review. Genes 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Sajjan, U.S. Host Evasion by Burkholderia cenocepacia. Front. Cell. Infect. Microbiol. 2012, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Leitão, J.H.; Sousa, S.A.; Ferreira, A.S.; Ramos, C.G.; Silva, I.N.; Moreira, L.M. Pathogenicity, virulence factors, and strategies to fight against Burkholderia cepacia complex pathogens and related species. Appl. Microbiol. Biotechnol. 2010, 87, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Tiringer, K.; Treis, A.; Fucik, P.; Gona, M.; Gruber, S.; Renner, S.; Dehlink, E.; Nachbaur, E.; Horak, F.; Jaksch, P.; et al. A Th17- and Th2-skewed Cytokine Profile in Cystic Fibrosis Lungs Represents a Potential Risk Factor for Pseudomonas aeruginosa Infection. Am. J. Respir. Crit. Care Med. 2013, 187, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Masson, A.; Launay, O.; Delaisi, B.; Bassinet, L.; Remus, N.; Lebourgeois, M.; Chedevergne, F.; Bailly, C.; Foucaud, P.; Corvol, H.; et al. Vaccine coverage in CF children: A French multicenter study. J. Cyst. Fibros. 2015, 14, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Malfroot, A.; Adam, G.; Ciofu, O.; Döring, G.; Knoop, C.; Lang, A.B.; Van Damme, P.; Dab, I.; Bush, A. Immunisation in the current management of cystic fibrosis patients. J. Cyst. Fibros. 2005, 4, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.J.; O’Connor, K.; Wijeyewickrema, L. Phage display for epitope determination: A paradigm for identifying receptor–ligand interactions. Biotechnol. Annu. Rev. 2004, 10, 151–188. [Google Scholar] [PubMed]
- Soria-Guerra, R.E.; Nieto-Gomez, R.; Govea-Alonso, D.O.; Rosales-Mendoza, S. An overview of bioinformatics tools for epitope prediction: Implications on vaccine development. J. Biomed. Inform. 2015, 53, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Escalona, E.; Sáez, D.; Oñate, A. Immunogenicity of a Multi-Epitope DNA Vaccine Encoding Epitopes from Cu-Zn Superoxide Dismutase and Open Reading Frames of Brucella abortus in Mice. Front. Immunol. 2017, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Wieser, A.; Magistro, G.; Nörenberg, D.; Hoffmann, C.; Schubert, S. First multi-epitope subunit vaccine against extraintestinal pathogenic Escherichia coli delivered by a bacterial type-3 secretion system (T3SS). Int. J. Med. Microbiol. 2012, 302, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Peri, C.; Gori, A.; Gagni, P.; Sola, L.; Girelli, D.; Sottotetti, S.; Cariani, L.; Chiari, M.; Cretich, M.; Colombo, G. Evolving serodiagnostics by rationally designed peptide arrays: The Burkholderia paradigm in Cystic Fibrosis. Sci. Rep. 2016, 6, 32873. [Google Scholar] [CrossRef] [PubMed]
- Gaudesi, D.; Peri, C.; Quilici, G.; Gori, A.; Ferrer-Navarro, M.; Conchillo-Solé, O.; Thomas, R.; Nithichanon, A.; Lertmemongkolchai, G.; Titball, R.; et al. Structure-Based Design of a B Cell Antigen from B. pseudomallei. ACS Chem. Biol. 2015, 10, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Nithichanon, A.; Rinchai, D.; Buddhisa, S.; Saenmuang, P.; Kewcharoenwong, C.; Kessler, B.; Khaenam, P.; Chetchotisakd, P.; Maillere, B.; Robinson, J.; et al. Immune Control of Burkholderia pseudomallei––Common, High-Frequency T-Cell Responses to a Broad Repertoire of Immunoprevalent Epitopes. Front. Immunol. 2018, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Zacharias, M. Prediction of protein-protein interaction sites using electrostatic desolvation profiles. Biophys. J. 2010, 98, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Potocnakova, L.; Bhide, M.; Pulzova, L.B. An Introduction to B-Cell Epitope Mapping and In Silico Epitope Prediction. J. Immunol. Res. 2016, 2016, 6760830. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, N.D.; Mayrose, I.; Halperin, D.; Yekutieli, D.; Gershoni, J.M.; Pupko, T. Computational characterization of B-cell epitopes. Mol. Immunol. 2008, 45, 3477–3489. [Google Scholar] [CrossRef] [PubMed]
- Capelli, R.; Matterazzo, E.; Amabili, M.; Peri, C.; Gori, A.; Gagni, P.; Chiari, M.; Lertmemongkolchai, G.; Cretich, M.; Bolognesi, M.; et al. Designing Probes for Immunodiagnostics: Structural Insights into an Epitope Targeting Burkholderia Infections. ACS Infect. Dis. 2017, 3, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Mayrose, I.; Penn, O.; Erez, E.; Rubinstein, N.D.; Shlomi, T.; Freund, N.T.; Bublil, E.M.; Ruppin, E.; Sharan, R.; Gershoni, J.M.; et al. Pepitope: Epitope mapping from affinity-selected peptides. Bioinformatics 2007, 23, 3244–3246. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, N.D.; Mayrose, I.; Martz, E.; Pupko, T. Epitopia: A web-server for predicting B-cell epitopes. BMC Bioinform. 2009, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, J.; Bui, H.-H.; Li, W.; Fusseder, N.; Bourne, P.E.; Sette, A.; Peters, B. ElliPro: A new structure-based tool for the prediction of antibody epitopes. BMC Bioinform. 2008, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 2017, 45, W24–W29. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Raghava, G.P.S. BcePred: Prediction of Continuous B-Cell Epitopes in Antigenic Sequences Using Physico-chemical Properties; Springer: Berlin/Heidelberg, Germany, 2004; pp. 197–204. [Google Scholar]
- Saha, S.; Raghava, G.P.S. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins Struct. Funct. Bioinform. 2006, 65, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Faraggi, E.; Zhou, Y.; Ruan, J.; Kurgan, L. BEST: Improved Prediction of B-Cell Epitopes from Antigen Sequences. PLoS ONE 2012, 7, e40104. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Zhang, L.; Liang, S.; Zhang, C. SVMTriP: A Method to Predict Antigenic Epitopes Using Support Vector Machine to Integrate Tri-Peptide Similarity and Propensity. PLoS ONE 2012, 7, e45152. [Google Scholar] [CrossRef] [PubMed]
- Davydov, Y.I.; Tonevitsky, A.G. Prediction of linear B-cell epitopes. Mol. Biol. 2009, 43, 150–158. [Google Scholar] [CrossRef]
- Sweredoski, M.J.; Baldi, P. COBEpro: A novel system for predicting continuous B-cell epitopes. Protein Eng. Des. Sel. 2009, 22, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H.; Yang, J.; Chou, K.-C. Prediction of linear B-cell epitopes using amino acid pair antigenicity scale. Amino Acids 2007, 33, 423–428. [Google Scholar] [CrossRef] [PubMed]
- El-Manzalawy, Y.; Dobbs, D.; Honavar, V. Predicting flexible length linear B-cell epitopes. Comput. Syst. Bioinform. Conf. 2008, 7, 121–132. [Google Scholar]
- EL-Manzalawy, Y.; Dobbs, D.; Honavar, V. Predicting linear B-cell epitopes using string kernels. J. Mol. Recognit. 2008, 21, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Ansari, H.R.; Raghava, G.P.S. Improved Method for Linear B-Cell Epitope Prediction Using Antigen’s Primary Sequence. PLoS ONE 2013, 8, e62216. [Google Scholar] [CrossRef] [PubMed]
- Ansari, H.R.; Raghava, G.P. Identification of conformational B-cell Epitopes in an antigen from its primary sequence. Immunome Res. 2010, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Sela-Culang, I.; Benhnia, M.R.-E.-I.; Matho, M.H.; Kaever, T.; Maybeno, M.; Schlossman, A.; Nimrod, G.; Li, S.; Xiang, Y.; Zajonc, D.; et al. Using a Combined Computational-Experimental Approach to Predict Antibody-Specific B Cell Epitopes. Structure 2014, 22, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Sweredoski, M.J.; Baldi, P. PEPITO: Improved discontinuous B-cell epitope prediction using multiple distance thresholds and half sphere exposure. Bioinformatics 2008, 24, 1459–1460. [Google Scholar] [CrossRef] [PubMed]
- Kringelum, J.V.; Lundegaard, C.; Lund, O.; Nielsen, M. Reliable B Cell Epitope Predictions: Impacts of Method Development and Improved Benchmarking. PLoS Comput. Biol. 2012, 8, e1002829. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zheng, D.; Zhang, C.; Zacharias, M. Prediction of antigenic epitopes on protein surfaces by consensus scoring. BMC Bioinform. 2009, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, K.; Liu, X.; Baker, T.; Shi, J.; Deane, C.M. Improving B-cell epitope prediction and its application to global antibody-antigen docking. Bioinformatics 2014, 30, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zheng, D.; Standley, D.M.; Yao, B.; Zacharias, M.; Zhang, C. EPSVR and EPMeta: Prediction of antigenic epitopes using support vector regression and multiple server results. BMC Bioinform. 2010, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.E. Recent advances in antigen processing and presentation. Nat. Immunol. 2007, 8, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Rammensee, H.-G.; Bachmann, J.; Emmerich, N.P.N.; Bachor, O.A.; Stevanović, S. SYFPEITHI: Database for MHC ligands and peptide motifs. Immunogenetics 1999, 50, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Doytchinova, I.A.; Zygouri, C.; Flower, D.R. MHCPred: A server for quantitative prediction of peptide-MHC binding. Nucleic Acids Res. 2003, 31, 3621–3624. [Google Scholar] [CrossRef] [PubMed]
- Reche, P.A.; Reinherz, E.L. Prediction of Peptide-MHC Binding Using Profiles. Methods Mol. Biol. 2007, 409, 185–200. [Google Scholar] [PubMed]
- Dönnes, P.; Kohlbacher, O. SVMHC: A server for prediction of MHC-binding peptides. Nucleic Acids Res. 2006, 34, W194–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wan, J.; Meng, X.; Flower, D.R.; Li, T. In Silico Prediction of Peptide-MHC Binding Affinity Using SVRMHC. Methods Mol. Biol. 2007, 409, 283–291. [Google Scholar] [PubMed]
- Vita, R.; Overton, J.A.; Greenbaum, J.A.; Ponomarenko, J.; Clark, J.D.; Cantrell, J.R.; Wheeler, D.K.; Gabbard, J.L.; Hix, D.; Sette, A.; et al. The immune epitope database (IEDB) 3.0. Nucleic Acids Res. 2015, 43, D405–D412. [Google Scholar] [CrossRef] [PubMed]
- Doytchinova, I.A.; Guan, P.; Flower, D.R. EpiJen: A server for multistep T cell epitope prediction. BMC Bioinform. 2006, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, M.; Raghava, G.P.S. A hybrid approach for predicting promiscuous MHC class I restricted T cell epitopes. J. Biosci. 2007, 32, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Raghava, G.P.S. ProPred1: Prediction of promiscuous MHC Class-I binding sites. Bioinformatics 2003, 19, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Andreatta, M.; Nielsen, M. Gapped sequence alignment using artificial neural networks: Application to the MHC class I system. Bioinformatics 2016, 32, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Schueler-Furman, O.; Altuvia, Y.; Sette, A.; Margalit, H. Structure-based prediction of binding peptides to MHC class I molecules: Application to a broad range of MHC alleles. Protein Sci. 2000, 9, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.V.; Lundegaard, C.; Lamberth, K.; Buus, S.; Lund, O.; Nielsen, M. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinform. 2007, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Raghava, G.P. ProPred: Prediction of HLA-DR binding sites. Bioinformatics 2001, 17, 1236–1237. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Bhasin, M.; Raghava, G.P.S. Application of Machine Learning Techniques in Predicting MHC Binders. Methods Mol. Biol. 2007, 409, 201–215. [Google Scholar] [PubMed]
| Tools | Link | Description | Reference |
|---|---|---|---|
| Pepitope | http://pepitope.tau.ac.il/ | Prediction of linear and discontinuous B-cell epitopes using Pepsurf or Mapitope algorithm | [66] |
| Epitopia | http://epitopia.tau.ac.il/ | Prediction of linear and discontinuous B-cell epitopes | [67] |
| Ellipro | http://tools.immuneepitope.org/ellipro/ | Prediction of linear and discontinuous B-cell epitopes based on the protein antigen’s 3D structure | [68] |
| BepiPred 2.0 | http://www.cbs.dtu.dk/services/BepiPred/ | Prediction of linear B-cell epitopes | [69] |
| Bcepred | http://crdd.osdd.net/raghava/bcepred/ | Prediction of linear B-cell epitopes using physicochemical properties | [70] |
| ABCPred | http://crdd.osdd.net/raghava/abcpred/ | Prediction of linear B-cell epitopes using recurrent neural network | [71] |
| BEST | http://biomine.cs.vcu.edu/datasets/BEST/ | Prediction of linear B-cell epitopes using support vector machine (SVM) tool | [72] |
| SVMTriP | http://sysbio.unl.edu/SVMTriP/prediction.php | Prediction of linear B-cell epitopes using SVM and combining tripeptide similarity and propensity scores | [73] |
| AAPPred | https://bioinf.ru/aappred/predict | Prediction of linear B-cell epitopes using amino acid pair antigenicity scale | [74] |
| COBEpro | http://scratch.proteomics.ics.uci.edu/ | Prediction of linear B-cell epitopes | [75] |
| BCPREDS | http://ailab.ist.psu.edu/bcpred/predict.html | Prediction of linear B-cell epitopes using AAP, BCPred, or FBCPred method | [76,77,78] |
| LBtope | http://crdd.osdd.net/raghava/lbtope/protein.php | Prediction of linear B-cell epitopes | [79] |
| CBTOPE | http://crdd.osdd.net/raghava/cbtope/submit.php | Prediction of discontinuous B-cell epitopes | [80] |
| PEASE | http://www.ofranlab.org/PEASE | Prediction of discontinuous B-cell epitopes | [81] |
| BEpro | http://pepito.proteomics.ics.uci.edu/ | Prediction of discontinuous B-cell epitopes | [82] |
| DiscoTope 2.0 | http://www.cbs.dtu.dk/services/DiscoTope/ | Prediction of discontinuous B-cell epitopes | [83] |
| EPCES | http://sysbio.unl.edu/EPCES/ | Prediction of discontinuous B-cell epitopes | [84] |
| EpiPred | http://opig.stats.ox.ac.uk/webapps/sabdab-sabpred/EpiPred.php | Prediction of discontinuous B-cell epitopes | [85] |
| EPSVR | http://sysbio.unl.edu/EPSVR/ | Prediction of discontinuous B-cell epitopes | [86] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, S.A.; Seixas, A.M.M.; Leitão, J.H. Postgenomic Approaches and Bioinformatics Tools to Advance the Development of Vaccines against Bacteria of the Burkholderia cepacia Complex. Vaccines 2018, 6, 34. https://doi.org/10.3390/vaccines6020034
Sousa SA, Seixas AMM, Leitão JH. Postgenomic Approaches and Bioinformatics Tools to Advance the Development of Vaccines against Bacteria of the Burkholderia cepacia Complex. Vaccines. 2018; 6(2):34. https://doi.org/10.3390/vaccines6020034
Chicago/Turabian StyleSousa, Sílvia A., António M. M. Seixas, and Jorge H. Leitão. 2018. "Postgenomic Approaches and Bioinformatics Tools to Advance the Development of Vaccines against Bacteria of the Burkholderia cepacia Complex" Vaccines 6, no. 2: 34. https://doi.org/10.3390/vaccines6020034
APA StyleSousa, S. A., Seixas, A. M. M., & Leitão, J. H. (2018). Postgenomic Approaches and Bioinformatics Tools to Advance the Development of Vaccines against Bacteria of the Burkholderia cepacia Complex. Vaccines, 6(2), 34. https://doi.org/10.3390/vaccines6020034







