pH-Responsive Micelle-Based Cytoplasmic Delivery System for Induction of Cellular Immunity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Micelles or Liposomes
2.3. Atomic Force Microscopy (AFM)
2.4. Cell Culture
2.5. Interaction of Micelles with Liposomes
2.6. Lipid Mixing Assay
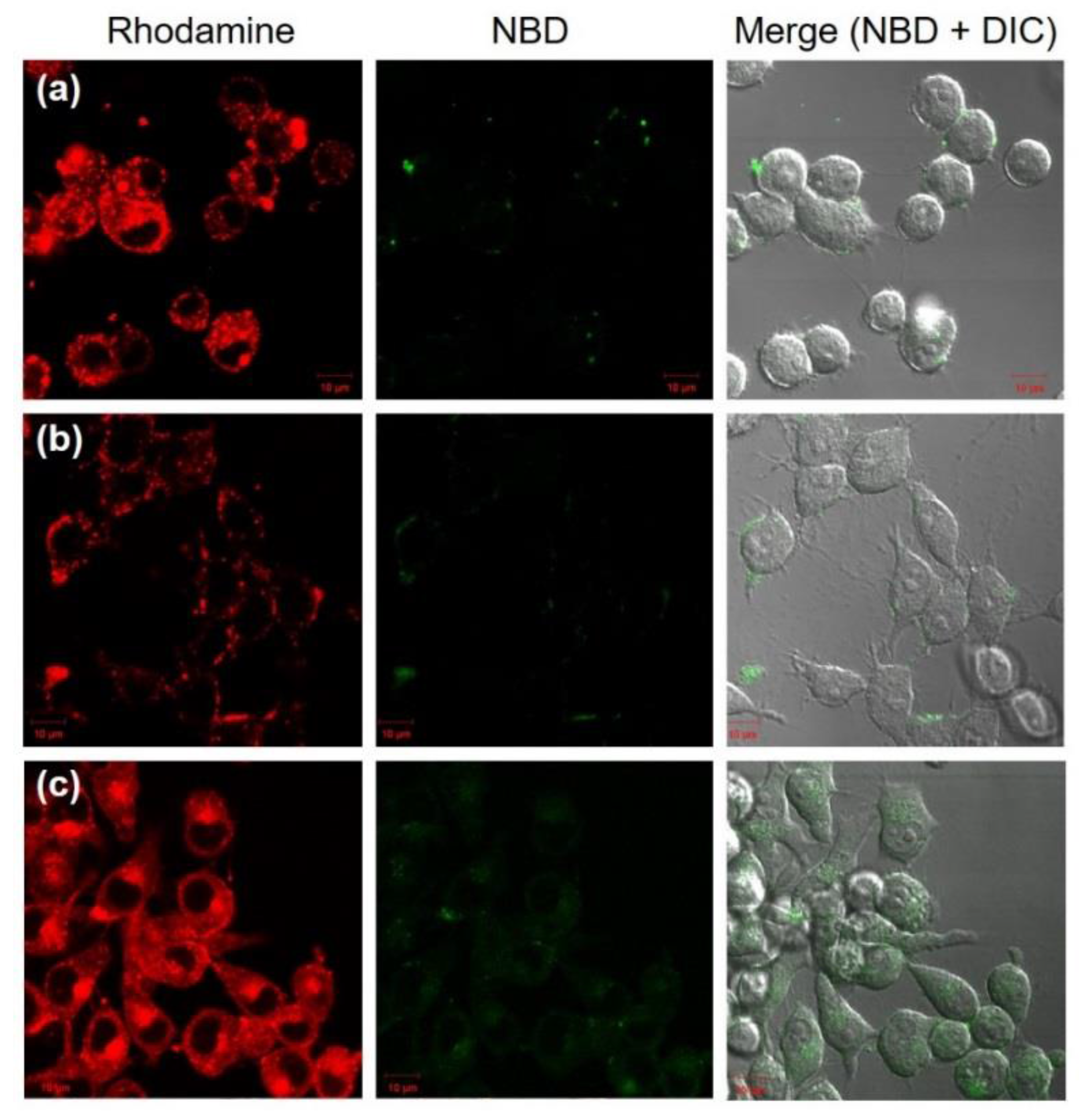
2.7. Cellular Association of Micelles
2.8. Detection of Intracellular Lipid Mixing
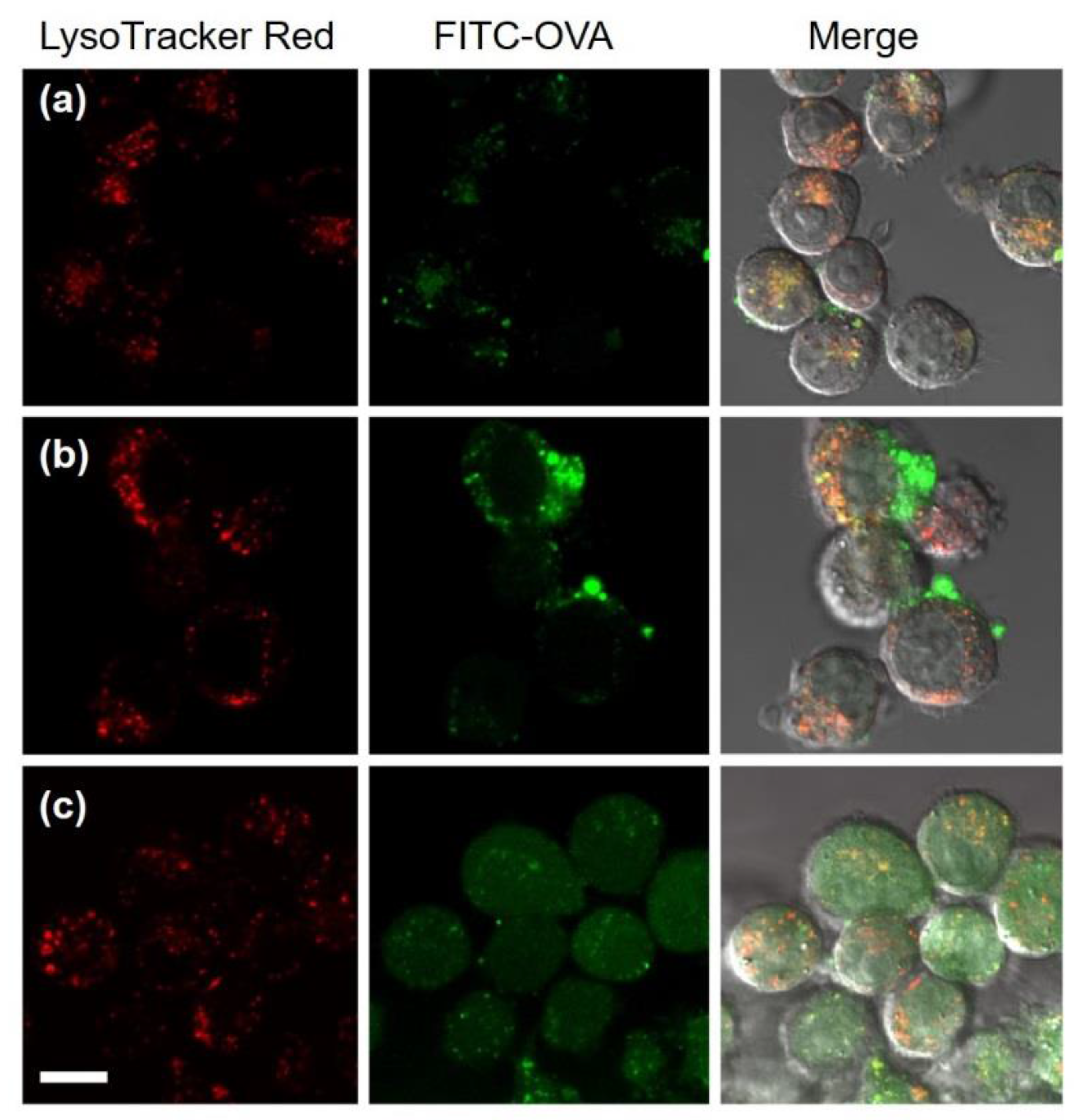
2.9. Cytoplasmic Delivery of Antigenic Proteins
2.10. Animals
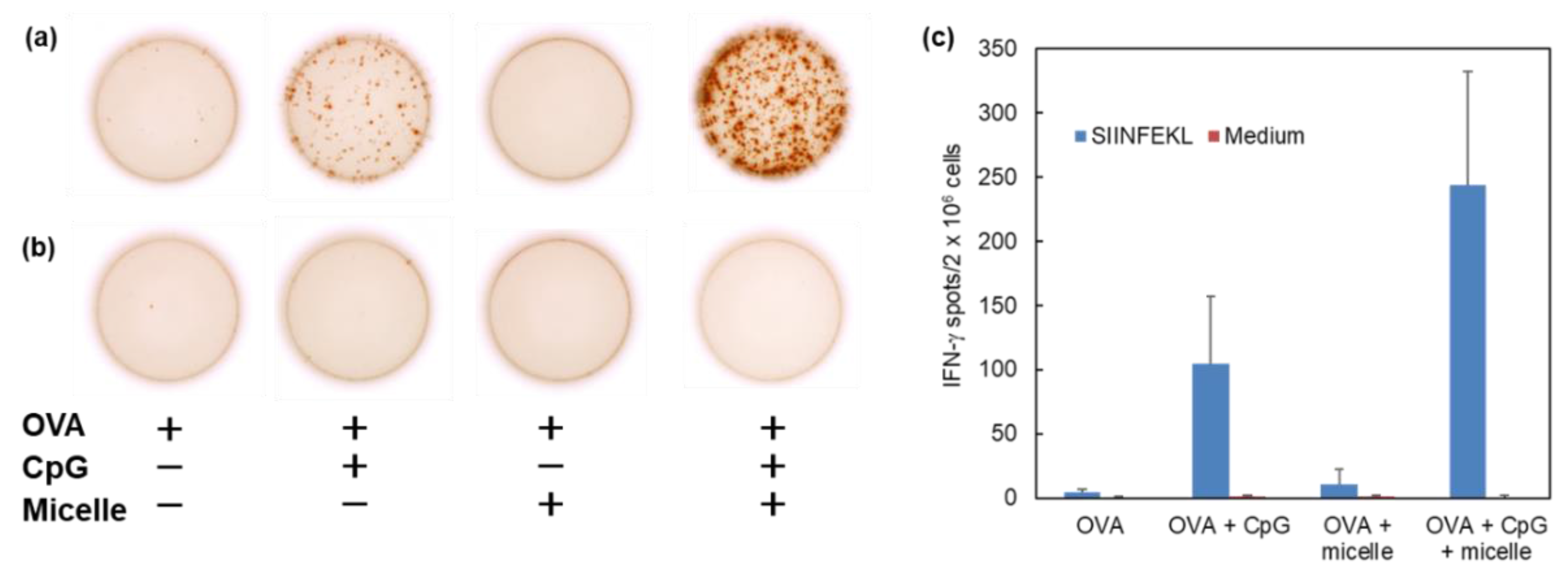
2.11. ELIspot Assay
2.12. Statistical Analysis
3. Results
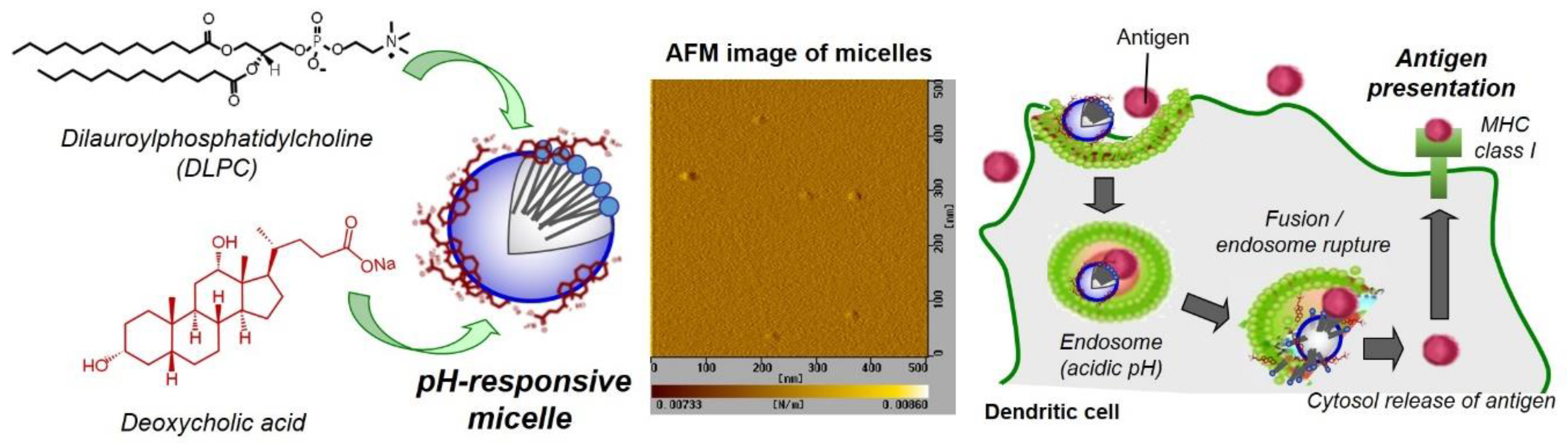
3.1. Characterization of DLPC/Deoxycholic Acid Micelles
3.2. Interaction of DLPC/Deoxycholic Acid Micelles with Dendritic Cells
3.3. Intracellular Behavior of DLPC/Deoxycholic Acid Micelles
3.4. Induction of Cellular Immunity In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.J.; Lalvani, A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Sandgren, K.J.; Bertram, K.; Cunningham, A.L. Understanding natural herpes simplex virus immunity to inform next-generation vaccine design. Clin. Transl. Immunol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Holz, L.E.; Fernandez-Ruiz, D.; Heath, W.R. Protective immunity to liver-stage malaria. Clin. Transl. Immunol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gattinoni, L.; Liu, F.; Ji, Y.; Yu, Z.; Restifo, N.P.; Rosenberg, S.A.; Morgan, R.A. In vitro generated anti-tumor T lymphocytes exhibit distinct subsets mimicking in vivo antigen-experienced cells. Cancer Immunol. Immunother. 2011, 60, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Steinman, R.M. Dendritic cells: Specialized and regulated antigen processing machines. Cell 2001, 106, 255–258. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Garulli, B.; Di Mario, G.; Sciaraffia, E.; Accapezzato, D.; Barnaba, V.; Castrucci, M.R. Enhancement of T cell-mediated immune responses to whole inactivated influenza virus by chloroquine treatment in vivo. Vaccine 2013, 31, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Z.; Huang, H.; Yang, Y.; Ding, Q.; Mai, J.; Guo, W.; Xu, Y. Improved antigen cross-presentation by polyethyleneimine-based nanoparticles. Int. J. Nanomed. 2011, 6, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bungener, L.; Serre, K.; Bijl, L.; Leserman, L.; Wilschut, J.; Daemen, T.; Machy, P. Virosome-mediated delivery of protein antigens to dendritic cells. Vaccine 2002, 20, 2287–2295. [Google Scholar] [CrossRef]
- Kunisawa, J.; Nakagawa, S.; Mayumi, T. Pharmacotherapy by intracellular delivery of drugs using fusogenic liposomes: Application to vaccine development. Adv. Drug Deliv. Rev. 2001, 52, 177–186. [Google Scholar] [CrossRef]
- Seki, K.; Tirrell, D.A. pH-Dependent complexation of poly(acrylic acid) derivatives with phospholipid vesicle membrane. Macromolecules 1984, 17, 1692–1698. [Google Scholar] [CrossRef]
- Murthy, N.; Robichaud, J.R.; Tirrell, D.A.; Stayton, P.S.; Hoffman, A.S. The design and synthesis of polymers for eukaryotic membrane disruption. J. Control. Release 1999, 61, 137–143. [Google Scholar] [CrossRef]
- Lackey, C.A.; Murthy, N.; Press, O.W.; Tirrell, D.A.; Hoffman, A.S.; Stayton, P.S. Hemolytic activity of pH-responsive polymer-streptavidin bioconjugates. Bioconj. Chem. 1999, 10, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Flanary, S.; Hoffman, A.S.; Stayton, P.S. Antigen delivery with poly(propylacrylic acid) conjugation enhances MHC-1 presentation and T-cell activation. Bioconj. Chem. 2009, 20, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Duval, C.L.; Crownover, E.F.; Hoffman, A.S.; Stayton, P.S. Intracellular delivery of a protein antigen with an endosomal-releasing polymer enhances CD8 T-cell production and prophylactic vaccine efficacy. Bioconj. Chem. 2010, 21, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Teranishi, R.; Yuba, E.; Kono, K. Effect of the side chain spacer structure on the pH-responsive properties of polycarboxylates. J. Biomater. Sci. Polym. Ed. 2017, 28, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Igawa, T.; Takagishi, T. Cytoplasmic delivery of calcein mediated by liposomes modified with a pH-sensitive poly(ethylene glycol) derivative. Biochim. Biophys. Acta 1997, 1325, 143–154. [Google Scholar] [CrossRef]
- Sakaguchi, N.; Kojima, C.; Harada, A.; Kono, K. Preparation of pH-sensitive poly(glycidol) derivatives with varying hydrophobicities: Their ability to sensitize stable liposomes to pH. Bioconj. Chem. 2008, 19, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Tajima, N.; Yoshizaki, Y.; Harada, A.; Hayashi, H.; Kono, K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials 2014, 35, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Yamaguchi, A.; Yoshizaki, Y.; Harada, A.; Kono, K. Bioactive polysaccharide-based pH-sensitive polymers for cytoplasmic delivery of antigen and activation of antigen-specific immunity. Biomaterials 2017, 120, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Uesugi, S.; Miyazaki, M.; Kado, Y.; Harada, A.; Kono, K. Development of pH-sensitive dextran derivatives with strong adjuvant function and their application to antigen delivery. Membranes 2017, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Kono, K. Carboxylated hyperbranched poly(glycidol)s for preparation of pH-sensitive liposomes. J. Control. Release 2011, 149, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Watarai, S.; Kono, K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials 2013, 34, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Fujii, S.; Mochizuki, S.; Sakurai, K.; Sakaguchi, N.; Koiwai, K. A two-component micelle with emergent pH responsiveness by mixing dilauroyl phosphocholine and deoxycholic acid and its delivery of proteins into the cytosol. Colloids Surf. B Biointerfaces 2017, 154, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Hafez, I.M.; Cullis, P.R. Cholesteryl hemisuccinate exhibits pH sensitive polymorphic phase behavior. Biochim. Biophys. Acta 2000, 1463, 107–114. [Google Scholar] [CrossRef]
- Liu, D.; Huang, L. Role of cholesterol in the stability of pH-sensitive, large unilamellar liposomes prepared by the detergent-dialysis method. Biochim. Biophys. Acta 1989, 981, 254–260. [Google Scholar] [CrossRef]
- Shen, Z.; Reznikoff, G.; Dranoff, G.; Rock, K.L. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 1997, 158, 2723–2730. [Google Scholar] [PubMed]
- Yamada, M.; Oeda, A.; Jung, J.; Iijima, M.; Yoshimoto, N.; Niimi, T.; Jeong, S.Y.; Choi, E.K.; Tanizawa, K.; Kuroda, S. Hepatitis B virus envelope L protein-derived bio-nanocapsules: Mechanisms of cellular attachment and entry into human hepatic cells. J. Control. Release 2012, 160, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Kogure, K.; Futaki, S.; Harashima, H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J. Biol. Chem. 2006, 281, 3544–3551. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Bachelder, E.M.; Beaudette, T.T.; Broaders, K.E.; Fréchet, J.M.J.; Albrecht, M.T.; Mateczun, A.J.; Ainslie, K.M.; Pesce, J.T.; Keane-Myers, A.M. In vitro analysis of acetalated dextran microparticles as a potent delivery platform for vaccine adjuvants. Mol. Pharm. 2010, 7, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Akiyoshi, K. Current advances in self-assembled nanogel delivery systems for immunotherapy. Adv. Drug Deliv. Rev. 2015, 95, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Sapay, N.; Bennett, W.F.D.; Tieleman, D.P. Thermodynamics of flip-flop and desorption for a systematic series of phosphatidylcholine lipids. Soft Matter 2009, 5, 3295–3302. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Q.; Liang, L.; Li, J.; Wang, K.; Li, J.; Lv, M.; Chen, N.; Song, H.; Lee, J.; et al. Real-time visualization of clustering and intracellular transport of gold nanoparticles by correlative imaging. Nat. Commun. 2017, 8, 15646. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, Y.K.; Mamrosh, J.L.; Busby, S.A.; Griffin, P.R.; Pathak, M.C.; Ortlund, E.A.; Moore, D.D. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature 2011, 474, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Ganley, D.J.; Wilt, J. Methods for the Synthesis and Purification of Deoxycholic Acid. Patent WO 2012174229 A2, 20 December 2012. [Google Scholar]
- Saari, M.; Vidgren, M.T.; Koskinen, M.O.; Turjanmaa, V.M.; Nieminen, M.M. Pulmonary distribution and clearance of two beclomethasone liposome formulations in healthy volunteers. Int. J. Plast. 1999, 181, 1–9. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuba, E.; Sakaguchi, N.; Kanda, Y.; Miyazaki, M.; Koiwai, K. pH-Responsive Micelle-Based Cytoplasmic Delivery System for Induction of Cellular Immunity. Vaccines 2017, 5, 41. https://doi.org/10.3390/vaccines5040041
Yuba E, Sakaguchi N, Kanda Y, Miyazaki M, Koiwai K. pH-Responsive Micelle-Based Cytoplasmic Delivery System for Induction of Cellular Immunity. Vaccines. 2017; 5(4):41. https://doi.org/10.3390/vaccines5040041
Chicago/Turabian StyleYuba, Eiji, Naoki Sakaguchi, Yuhei Kanda, Maiko Miyazaki, and Kazunori Koiwai. 2017. "pH-Responsive Micelle-Based Cytoplasmic Delivery System for Induction of Cellular Immunity" Vaccines 5, no. 4: 41. https://doi.org/10.3390/vaccines5040041
APA StyleYuba, E., Sakaguchi, N., Kanda, Y., Miyazaki, M., & Koiwai, K. (2017). pH-Responsive Micelle-Based Cytoplasmic Delivery System for Induction of Cellular Immunity. Vaccines, 5(4), 41. https://doi.org/10.3390/vaccines5040041





