Maternal Immunization: New Perspectives on Its Application Against Non-Infectious Related Diseases in Newborns
Abstract
1. Introduction
2. Maternal Immunization
2.1. The Original Concept
2.2. Milestones, Claims and New Insights
2.3. Maternal Immunization can Confer Active Immunity to Offspring via Breastfeeding
3. Maternal Immunization can Prevent Non-Infectious Diseases in Offspring
4. Maternal Immunization Against Tumor Associated Antigens: The Potential of DNA Vaccination
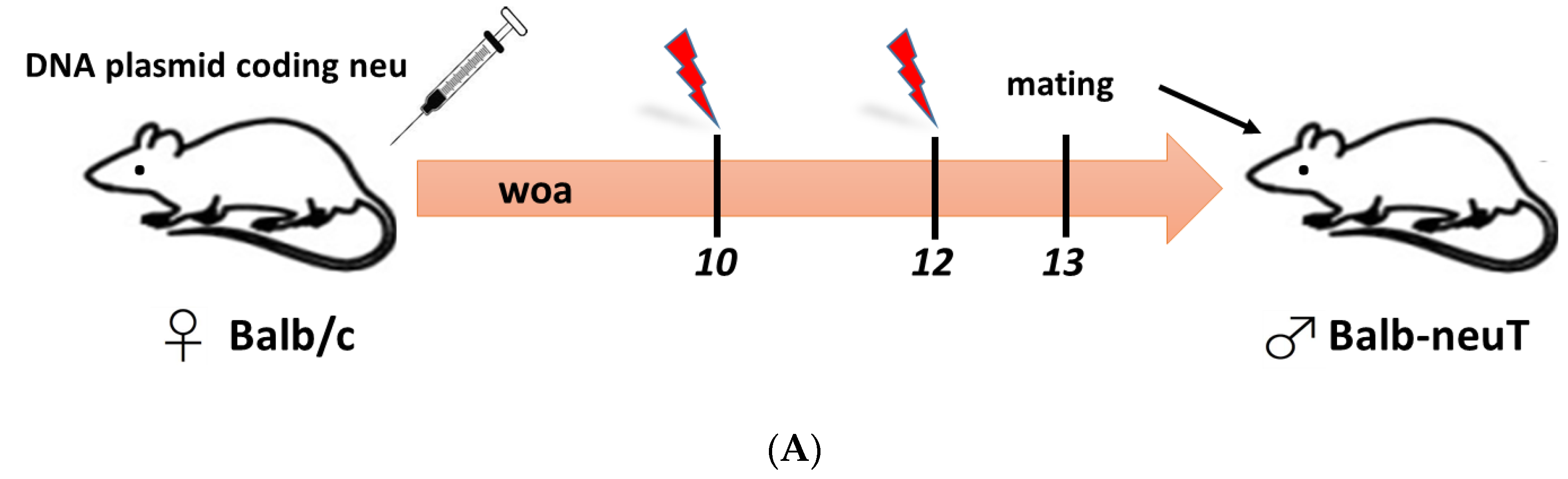
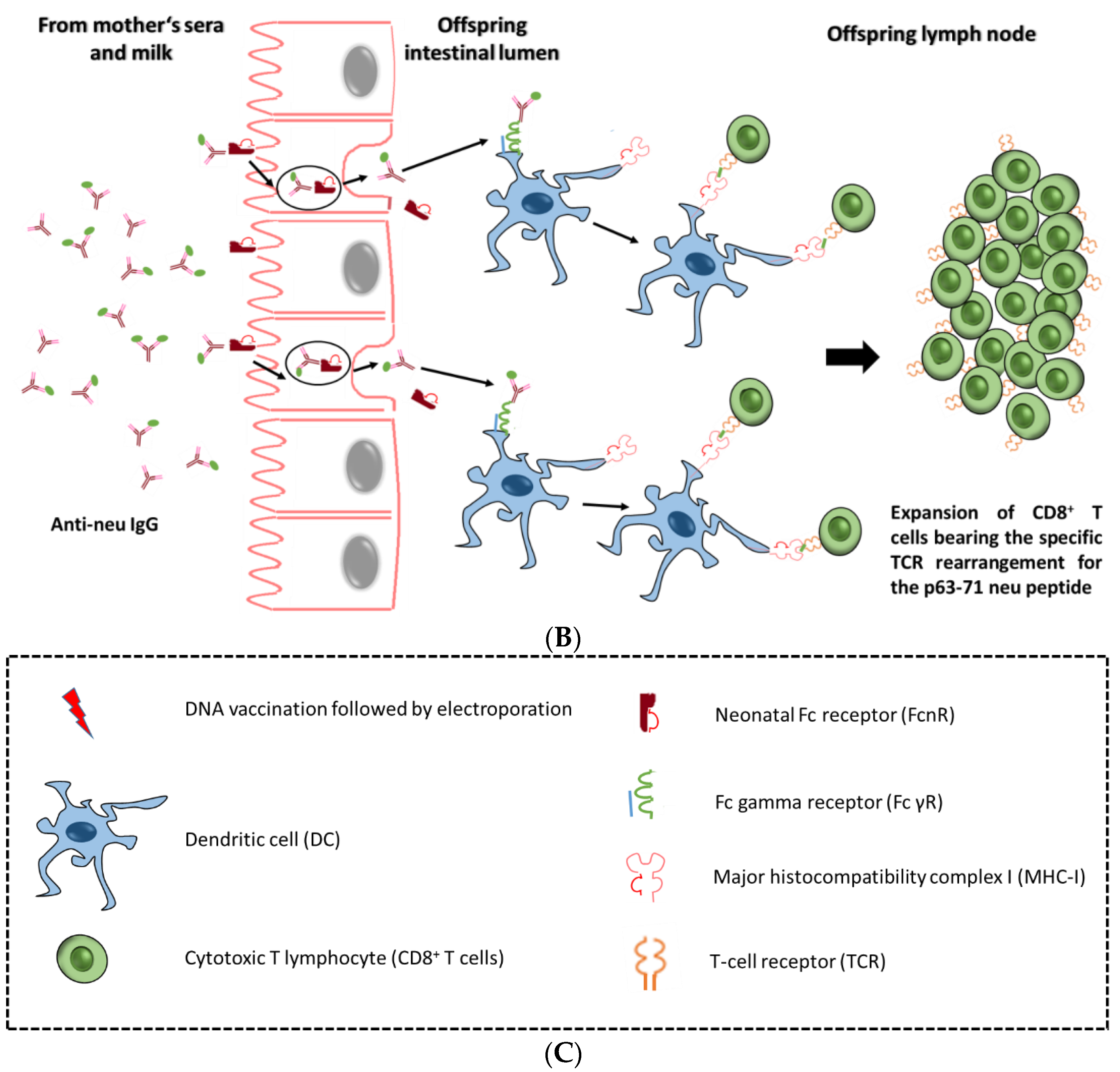
4.1. Maternal Immunization can Confer Anti-Tumor Immunity Against Her2-neu
5. The Rationale for the Application of Maternal Immunization Against Childhood Cancer
Towards the Application of Maternal Immunization against Neuroblastoma
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thwaites, C.L.; Loan, H.T. Eradication of tetanus. Br. Med. Bull. 2015, 116, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, M.C.; Whitney, C.G.; Messonnier, N.E.; Zell, E.R.; Lynfield, R.; Hadler, J.L.; Harrison, L.H.; Farley, M.M.; Reingold, A.; Bennett, N.M.; et al. Bacterial meningitis in the United States, 1998–2007. N. Engl. J. Med. 2011, 364, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Saliou, P. Eradication of infectious diseases by vaccination. Med. Trop. (Mars) 2007, 67, 321–327. [Google Scholar] [PubMed]
- Forni, G.; Lollini, P.L.; Musiani, P.; Colombo, M.P. Immunoprevention of cancer: Is the time ripe? Cancer Res. 2000, 60, 2571–2575. [Google Scholar] [PubMed]
- Lollini, P.L.; Cavallo, F.; Nanni, P.; Forni, G. Vaccines for tumour prevention. Nat. Rev. Cancer 2006, 6, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, F.; De Giovanni, C.; Nanni, P.; Forni, G.; Lollini, P.L. 2011: The immune hallmarks of cancer. Cancer Immunol. Immunother. Cii 2011, 60, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Bot, A.; Marincola, F.; Smith, K.A. Repositioning therapeutic cancer vaccines in the dawning era of potent immune interventions. Expert Rev. Vaccines 2013, 12, 1219–1234. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Chen, T.; Fan, C.; Zhan, Q.; Wang, Y.; Lu, J.; Lu, L.L.; Ni, Z.; Huang, F.; Yao, H.; et al. Efficacy of neonatal HBV vaccination on liver cancer and other liver diseases over 30-year follow-up of the Qidong hepatitis B intervention study: A cluster randomized controlled trial. PLoS Med. 2014, 11, e1001774. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S. Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr. Opin. Immunol. 2004, 16, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.A.; Jaffee, E.M.; Lutz, E.R. Cancer immunoprevention--the next frontier. Cancer Prev. Res. (Phila) 2014, 7, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J.; Beatty, P.L. Cancer immunoprevention. Curr. Opin. Immunol. 2016, 39, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Lohmueller, J.J.; Sato, S.; Popova, L.; Chu, I.M.; Tucker, M.A.; Barberena, R.; Innocenti, G.M.; Cudic, M.; Ham, J.D.; Cheung, W.C.; et al. Antibodies elicited by the first non-viral prophylactic cancer vaccine show tumor-specificity and immunotherapeutic potential. Sci. Rep. 2016, 6, 31740. [Google Scholar] [CrossRef] [PubMed]
- Lollini, P.L.; De Giovanni, C.; Pannellini, T.; Cavallo, F.; Forni, G.; Nanni, P. Cancer immunoprevention. Future Oncol. 2005, 1, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lollini, P.L.; Cavallo, F.; Nanni, P.; Quaglino, E. The Promise of Preventive Cancer Vaccines. Vaccines (Basel) 2015, 3, 467–489. [Google Scholar] [CrossRef] [PubMed]
- PrabhuDas, M.; Bonney, E.; Caron, K.; Dey, S.; Erlebacher, A.; Fazleabas, A.; Fisher, S.; Golos, T.; Matzuk, M.; McCune, J.M.; et al. Immune mechanisms at the maternal-fetal interface: Perspectives and challenges. Nat. Immunol. 2015, 16, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Vernochet, C.; Caucheteux, S.M.; Kanellopoulos-Langevin, C. Bi-directional cell trafficking between mother and fetus in mouse placenta. Placenta 2007, 28, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Sunami, R.; Komuro, M.; Yuminamochi, T.; Hoshi, K.; Hirata, S. Fetal cell microchimerism develops through the migration of fetus-derived cells to the maternal organs early after implantation. J. Reprod. Immunol. 2010, 84, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Dawe, G.S.; Tan, X.W.; Xiao, Z.C. Cell migration from baby to mother. Cell Adh Migr 2007, 1, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Darmstadt, G.L.; Zaidi, A.K.M.; Stoll, B.J. Neonatal Infections: A Global Perspective. In Infectious Diseases of the Fetus and Newborn Infant; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 24–51. [Google Scholar]
- Holt, P.G.; Jones, C.A. The development of the immune system during pregnancy and early life. Allergy 2000, 55, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Mooi, F.R.; de Greeff, S.C. The case for maternal vaccination against pertussis. Lancet Infect. Dis. 2007, 7, 614–624. [Google Scholar] [CrossRef]
- Jones, C.; Heath, P. Antenatal immunization. Hum. Vaccin Immunother. 2014, 10, 2118–2122. [Google Scholar] [CrossRef] [PubMed]
- Roper, M.H.; Vandelaer, J.H.; Gasse, F.L. Maternal and neonatal tetanus. Lancet 2007, 370, 1947–1959. [Google Scholar] [CrossRef]
- Demicheli, V.; Barale, A.; Rivetti, A. Vaccines for women to prevent neonatal tetanus. Cochrane Database Sys. Rev. 2013, 31, CD002959. [Google Scholar] [CrossRef]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E.; Omer, S.B.; Shahid, N.S.; Breiman, R.F.; Steinhoff, M.C. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008, 359, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Nitsch-Osuch, A.; Korzeniewski, K.; Gawlak, M.; Zycinska, K.; Wardyn, K.; Kuchar, E. Epidemiological and clinical reasons for vaccination against pertussis and influenza in pregnant women. Adv. Exp. Med. Biol. 2015, 849, 11–21. [Google Scholar] [PubMed]
- Healy, C.M. Vaccines in pregnant women and research initiatives. Clin. Obstet. Gynecol. 2012, 55, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Nickell, S.; Powell, M.; Harriman, K. Effectiveness of Prenatal Versus Postpartum Tetanus, Diphtheria, and Acellular Pertussis Vaccination in Preventing Infant Pertussis. Clin. Infect. Dis. 2017, 64, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Cherry, J.D.; Harriman, K. Effectiveness of Prenatal Tetanus, Diphtheria, and Acellular Pertussis Vaccination on Pertussis Severity in Infants. Clin. Infect. Dis. 2017, 64, 9–14. [Google Scholar] [CrossRef] [PubMed]
- McMillan, M.; Clarke, M.; Parrella, A.; Fell, D.B.; Amirthalingam, G.; Marshall, H.S. Safety of Tetanus, Diphtheria, and Pertussis Vaccination During Pregnancy: A Systematic Review. Obstet. Gynecol. 2017, 129, 560–573. [Google Scholar] [CrossRef] [PubMed]
- De Martino, M. Dismantling the Taboo against Vaccines in Pregnancy. Int. J. Mol. Sci. 2016, 17, E894. [Google Scholar] [CrossRef] [PubMed]
- Regan, A.K. The safety of maternal immunization. Hum. Vaccin Immunother. 2016, 12, 3132–3136. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.M. Respiratory syncytial virus in infants: Is maternal vaccination a realistic strategy? Curr. Opin. Infect. Dis. 2015, 28, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Atwell, J.E.; Karron, R.A. Vaccination against respiratory syncytial virus in pregnancy. Lancet Infect. Dis. 2016, 16, 1330–1331. [Google Scholar] [CrossRef]
- Pass, R.F.; Zhang, C.; Evans, A.; Simpson, T.; Andrews, W.; Huang, M.L.; Corey, L.; Hill, J.; Davis, E.; Flanigan, C.; et al. Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 2009, 360, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Englund, J.A. Maternal immunization. Clin. Infect. Dis. 2014, 59, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Delany, I.; Rappuoli, R.; De Gregorio, E. Vaccines for the 21st century. EMBO Mol. Med. 2014, 6, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Cutland, C.L.; Jose, L.; Koen, A.; Govender, N.; Wittke, F.; Olugbosi, M.; Meulen, A.S.; Baker, S.; Dull, P.M.; et al. Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in healthy women and their infants: A randomised phase 1b/2 trial. Lancet Infect. Dis. 2016, 16, 923–934. [Google Scholar] [CrossRef]
- Donders, G.G.; Halperin, S.A.; Devlieger, R.; Baker, S.; Forte, P.; Wittke, F.; Slobod, K.S.; Dull, P.M. Maternal Immunization With an Investigational Trivalent Group B Streptococcal Vaccine: A Randomized Controlled Trial. Obstet. Gynecol. 2016, 127, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Dormitzer, P.R.; Nokes, D.J.; Rappuoli, R.; Roca, A.; Graham, B.S. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 2013, 31 (Suppl 2), B209–B215. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.F.; Suter, M.; Schraner, E.M.; Humbel, B.M.; Tobler, K.; Ackermann, M.; Laimbacher, A.S. Transfer of Anti-Rotavirus Antibodies during Pregnancy and in Milk Following Maternal Vaccination with a Herpes Simplex Virus Type-1 Amplicon Vector. Int. J. Mol. Sci. 2017, 18, E431. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Nelson, A.; Luquero, F.J.; Azman, A.S.; Debes, A.K.; M’Bang’ombe, M.M.; Seyama, L.; Kachale, E.; Zuze, K.; Malichi, D.; et al. Safety of a killed oral cholera vaccine (Shanchol) in pregnant women in Malawi: An observational cohort study. Lancet. Infect. Dis. 2017, 17, 538–544. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects—Reviewing the Evidence for Causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Zika rewrites maternal immunization ethics. Science 2017, 357, 241. [Google Scholar] [CrossRef] [PubMed]
- Saji, F.; Samejima, Y.; Kamiura, S.; Koyama, M. Dynamics of immunoglobulins at the feto-maternal interface. Rev. Reprod. 1999, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Faucette, A.N.; Unger, B.L.; Gonik, B.; Chen, K. Maternal vaccination: Moving the science forward. Hum. Reprod. Update 2015, 21, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Kliwinski, C.; Cooper, P.R.; Perkinson, R.; Mabus, J.R.; Tam, S.H.; Wilkinson, T.M.; Giles-Komar, J.; Scallon, B.; Powers, G.D.; Hornby, P.J. Contribution of FcRn binding to intestinal uptake of IgG in suckling rat pups and human FcRn-transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G262–G270. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Kim, J.; Ganesan, L.P.; Phillips, G.S.; Hua, K.; Jarjoura, D.; Hayton, W.L.; Robinson, J.M.; Anderson, C.L. IgG is transported across the mouse yolk sac independently of FcgammaRIIb. J. Reprod. Immunol. 2010, 84, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Paveglio, S.; Puddington, L.; Rafti, E.; Matson, A.P. FcRn-mediated intestinal absorption of IgG anti-IgE/IgE immune complexes in mice. Clin. Exp. Allergy 2012, 42, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Bundhoo, A.; Paveglio, S.; Rafti, E.; Dhongade, A.; Blumberg, R.S.; Matson, A.P. Evidence that FcRn mediates the transplacental passage of maternal IgE in the form of IgG anti-IgE/IgE immune complexes. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Bourges, D.; Meurens, F.; Berri, M.; Chevaleyre, C.; Zanello, G.; Levast, B.; Melo, S.; Gerdts, V.; Salmon, H. New insights into the dual recruitment of IgA+ B cells in the developing mammary gland. Mol. Immunol. 2008, 45, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Cheung, M.C.; Chintalacharuvu, K.R.; Rey, J.; Corthesy, B.; Neutra, M.R. Selective adherence of IgA to murine Peyer’s patch M cells: Evidence for a novel IgA receptor. J. Immunol. 2002, 169, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A.; Rescigno, M. The biology of intestinal immunoglobulin A responses. Immunity 2008, 28, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Matysiak-Budnik, T.; Moura, I.C.; Arcos-Fajardo, M.; Lebreton, C.; Menard, S.; Candalh, C.; Ben-Khalifa, K.; Dugave, C.; Tamouza, H.; van Niel, G.; et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J. Exp. Med. 2008, 205, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Moura, I.C.; Centelles, M.N.; Arcos-Fajardo, M.; Malheiros, D.M.; Collawn, J.F.; Cooper, M.D.; Monteiro, R.C. Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J. Exp. Med. 2001, 194, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Salonen, E.M.; Hovi, T.; Meurman, O.; Vesikari, T.; Vaheri, A. Kinetics of specific IgA, IgD, IgE, IgG, and IgM antibody responses in rubella. J. Med. Virol. 1985, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.G.; Leslie, G.A. Immunoglobulin D in rat serum, saliva and milk. Immunology 1985, 55, 571–577. [Google Scholar] [PubMed]
- Litwin, S.D.; Zehr, B.D.; Insel, R.A. Selective concentration of IgD class-specific antibodies in human milk. Clin. Exp. Immunol. 1990, 80, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Swain, P.; Nayak, S.K. Role of maternally derived immunity in fish. Fish Shellfish Immunol. 2009, 27, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xu, W.; Wilson, M.; He, B.; Miller, N.W.; Bengten, E.; Edholm, E.S.; Santini, P.A.; Rath, P.; Chiu, A.; et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat. Immunol. 2009, 10, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.A.; Holloway, J.A.; Popplewell, E.J.; Shute, J.K.; Boughton, J.; Warner, J.O. Fetal exposure to intact immunoglobulin E occurs via the gastrointestinal tract. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2003, 33, 306–311. [Google Scholar] [CrossRef]
- Yoshida, M.; Claypool, S.M.; Wagner, J.S.; Mizoguchi, E.; Mizoguchi, A.; Roopenian, D.C.; Lencer, W.I.; Blumberg, R.S. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 2004, 20, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Favre, L.; Spertini, F.; Corthesy, B. Secretory IgA possesses intrinsic modulatory properties stimulating mucosal and systemic immune responses. J. Immunol. 2005, 175, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kobayashi, K.; Kuo, T.T.; Bry, L.; Glickman, J.N.; Claypool, S.M.; Kaser, A.; Nagaishi, T.; Higgins, D.E.; Mizoguchi, E.; et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J. Clin. Invest. 2006, 116, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Elahi, S.; Ertelt, J.M.; Kinder, J.M.; Jiang, T.T.; Zhang, X.; Xin, L.; Chaturvedi, V.; Strong, B.S.; Qualls, J.E.; Steinbrecher, K.A.; et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013, 504, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Gutle, D.; Walther, S.; Menard, S.; Bogdan, C.; Hornef, M.W. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 2006, 203, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Garly, M.L.; Bale, C.; Martins, C.L.; Monteiro, M.; George, E.; Kidd, M.; Dias, F.; Aaby, P.; Whittle, H.C. Measles antibody responses after early two dose trials in Guinea-Bissau with Edmonston-Zagreb and Schwarz standard-titre measles vaccine: Better antibody increase from booster dose of the Edmonston-Zagreb vaccine. Vaccine 2001, 19, 1951–1959. [Google Scholar] [CrossRef]
- Borras, E.; Urbiztondo, L.; Costa, J.; Batalla, J.; Torner, N.; Plasencia, A.; Salleras, L.; Dominguez, A.; Working Group for the Study of Measles Immunity in Children. Measles antibodies and response to vaccination in children aged less than 14 months: Implications for age of vaccination. Epidemiol. Infect. 2012, 140, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, Q.; Xu, B.; Zhou, Z.; Wang, Z.; Zhou, Y.H. Influence of maternal antibody against hepatitis B surface antigen on active immune response to hepatitis B vaccine in infants. Vaccine 2008, 26, 6064–6067. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Pollock, L.; Barnett, S.M.; Battersby, A.; Kampmann, B. The relationship between concentration of specific antibody at birth and subsequent response to primary immunization. Vaccine 2014, 32, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Leuridan, E.; Maertens, K.; Nguyen, T.D.; Hens, N.; Vu, N.H.; Cabore, R.N.; Duong, H.T.; Huygen, K.; Van Damme, P.; et al. Pertussis vaccination during pregnancy in Vietnam: Results of a randomized controlled trial Pertussis vaccination during pregnancy. Vaccine 2016, 34, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.M.; Bond, N.H.; Maccato, M.; Pinell, P.; Hammill, H.A.; Swamy, G.K.; Walter, E.B.; Jackson, L.A.; Englund, J.A.; Edwards, M.S.; et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: A randomized clinical trial. JAMA 2014, 311, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Maertens, K.; Cabore, R.N.; Huygen, K.; Hens, N.; Van Damme, P.; Leuridan, E. Pertussis vaccination during pregnancy in Belgium: Results of a prospective controlled cohort study. Vaccine 2016, 34, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Bertley, F.M.; Ibrahim, S.A.; Libman, M.; Ward, B.J. Measles vaccination in the presence of maternal antibodies primes for a balanced humoral and cellular response to revaccination. Vaccine 2004, 23, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Amir, J.; Mijalovsky, A.; Kalmanovitch, I.; Bar-Yochai, A.; Thoelen, S.; Safary, A.; Ashkenazi, S. Immunization against hepatitis A in the first year of life: Priming despite the presence of maternal antibody. Pediatr. Infect. Dis. J. 2000, 19, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.A. Mechanisms by which maternal antibodies influence infant vaccine responses: Review of hypotheses and definition of main determinants. Vaccine 2003, 21, 3406–3412. [Google Scholar] [CrossRef]
- Dagan, R.; Eskola, J.; Leclerc, C.; Leroy, O. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect. Immun. 1998, 66, 2093–2098. [Google Scholar] [PubMed]
- Niewiesk, S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2014, 5, 446. [Google Scholar] [CrossRef] [PubMed]
- Feunou, P.F.; Mielcarek, N.; Locht, C. Reciprocal interference of maternal and infant immunization in protection against pertussis. Vaccine 2016, 34, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Maertens, K.; Hoang, T.T.; Nguyen, T.D.; Cabore, R.N.; Duong, T.H.; Huygen, K.; Hens, N.; Van Damme, P.; Dang, D.A.; Leuridan, E. The Effect of Maternal Pertussis Immunization on Infant Vaccine Responses to a Booster Pertussis-Containing Vaccine in Vietnam. Clin. Infect. Dis. 2016, 63, S197–S204. [Google Scholar] [CrossRef] [PubMed]
- Manickan, E.; Yu, Z.; Rouse, B.T. DNA immunization of neonates induces immunity despite the presence of maternal antibody. J. Clin. Invest. 1997, 100, 2371–2375. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, E.M.; Moonen-Leusen, H.W.; de Visser, Y.E.; Middel, W.G.; Boersma, W.J.; Bianchi, A.T. A DNA vaccine coding for gB and gD of pseudorabies virus (suid herpes type 1) primes the immune system in the presence of maternal immunity more efficiently than conventional vaccines. Vaccine 2006, 24, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.A. Breastfeeding provides passive and likely long-lasting active immunity. Ann. Allergy Asthma Immunol. 1998, 81, 523–533; quiz 533–534, 537. [Google Scholar] [CrossRef]
- Pabst, H.F.; Spady, D.W.; Pilarski, L.M.; Carson, M.M.; Beeler, J.A.; Krezolek, M.P. Differential modulation of the immune response by breast- or formula-feeding of infants. Acta Paediatr. 1997, 86, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Zoric, M.; Fulconis, F.; Minoli, I.; Moro, G.; Carlsson, B.; Bottiger, M.; Raiha, N.; Hanson, L.A. Antibody responses to parenteral and oral vaccines are impaired by conventional and low protein formulas as compared to breast-feeding. Acta Paediatr. Scand. 1990, 79, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Zoric, M.; Carlsson, B.; Jeansson, S.; Ekre, H.P.; Osterhaus, A.D.; Roberton, D.; Hanson, L.A. Anti-idiotypic antibodies to poliovirus antibodies in commercial immunoglobulin preparations, human serum, and milk. Pediatr. Res. 1993, 33, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, M.F.; Jenmalm, M.C.; Garofalo, R.P.; Bjorksten, B. Cytokines in breast milk from allergic and nonallergic mothers. Pediatr. Res. 2000, 47, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.A.; Korotkova, M.; Haversen, L.; Mattsby-Baltzer, I.; Hahn-Zoric, M.; Silfverdal, S.A.; Strandvik, B.; Telemo, E. Breast-feeding, a complex support system for the offspring. Pediatr. Int. 2002, 44, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Elahi, S.; Thompson, D.R.; Van Kessel, J.; Babiuk, L.A.; Gerdts, V. The protective role of passively transferred maternal cytokines against Bordetella pertussis infection in newborn piglets. Infect. Immun. 2017, 85, e01063-16. [Google Scholar] [CrossRef] [PubMed]
- Wirt, D.P.; Adkins, L.T.; Palkowetz, K.H.; Schmalstieg, F.C.; Goldman, A.S. Activated and memory T lymphocytes in human milk. Cytometry 1992, 13, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Huang, Y.Y.; Goji, H. Antibody production in early life supported by maternal lymphocyte factors. Biochim. Biophys. Acta 2003, 1637, 55–58. [Google Scholar] [CrossRef]
- Hanson, L.A.; Silfverdal, S.A.; Korotkova, M.; Erling, V.; Strombeck, L.; Olcen, P.; Ulanova, M.; Hahn-Zoric, M.; Zaman, S.; Ashraf, R.; et al. Immune system modulation by human milk. Adv. Exp. Med. Biol. 2002, 503, 99–106. [Google Scholar] [PubMed]
- Cabinian, A.; Sinsimer, D.; Tang, M.; Zumba, O.; Mehta, H.; Toma, A.; Sant’Angelo, D.; Laouar, Y.; Laouar, A. Transfer of Maternal Immune Cells by Breastfeeding: Maternal Cytotoxic T Lymphocytes Present in Breast Milk Localize in the Peyer’s Patches of the Nursed Infant. PLoS ONE 2016, 11, e0156762. [Google Scholar] [CrossRef] [PubMed]
- Tuboly, S.; Bernath, S.; Glavits, R.; Kovacs, A.; Megyeri, Z. Intestinal absorption of colostral lymphocytes in newborn lambs and their role in the development of immune status. Acta Vet. Hung. 1995, 43, 105–115. [Google Scholar] [PubMed]
- Bandrick, M.; Theis, K.; Molitor, T.W. Maternal immunity enhances Mycoplasma hyopneumoniae vaccination induced cell-mediated immune responses in piglets. BMC Vet. Res. 2014, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.; Rath, T.; Pyzik, M.; Blumberg, R.S. The Role of FcRn in Antigen Presentation. Front. Immunol. 2014, 5, 408. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Freigang, S.; Eberle, C.; Pattison, J.; Gupta, S.; Napoli, C.; Palinski, W. Maternal immunization programs postnatal immune responses and reduces atherosclerosis in offspring. Circ. Res. 2006, 99, e51–e64. [Google Scholar] [CrossRef] [PubMed]
- Eberle, C.; Merki, E.; Yamashita, T.; Johnson, S.; Armando, A.M.; Quehenberger, O.; Napoli, C.; Palinski, W. Maternal immunization affects in utero programming of insulin resistance and type 2 diabetes. PLoS ONE 2012, 7, e45361. [Google Scholar] [CrossRef] [PubMed]
- Melkild, I.; Groeng, E.C.; Leikvold, R.B.; Granum, B.; Lovik, M. Maternal allergen immunization during pregnancy in a mouse model reduces adult allergy-related antibody responses in the offspring. Clin. Exp. Allergy 2002, 32, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, A.E.; Brito, C.A.; Victor, J.R.; Rigato, P.O.; Goldoni, A.L.; Duarte, A.J.; Sato, M.N. Maternal-fetal interaction: Preconception immunization in mice prevents neonatal sensitization induced by allergen exposure during pregnancy and breastfeeding. Immunology 2007, 122, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Seeger, M.; Thierse, H.J.; Lange, H.; Shaw, L.; Hansen, H.; Lemke, H. Antigen-independent suppression of the IgE immune response to bee venom phospholipase A2 by maternally derived monoclonal IgG antibodies. Eur. J. Immunol. 1998, 28, 2124–2130. [Google Scholar] [CrossRef]
- Polte, T.; Hansen, G. Maternal tolerance achieved during pregnancy is transferred to the offspring via breast milk and persistently protects the offspring from allergic asthma. Clin. Exp. Allergy 2008, 38, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, E.; Rekima, A.; Seitz-Polski, B.; Kanda, A.; Fleury, S.; Tissandie, E.; Monteiro, R.; Dombrowicz, D.D.; Julia, V.; Glaichenhaus, N.; et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol. 2010, 3, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.R.; Muniz, B.P.; Fusaro, A.E.; de Brito, C.A.; Taniguchi, E.F.; Duarte, A.J.; Sato, M.N. Maternal immunization with ovalbumin prevents neonatal allergy development and up-regulates inhibitory receptor Fc gamma RIIB expression on B cells. BMC Immunol. 2010, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Muniz, B.P.; Victor, J.R.; de Mendonca Oliveira, L.; de Lima Lira, A.A.; Perini, A.; Olivo, C.R.; Arantes-Costa, F.M.; Martins, M.A.; da Silva Duarte, A.J.; Sato, M.N. Tolerogenic microenvironment in neonatal period induced by maternal immunization with ovalbumin. Immunobiology 2014, 219, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.R. Influence of maternal immunization with allergens on the thymic maturation of lymphocytes with regulatory potential in children: A broad field for further exploration. J. Immunol. Res. 2014, 2014, 780386. [Google Scholar] [CrossRef] [PubMed]
- Gerhold, K.; Avagyan, A.; Reichert, E.; Seib, C.; Van, D.V.; Luger, E.O.; Hutloff, A.; Hamelmann, E. Prenatal allergen exposures prevent allergen-induced sensitization and airway inflammation in young mice. Allergy 2012, 67, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Sandler, B.; Smirnoff, P.; Gurevich, P.; Zusman, I. Transplacental tumor-preventive effects of polyclonal antibodies generated against the soluble 53 kDa antigen on mammary tumorigenesis in offspring. Oncol. Rep. 1999, 6, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hur, H.; Plonsky, E.; Berman, V.; Tendler, Y.; Gurevich, P.; Moriel, E.; Guy, M.; Sandler, B.; Zusman, I. Transplacental effects of IgG generated against soluble 53 kDa protein on the splenic lymph system of rat progeny exposed to carcinogen: Rate of apoptosis, proliferation of lymphocytes and expression of Fas and Fas ligand proteins. Eur. J. Gynaecol. Oncol. 1999, 20, 306–310. [Google Scholar] [PubMed]
- Chu, N.J.; Armstrong, T.D.; Jaffee, E.M. Nonviral oncogenic antigens and the inflammatory signals driving early cancer development as targets for cancer immunoprevention. Clin. Cancer Res. 2015, 21, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Blixt, O.; Bueti, D.; Burford, B.; Allen, D.; Julien, S.; Hollingsworth, M.; Gammerman, A.; Fentiman, I.; Taylor-Papadimitriou, J.; Burchell, J.M. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011, 13, R25. [Google Scholar] [CrossRef] [PubMed]
- Daudi, S.; Eng, K.H.; Mhawech-Fauceglia, P.; Morrison, C.; Miliotto, A.; Beck, A.; Matsuzaki, J.; Tsuji, T.; Groman, A.; Gnjatic, S.; et al. Expression and immune responses to MAGE antigens predict survival in epithelial ovarian cancer. PLoS ONE 2014, 9, e104099. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, N.; Gnjatic, S.; Ritter, E.; Mangone, M.; Austin, W.; Reyner, K.; Jayabalan, D.; Niesvizky, R.; Jagannath, S.; Bhardwaj, N.; et al. Cellular immune responses against CT7 (MAGE-C1) and humoral responses against other cancer-testis antigens in multiple myeloma patients. Cancer Immun. 2010, 10, 4. [Google Scholar] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Arigoni, M.; Barutello, G.; Lanzardo, S.; Longo, D.; Aime, S.; Curcio, C.; Iezzi, M.; Zheng, Y.; Barkefors, I.; Holmgren, L.; et al. A vaccine targeting angiomotin induces an antibody response which alters tumor vessel permeability and hampers the growth of established tumors. Angiogenesis 2012, 15, 305–316. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, C.; Nicoletti, G.; Quaglino, E.; Landuzzi, L.; Palladini, A.; Ianzano, M.L.; Dall’Ora, M.; Grosso, V.; Ranieri, D.; Laranga, R.; et al. Vaccines against human HER2 prevent mammary carcinoma in mice transgenic for human HER2. Breast Cancer Res. BCR 2014, 16, R10. [Google Scholar] [CrossRef] [PubMed]
- Lanzardo, S.; Conti, L.; Rooke, R.; Ruiu, R.; Accart, N.; Bolli, E.; Arigoni, M.; Macagno, M.; Barrera, G.; Pizzimenti, S.; et al. Immunotargeting of Antigen xCT Attenuates Stem-like Cell Behavior and Metastatic Progression in Breast Cancer. Cancer Res. 2016, 76, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.N.; Ferraro, B.; Duperret, E.K.; Kraynyak, K.A.; Chu, J.; Saint-Fleur, A.; Yan, J.; Levitsky, H.; Khan, A.S.; Sardesai, N.Y.; et al. A Novel DNA Vaccine Platform Enhances Neo-antigen-like T Cell Responses against WT1 to Break Tolerance and Induce Anti-tumor Immunity. Mol. Ther. 2017, 25, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, F.; Iussich, S.; Maniscalco, L.; Lorda Mayayo, S.; La Rosa, G.; Arigoni, M.; De Maria, R.; Gattino, F.; Lanzardo, S.; Lardone, E.; et al. CSPG4-specific immunity and survival prolongation in dogs with oral malignant melanoma immunized with human CSPG4 DNA. Clin. Cancer Res. 2014, 20, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Olgasi, C.; Dentelli, P.; Rosso, A.; Iavello, A.; Togliatto, G.; Toto, V.; Liberatore, M.; Barutello, G.; Musiani, P.; Cavallo, F.; et al. DNA vaccination against membrane-bound Kit ligand: A new approach to inhibiting tumour growth and angiogenesis. Eur. J. Cancer 2014, 50, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.K.; Palucka, K. Understanding and activating immunity against human cancer. Curr. Opin. Immunol. 2010, 22, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.P.; Ciernik, I.F.; Kelley, M.J.; Smith, M.C.; Nadaf, S.; Kavanaugh, D.; Maher, V.E.; Stipanov, M.; Contois, D.; Johnson, B.E.; et al. Immunization with mutant p53- and K-ras-derived peptides in cancer patients: Immune response and clinical outcome. J. Clin. Oncol. 2005, 23, 5099–5107. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Sweeney, P.; Sullivan, G.C.; Tangney, M. DNA vaccination for prostate cancer, from preclinical to clinical trials—where we stand? Genet. Vaccines Ther. 2012, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.F. DNA vaccine for cancer immunotherapy. Hum. Vaccin. Immunother. 2014, 10, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Pertmer, T.M.; Oran, A.E.; Moser, J.M.; Madorin, C.A.; Robinson, H.L. DNA vaccines for influenza virus: Differential effects of maternal antibody on immune responses to hemagglutinin and nucleoprotein. J. Virol. 2000, 74, 7787–7793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, J.; Fang, F.; Zhou, Y.; Wu, J.; Chang, H.; Zhang, R.; Wang, F.; Li, X.; Wang, H.; et al. Maternal immunization with both hemagglutinin- and neuraminidase-expressing DNAs provides an enhanced protection against a lethal influenza virus challenge in infant and adult mice. DNA Cell Biol. 2005, 24, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, F.; Fang, F.; Chang, H.; Chen, Z. Vaccination with hemagglutinin or neuraminidase DNA protects BALB/c mice against influenza virus infection in presence of maternal antibody. Bmc. Infect. Dis. 2007, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Van Drunen Littel-van den Hurk, S.; Braun, R.P.; Lewis, P.J.; Karvonen, B.C.; Babiuk, L.A.; Griebel, P.J. Immunization of neonates with DNA encoding a bovine herpesvirus glycoprotein is effective in the presence of maternal antibodies. Viral. Immunol. 1999, 12, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.J.; van Drunen Littel-Van Den, H.; Babiuk, L.A. Induction of immune responses to bovine herpesvirus type 1 gD in passively immune mice after immunization with a DNA-based vaccine. J. Gen. Virol. 1999, 80 Pt 11, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Premenko-Lanier, M.; Rota, P.A.; Rhodes, G.; Verhoeven, D.; Barouch, D.H.; Lerche, N.W.; Letvin, N.L.; Bellini, W.J.; McChesney, M.B. DNA vaccination of infants in the presence of maternal antibody: A measles model in the primate. Virology 2003, 307, 67–75. [Google Scholar] [CrossRef]
- Hamers, C.; Juillard, V.; Fischer, L. DNA vaccination against pseudorabies virus and bovine respiratory syncytial virus infections of young animals in the face of maternally derived immunity. J. Comp. Pathol. 2007, 137 (Suppl 1), S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, F.; Calogero, R.A.; Forni, G. Are oncoantigens suitable targets for anti-tumour therapy? Nat. Rev. Cancer 2007, 7, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, M.; Quaglino, E.; Amici, A.; Lollini, P.L.; Forni, G.; Cavallo, F. DNA vaccination against oncoantigens: A promise. Oncoimmunology 2012, 1, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Boggio, K.; Nicoletti, G.; Di Carlo, E.; Cavallo, F.; Landuzzi, L.; Melani, C.; Giovarelli, M.; Rossi, I.; Nanni, P.; De Giovanni, C.; et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J. Exp. Med. 1998, 188, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, E.; Mastini, C.; Forni, G.; Cavallo, F. ErbB2 transgenic mice: A tool for investigation of the immune prevention and treatment of mammary carcinomas. Curr. Protoc. Immunol. 2008. [Google Scholar] [CrossRef]
- Conti, L.; Ruiu, R.; Barutello, G.; Macagno, M.; Bandini, S.; Cavallo, F.; Lanzardo, S. Microenvironment, oncoantigens, and antitumor vaccination: Lessons learned from BALB-neuT mice. Biomed. Res. Int. 2014, 2014, 534969. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, A.; Landuzzi, L.; Nicoletti, G.; De Giovanni, C.; Croci, S.; Palladini, A.; Ferrini, S.; Iezzi, M.; Musiani, P.; Cavallo, F.; et al. Gene expression analysis of immune-mediated arrest of tumorigenesis in a transgenic mouse model of HER-2/neu-positive basal-like mammary carcinoma. Am. J. Pathol. 2005, 166, 1205–1216. [Google Scholar] [CrossRef]
- Quaglino, E.; Iezzi, M.; Mastini, C.; Amici, A.; Pericle, F.; Di Carlo, E.; Pupa, S.M.; De Giovanni, C.; Spadaro, M.; Curcio, C.; et al. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res. 2004, 64, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, E.; Mastini, C.; Amici, A.; Marchini, C.; Iezzi, M.; Lanzardo, S.; De Giovanni, C.; Montani, M.; Lollini, P.L.; Masucci, G.; et al. A better immune reaction to Erbb-2 tumors is elicited in mice by DNA vaccines encoding rat/human chimeric proteins. Cancer Res. 2010, 70, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.; Khan, A.S.; Amici, A.; Spadaro, M.; Quaglino, E.; Cavallo, F.; Forni, G.; Draghia-Akli, R. DNA immunization using constant-current electroporation affords long-term protection from autochthonous mammary carcinomas in cancer-prone transgenic mice. Cancer Gene Ther. 2008, 15, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Rolla, S.; Marchini, C.; Malinarich, S.; Quaglino, E.; Lanzardo, S.; Montani, M.; Iezzi, M.; Angeletti, M.; Ramadori, G.; Forni, G.; et al. Protective immunity against neu-positive carcinomas elicited by electroporation of plasmids encoding decreasing fragments of rat neu extracellular domain. Hum. Gene Ther. 2008, 19, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, E.; Riccardo, F.; Macagno, M.; Bandini, S.; Cojoca, R.; Ercole, E.; Amici, A.; Cavallo, F. Chimeric DNA Vaccines against ErbB2+ Carcinomas: From Mice to Humans. Cancers 2011, 3, 3225–3241. [Google Scholar] [CrossRef] [PubMed]
- Barutello, G.; Curcio, C.; Spadaro, M.; Arigoni, M.; Trovato, R.; Bolli, E.; Zheng, Y.; Ria, F.; Quaglino, E.; Iezzi, M.; et al. Antitumor immunization of mothers delays tumor development in cancer-prone offspring. Oncoimmunology 2015, 4, e1005500. [Google Scholar] [CrossRef] [PubMed]
- Press, M.F.; Cordon-Cardo, C.; Slamon, D.J. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 1990, 5, 953–962. [Google Scholar] [PubMed]
- Lo Iacono, M.; Cavallo, F.; Quaglino, E.; Rolla, S.; Iezzi, M.; Pupa, S.M.; De Giovanni, C.; Lollini, P.L.; Musiani, P.; Forni, G.; et al. A limited autoimmunity to p185neu elicited by DNA and allogeneic cell vaccine hampers the progression of preneoplastic lesions in HER-2/NEU transgenic mice. Int. J. Immunopathol. Pharmacol. 2005, 18, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, E.; Rolla, S.; Iezzi, M.; Spadaro, M.; Musiani, P.; De Giovanni, C.; Lollini, P.L.; Lanzardo, S.; Forni, G.; Sanges, R.; et al. Concordant morphologic and gene expression data show that a vaccine halts HER-2/neu preneoplastic lesions. J. Clin. Invest. 2004, 113, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012, 119, 5640–5649. [Google Scholar] [CrossRef] [PubMed]
- Rolla, S.; Nicolo, C.; Malinarich, S.; Orsini, M.; Forni, G.; Cavallo, F.; Ria, F. Distinct and non-overlapping T cell receptor repertoires expanded by DNA vaccination in wild-type and HER-2 transgenic BALB/c mice. J. Immunol. 2006, 177, 7626–7633. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Gauchez, A.S.; Jacot, W.; Lamy, P.J. HER2 shedding and serum HER2 extracellular domain: Biology and clinical utility in breast cancer. Cancer Treat. Rev. 2012, 38, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Cecen, E.; Gunes, D.; Uysal, K.M.; Yuceer, N.; Ozer, E. Atypical teratoid/rhabdoid tumor in an infant conceived by in vitro fertilization. Child Nerv. Syst. 2010, 26, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Beasley, M.; Pang, D.; Macfarlane, G.J. Maternal and perinatal risk factors for childhood cancer: Record linkage study. BMJ Open 2014, 4, e003656. [Google Scholar] [CrossRef] [PubMed]
- Strahm, B.; Malkin, D. Hereditary cancer predisposition in children: Genetic basis and clinical implications. Int. J. Cancer 2006, 119, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Salas, P.C.; Lapunzina, P.; Perez-Martinez, A. [Genetic predisposition to childhood cancer]. An. Pediatr. (Barc) 2017. [Google Scholar] [CrossRef]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Orbach, D.; Sarnacki, S.; Brisse, H.J.; Gauthier-Villars, M.; Jarreau, P.H.; Tsatsaris, V.; Baruchel, A.; Zerah, M.; Seigneur, E.; Peuchmaur, M.; et al. Neonatal cancer. Lancet Oncol. 2013, 14, e609–e620. [Google Scholar] [CrossRef]
- Marshall, G.M.; Carter, D.R.; Cheung, B.B.; Liu, T.; Mateos, M.K.; Meyerowitz, J.G.; Weiss, W.A. The prenatal origins of cancer. Nat. Rev. Cancer 2014, 14, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, J.B. Precursor lesions of Wilms tumor: Clinical and biological implications. Med. Pediatr. Oncol. 1993, 21, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Nuchtern, J.G.; London, W.B.; Barnewolt, C.E.; Naranjo, A.; McGrady, P.W.; Geiger, J.D.; Diller, L.; Schmidt, M.L.; Maris, J.M.; Cohn, S.L.; et al. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: A Children’s Oncology Group study. Ann. Surg. 2012, 256, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Saylors, R.L., 3rd; Cohn, S.L.; Morgan, E.R.; Brodeur, G.M. Prenatal detection of neuroblastoma by fetal ultrasonography. Am. J. Pediatr. Hematol. Oncol. 1994, 16, 356–360. [Google Scholar] [PubMed]
- Vadeyar, S.; Ramsay, M.; James, D.; O’Neill, D. Prenatal diagnosis of congenital Wilms’ tumor (nephroblastoma) presenting as fetal hydrops. Ultrasound Obstet. Gynecol. 2000, 16, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Thankamony, A.; Harlow, F.H.; Ponnampalam, J.; Clarke, P. Congenital brain tumour mimicking fetal intracranial haemorrhage. J. Obstet. Gynaecol. 2007, 27, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Hillenbrand, C.M.; Reykowski, A. MR Imaging of the Newborn: A technical perspective. Magn. Reson. Imaging Clin. N. Am. 2012, 20, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Faas, B.H.; de Ligt, J.; Janssen, I.; Eggink, A.J.; Wijnberger, L.D.; van Vugt, J.M.; Vissers, L.; Geurts van Kessel, A. Non-invasive prenatal diagnosis of fetal aneuploidies using massively parallel sequencing-by-ligation and evidence that cell-free fetal DNA in the maternal plasma originates from cytotrophoblastic cells. Expert Opin. Biol. Ther. 2012, 12 (Suppl 1), S19–S26. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Tzika, A.; Wood, H.; Berri, S.; Roberts, P.; Mason, G.; Sheridan, E. Diagnosis of fetal submicroscopic chromosomal abnormalities in failed array CGH samples: Copy number by sequencing as an alternative to microarrays for invasive fetal testing. Ultrasound Obstet. Gynecol. 2015, 45, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Nepomnyashchaya, Y.N.; Artemov, A.V.; Roumiantsev, S.A.; Roumyantsev, A.G.; Zhavoronkov, A. Non-invasive prenatal diagnostics of aneuploidy using next-generation DNA sequencing technologies, and clinical considerations. Clin. Chem. Lab. Med. 2013, 51, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Barrett, A.N.; Tan, T.Z.; Huang, Z.; Mahyuddin, A.P.; Ponnusamy, S.; Sandhu, J.S.; Ho, S.S.; Chan, J.K.; Chong, S.; et al. Detection of aneuploidy from single fetal nucleated red blood cells using whole genome sequencing. Prenat. Diagn. 2015, 35, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Quinlan-Jones, E.; Williams, D.; Bell, C.; Miller, C.; Gokhale, C.; Kilby, M.D. Prenatal Detection of PIK3CA-related Overgrowth Spectrum in Cultured Amniocytes Using Long-range PCR and Next-generation Sequencing. Pediatr. Dev. Pathol. 2017, 20, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Young, E.; Bowns, B. Noninvasive prenatal diagnosis for single gene disorders. Curr. Opin. Obstet. Gynecol. 2017, 29, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bourdeaut, F.; Ferrand, S.; Brugieres, L.; Hilbert, M.; Ribeiro, A.; Lacroix, L.; Benard, J.; Combaret, V.; Michon, J.; Valteau-Couanet, D.; et al. ALK germline mutations in patients with neuroblastoma: A rare and weakly penetrant syndrome. Eur. J. Hum. Genet. 2012, 20, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Aretz, S.; Koch, A.; Uhlhaas, S.; Friedl, W.; Propping, P.; von Schweinitz, D.; Pietsch, T. Should children at risk for familial adenomatous polyposis be screened for hepatoblastoma and children with apparently sporadic hepatoblastoma be screened for APC germline mutations? Pediatr. Blood Cancer 2006, 47, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Rubie, H.; De Bernardi, B.; Gerrard, M.; Canete, A.; Ladenstein, R.; Couturier, J.; Ambros, P.; Munzer, C.; Pearson, A.D.; Garaventa, A.; et al. Excellent outcome with reduced treatment in infants with nonmetastatic and unresectable neuroblastoma without MYCN amplification: Results of the prospective INES 99.1. J. Clin. Oncol. 2011, 29, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Creutzig, U.; Zimmermann, M.; Bourquin, J.P.; Dworzak, M.N.; Kremens, B.; Lehrnbecher, T.; von Neuhoff, C.; Sander, A.; von Stackelberg, A.; Schmid, I.; et al. Favorable outcome in infants with AML after intensive first- and second-line treatment: An AML-BFM study group report. Leukemia 2012, 26, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, I.C.; Tuamokumo, N.; Yock, T.I. Role of radiation therapy in pediatric cancer. Hematol. Oncol. Clin. N. Am. 2006, 20, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Yankelevich, M.; Kondadasula, S.V.; Thakur, A.; Buck, S.; Cheung, N.K.; Lum, L.G. Anti-CD3 x anti-GD2 bispecific antibody redirects T-cell cytolytic activity to neuroblastoma targets. Pediatr. Blood Cancer 2012, 59, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.U.; Savoldo, B.; Dotti, G.; Pule, M.; Yvon, E.; Myers, G.D.; Rossig, C.; Russell, H.V.; Diouf, O.; Liu, E.; et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011, 118, 6050–6056. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.A.; Krishnadas, D.K.; Lucas, K.G. Cellular and Antibody Based Approaches for Pediatric Cancer Immunotherapy. J. Immunol. Res. 2015, 2015, 675269. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, R.F.; Haight, A.E.; Hirschmann-Jax, C.; Yvon, E.S.; Rill, D.R.; Mei, Z.; Smith, S.C.; Inman, S.; Cooper, K.; Alcoser, P.; et al. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood 2003, 101, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Krishnadas, D.K.; Shapiro, T.; Lucas, K. Complete remission following decitabine/dendritic cell vaccine for relapsed neuroblastoma. Pediatrics 2013, 131, e336–e341. [Google Scholar] [CrossRef] [PubMed]
- Lasky, J.L., 3rd; Panosyan, E.H.; Plant, A.; Davidson, T.; Yong, W.H.; Prins, R.M.; Liau, L.M.; Moore, T.B. Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer Res. 2013, 33, 2047–2056. [Google Scholar] [PubMed]
- Yuki, N.; Yamada, M.; Tagawa, Y.; Takahashi, H.; Handa, S. Pathogenesis of the neurotoxicity caused by anti-GD2 antibody therapy. J. Neurol. Sci. 1997, 149, 127–130. [Google Scholar] [CrossRef]
- Bonifant, C.L.; Jackson, H.J.; Brentjens, R.J.; Curran, K.J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 2016, 3, 16011. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Barrett, D.; Teachey, D.T.; Grupp, S.A. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014, 20, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Thurner, B.; Haendle, I.; Roder, C.; Dieckmann, D.; Keikavoussi, P.; Jonuleit, H.; Bender, A.; Maczek, C.; Schreiner, D.; von den Driesch, P.; et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J. Exp. Med. 1999, 190, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Mettling, K.; Murcek, K.; Rubarth, L.B. Malignancies and tumors in the neonate. Neonatal. Netw. 2013, 32, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Tweddle, D.A. Neonatal neuroblastoma. Semin. Fetal Neonatal Med. 2012, 17, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Mosse, Y.P.; Laudenslager, M.; Longo, L.; Cole, K.A.; Wood, A.; Attiyeh, E.F.; Laquaglia, M.J.; Sennett, R.; Lynch, J.E.; Perri, P.; et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 2008, 455, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Mosse, Y.P.; Laudenslager, M.; Khazi, D.; Carlisle, A.J.; Winter, C.L.; Rappaport, E.; Maris, J.M. Germline PHOX2B mutation in hereditary neuroblastoma. Am. J. Hum. Genet. 2004, 75, 727–730. [Google Scholar] [CrossRef] [PubMed]
- George, R.E.; Sanda, T.; Hanna, M.; Frohling, S.; Luther, W., 2nd; Zhang, J.; Ahn, Y.; Zhou, W.; London, W.B.; McGrady, P.; et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 2008, 455, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M.; Seeger, R.C.; Schwab, M.; Varmus, H.E.; Bishop, J.M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984, 224, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.; Varmus, H.E.; Bishop, J.M.; Grzeschik, K.H.; Naylor, S.L.; Sakaguchi, A.Y.; Brodeur, G.; Trent, J. Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature 1984, 308, 288–291. [Google Scholar] [CrossRef]
- Berry, T.; Luther, W.; Bhatnagar, N.; Jamin, Y.; Poon, E.; Sanda, T.; Pei, D.; Sharma, B.; Vetharoy, W.R.; Hallsworth, A.; et al. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell 2012, 22, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, K.; Kageyama, S.; Tao, H.; Bunai, T.; Suzuki, M.; Kamo, T.; Takamochi, K.; Suzuki, K.; Tanahashi, M.; Niwa, H.; et al. EML4-ALK fusion transcripts, but no NPM-, TPM3-, CLTC-, ATIC-, or TFG-ALK fusion transcripts, in non-small cell lung carcinomas. Lung Cancer 2008, 61, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Chiarle, R.; Voena, C.; Ambrogio, C.; Piva, R.; Inghirami, G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat. Rev. Cancer 2008, 8, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Corao, D.A.; Biegel, J.A.; Coffin, C.M.; Barr, F.G.; Wainwright, L.M.; Ernst, L.M.; Choi, J.K.; Zhang, P.J.; Pawel, B.R. ALK expression in rhabdomyosarcomas: Correlation with histologic subtype and fusion status. Pediatr. Dev. Pathol. 2009, 12, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Pillay, K.; Govender, D.; Chetty, R. ALK protein expression in rhabdomyosarcomas. Histopathology 2002, 41, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, J.C.; Flucke, U.E.; Roeffen, M.H.; de Bont, E.S.; Sleijfer, S.; Mavinkurve-Groothuis, A.M.; Suurmeijer, A.J.; van der Graaf, W.T.; Versleijen-Jonkers, Y.M. Anaplastic lymphoma kinase aberrations in rhabdomyosarcoma: Clinical and prognostic implications. J. Clin. Oncol. 2012, 30, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Ameur, N.; Yilmaz, I.; Nafa, K.; Lau, C.Y.; Marchetti, A.; Borsu, L.; Barr, F.G.; Ladanyi, M. Oncogene mutation profiling of pediatric solid tumors reveals significant subsets of embryonal rhabdomyosarcoma and neuroblastoma with mutated genes in growth signaling pathways. Clin. Cancer Res. 2012, 18, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Onoda, T.; Kanno, M.; Sato, H.; Takahashi, N.; Izumino, H.; Ohta, H.; Emura, T.; Katoh, H.; Ohizumi, H.; Ohtake, H.; et al. Identification of novel ALK rearrangement A2M-ALK in a neonate with fetal lung interstitial tumor. Genes Chromosom. Cancer 2014, 53, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Brackett, N.; Johnson, R.; Schindel, D.T.; Koduru, P.; Cope-Yokoyama, S. A novel ALK rearrangement in an inflammatory myofibroblastic tumor in a neonate. Cancer Genet. 2013, 206, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Bresler, S.C.; Wood, A.C.; Haglund, E.A.; Courtright, J.; Belcastro, L.T.; Plegaria, J.S.; Cole, K.; Toporovskaya, Y.; Zhao, H.; Carpenter, E.L.; et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci. Transl. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Humphreys, A.; Turnbull, L.; Bellini, A.; Schleiermacher, G.; Salwen, H.; Cohn, S.L.; Bown, N.; Tweddle, D.A. Identification of different ALK mutations in a pair of neuroblastoma cell lines established at diagnosis and relapse. Oncotarget 2016, 7, 87301–87311. [Google Scholar] [CrossRef] [PubMed]
- Chiarle, R.; Martinengo, C.; Mastini, C.; Ambrogio, C.; D’Escamard, V.; Forni, G.; Inghirami, G. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nat. Med. 2008, 14, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Voena, C.; Menotti, M.; Mastini, C.; Di Giacomo, F.; Longo, D.L.; Castella, B.; Merlo, M.E.; Ambrogio, C.; Wang, Q.; Minero, V.G.; et al. Efficacy of a Cancer Vaccine against ALK-Rearranged Lung Tumors. Cancer Immunol. Res. 2015, 3, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Duyster, J.; Bai, R.Y.; Morris, S.W. Translocations involving anaplastic lymphoma kinase (ALK). Oncogene 2001, 20, 5623–5637. [Google Scholar] [CrossRef] [PubMed]
| Target Population | Vaccine | Type/Form | Recommendation |
|---|---|---|---|
| All pregnant women | Influenza | Inactivated | 1 dose administered during flu at any gestational ages |
| Tetanus, Diphtheria and acellular Pertussis (Tdap) | Toxoid/inactivated bacteria | 1 dose ideally between 27 and 36 weeks of gestation | |
| Pregnant women with specific risk factors | Hepatitis A | Inactivated whole-cell viral | 2 doses; allowed in some circumstances |
| Hepatitis B | Inactivated viral recombinant subunit | 3 doses; allowed in some circumstances | |
| Pneumococcal | Inactivated bacteria polysaccharide | 1 dose if there is risk factor | |
| Meningococcal | Inactivated bacteria polysaccharide | 1 dose if there is risk factor | |
| Conjugate | |||
| Yellow fever | Live-attenuated viral | 1 doses during epidemics and in case of travel to endemic regions. (Should be avoided during breastfeeding) | |
| Japanese Encephalitis | Live-attenuated viral | 1 doses during epidemics and in case of travel to endemic regions | |
| Typhoid | Live-attenuated bacterial recombinant | Insufficient data for recommendation | |
| Anthrax | Inactivated subunit | Post-exposure prophylaxis; pre-exposure prophylaxis is not recommended | |
| Rabies | Inactivated whole-cell viral | Post-exposure prophylaxis; consider pre-exposure prophylaxis if risk of exposure is very high | |
| Tetanus and Diphteria (Td) | Inactivated bacterial toxoids | Allowed in some circumstances (Tdap preferred) | |
| Smallpox | Live-attenuated viral | Post-exposure prophylaxis; pre-exposure prophylaxis is not recommended | |
| Postpartum women (contraindicated in pregnancy) | MMR (Measles, Mumps, Rubella) | Live-attenuated viral | 1 dose immediately postpartum if susceptible to rubella |
| Varicella | Live-attenuated viral | 1 dose immediately postpartum if susceptible |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccardo, F.; Réal, A.; Voena, C.; Chiarle, R.; Cavallo, F.; Barutello, G. Maternal Immunization: New Perspectives on Its Application Against Non-Infectious Related Diseases in Newborns. Vaccines 2017, 5, 20. https://doi.org/10.3390/vaccines5030020
Riccardo F, Réal A, Voena C, Chiarle R, Cavallo F, Barutello G. Maternal Immunization: New Perspectives on Its Application Against Non-Infectious Related Diseases in Newborns. Vaccines. 2017; 5(3):20. https://doi.org/10.3390/vaccines5030020
Chicago/Turabian StyleRiccardo, Federica, Aline Réal, Claudia Voena, Roberto Chiarle, Federica Cavallo, and Giuseppina Barutello. 2017. "Maternal Immunization: New Perspectives on Its Application Against Non-Infectious Related Diseases in Newborns" Vaccines 5, no. 3: 20. https://doi.org/10.3390/vaccines5030020
APA StyleRiccardo, F., Réal, A., Voena, C., Chiarle, R., Cavallo, F., & Barutello, G. (2017). Maternal Immunization: New Perspectives on Its Application Against Non-Infectious Related Diseases in Newborns. Vaccines, 5(3), 20. https://doi.org/10.3390/vaccines5030020






