Mitochondrion: A Promising Target for Nanoparticle-Based Vaccine Delivery Systems
Abstract
:1. Introduction
2. NP-based Vaccine Delivery Systems
2.1. Polymeric NPs as Antigen Carriers
2.2. Liposomal Systems as Antigen Carrier/Adjuvant
2.3. Others Types of NP-based Systems as Antigen Carrier/Adjuvant
3. Mitochondria Targeting Moiety
4. Examples of Targeted NP-Based Vaccine
5. Conclusions and Future Outlook
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Liu, M.A. Immunologic basis of vaccine vectors. Immunity 2010, 33, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Geels, M.; Ye, K. Developments in high-yield system expressed vaccines and immunotherapy. Recent Pat. Biotechnol. 2010, 4, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Hanson, M.C.; Rakhra, K.; Tokatlian, T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015, 115, 11109–11146. [Google Scholar] [CrossRef] [PubMed]
- Rice-Ficht, A.C.; Arenas-Gamboa, A.M.; Kahl-McDonagh, M.M.; Ficht, T.A. Polymeric particles in vaccine delivery. Curr. Opin. Microbiol. 2010, 13, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Banik, B.; Pathak, R.K.; Kumar, A.; Kolishetti, N.; Dhar, S. Nanotechnology inspired tools for mitochondrial dysfunction related diseases. Adv. Drug Deliv. Rev. 2016, 99, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Mitochondria: Dynamic organelles in disease, aging, and development. Cell 2006, 125, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Kolishetti, N.; Dhar, S. Targeted nanoparticles in mitochondrial medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Duchen, M.R. Mitochondria in health and disease: Perspectives on a new mitochondrial biology. Mol. Aspects Med. 2004, 25, 365–451. [Google Scholar] [CrossRef] [PubMed]
- Marrache, S.; Kumar Pathak, R.; Darley, K.L.; Choi, J.H.; Zaver, D.; Kolishetti, N.; Dhar, S. Nanocarriers for tracking and treating diseases. Curr. Med. Chem. 2013, 20, 3500–3514. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013, 38, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Chen, W.; Huang, L. Reactive oxygen species play a central role in the activity of cationic liposome based cancer vaccine. J. Control. Release 2008, 130, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, S.; Danner, D.; Mutimer, D.; Fussey, S.; James, O.; Bassendine, M. Primary biliary cirrhosis: Identification of two major M2 mitochondrial autoantigens. Lancet 1988, 331, 1067–1070. [Google Scholar] [CrossRef]
- Fussey, S.P.; Lindsay, J.G.; Fuller, C.; Perham, R.N.; Dale, S.; James, O.F.; Bassendine, M.F.; Yeaman, S.J. Autoantibodies in primary biliary cirrhosis: Analysis of reactivity against eukaryotic and prokaryotic 2-oxo acid dehydrogenase complexes. Hepatology 1991, 13, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Moteki, S.; Leung, P.; Dickson, E.R.; Van Thiel, D.H.; Galperin, C.; Buch, T.; Alarcon-Segovia, D.; Kershenobich, D.; Kawano, K.; Coppel, R.L. Epitope mapping and reactivity of autoantibodies to the E2 component of 2-oxoglutarate dehydrogenase complex in primary biliary cirrhosis using recombinant 2*oxoglutarate dehydrogenase complex. Hepatology 1996, 23, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Duvvuri, B.; Grigull, J.; Jamnik, R.; Wither, J.E.; Wu, G.E. Experimental evidence that mutated-self peptides derived from mitochondrial DNA somatic mutations have the potential to trigger autoimmunity. Hum. Immunol. 2014, 75, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Gershwin, M.E.; Mackay, I.; Sturgess, A.; Coppel, R. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J. Immunol. 1987, 138, 3525–3531. [Google Scholar] [PubMed]
- Marrache, S.; Tundup, S.; Harn, D.A.; Dhar, S. Ex vivo programming of dendritic cells by mitochondria-targeted nanoparticles to produce interferon-gamma for cancer immunotherapy. ACS Nano 2013, 7, 7392–7402. [Google Scholar] [CrossRef] [PubMed]
- Pierini, S.; Fang, C.; Rafail, S.; Facciponte, J.G.; Huang, J.; De Sanctis, F.; Morgan, M.A.; Uribe-Herranz, M.; Tanyi, J.L.; Facciabene, A. A tumor mitochondria vaccine protects against experimental renal cell carcinoma. J. Immunol. 2015, 195, 4020–4027. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Huston, M.M.; Jenkins, R.N.; Huston, D.P.; Rich, R.R. Mitochondria control expression of a murine cell surface antigen. Nature 1983, 306, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Loveland, B.; Wang, C.R.; Yonekawa, H.; Hermel, E.; Lindahl, K.F. Maternally transmitted histocompatibility antigen of mice: A hydrophobic peptide of a mitochondrially encoded protein. Cell 1990, 60, 971–980. [Google Scholar] [CrossRef]
- Kita, H.; Matsumura, S.; He, X.S.; Ansari, A.A.; Lian, Z.X.; Van de Water, J.; Coppel, R.L.; Kaplan, M.M.; Gershwin, M.E. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J. Clin. Invest. 2002, 109, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Berard, M.; Mondière, P.; Casamayor-Pallejà, M.; Hennino, A.; Bella, C.; Defrance, T. Mitochondria connects the antigen receptor to effector caspases during B cell receptor-induced apoptosis in normal human B cells. J. Immunol. 1999, 163, 4655–4662. [Google Scholar] [PubMed]
- Marrache, S.; Tundup, S.; Harn, D.A.; Dhar, S. Ex Vivo Generation of functional immune cells by mitochondria-targeted photosensitization of cancer cells. Methods Mol. Biol. 2015, 2, 113–122. [Google Scholar]
- Marrache, S.; Choi, J.H.; Tundup, S.; Zaver, D.; Harn, D.A.; Dhar, S. Immune stimulating photoactive hybrid nanoparticles for metastatic breast cancer. Integr. Biol. 2013, 5, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Singh, L.; Selvarajan, V.; Chng, W.J.; Ng, S.; Tan, N.; Ho, B.; Chen, J.; Ding, J.L. T-cell death following immune activation is mediated by mitochondria-localized SARM. Cell Death Differ. 2013, 20, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Van de Water, J.; Ansari, A.; Surh, C.; Coppel, R.; Roche, T.; Bonkovsky, H.; Kaplan, M.; Gershwin, M. Evidence for the targeting by 2-oxo-dehydrogenase enzymes in the T cell response of primary biliary cirrhosis. J. Immunol. 1991, 146, 89–94. [Google Scholar] [PubMed]
- Murphy, M.P.; Siegel, R.M. Mitochondrial ROS fire up T cell activation. Immunity 2013, 38, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Storni, T.; Kündig, T.M.; Senti, G.; Johansen, P. Immunity in response to particulate antigen-delivery systems. Adv. Drug Deliv. Rev. 2005, 57, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Jewell, C.M.; López, S.C.; Irvine, D.J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl. Acad. Sci. USA 2011, 108, 15745–15750. [Google Scholar] [CrossRef] [PubMed]
- Demento, S.L.; Cui, W.; Criscione, J.M.; Stern, E.; Tulipan, J.; Kaech, S.M.; Fahmy, T.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012, 33, 4957–4964. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, S.; Kleinovink, J.W.; Fransen, M.F.; Mezzanotte, L.; Gold, H.; Wisse, P.; Overkleeft, H.; Amidi, M.; Jiskoot, W.; Löwik, C.W. Near-infrared labeled, ovalbumin loaded polymeric nanoparticles based on a hydrophilic polyester as model vaccine: In vivo tracking and evaluation of antigen-specific CD8+ T cell immune response. Biomaterials 2015, 37, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, S.; Fransen, M.F.; Kleinovink, J.W.; Christensen, J.R.; Amidi, M.; Hennink, W.E.; Ossendorp, F. Polymeric nanoparticles for co-delivery of synthetic long peptide antigen and poly IC as therapeutic cancer vaccine formulation. J. Control. Release 2015, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; Tacken, P.J.; Fokkink, R.; Joosten, B.; Stuart, M.C.; Albericio, F.; Torensma, R.; Figdor, C.G. Targeted PLGA nano-but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J. Control. Release 2010, 144, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.S.; Cao, M.; Wong, W.W.; Fischer, K.P.; Addison, W.R.; Kwon, G.S.; Tyrrell, D.L.; Samuel, J. Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine delivery. J. Control. Release 2005, 102, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Wendorf, J.; Chesko, J.; Kazzaz, J.; Ugozzoli, M.; Vajdy, M.; O’Hagan, D.; Singh, M. A comparison of anionic nanoparticles and microparticles as vaccine delivery systems. Hum. Vaccin. 2008, 4, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Solbrig, C.; Saucier-Sawyer, J.; Cody, V.; Saltzman, W.; Hanlon, D. Polymer nanoparticles for immunotherapy from encapsulated tumor-associated antigens and whole tumor cells. Mol. Pharm. 2007, 4, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Diwan, M.; Elamanchili, P.; Cao, M.; Samuel, J. Dose sparing of CpG oligodeoxynucleotide vaccine adjuvants by nanoparticle delivery. Curr. Drug Deliv. 2004, 1, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, S.; Molavi, O.; Ma, Z.; Haddadi, A.; Alshamsan, A.; Gobti, Z.; Elhasi, S.; Samuel, J.; Lavasanifar, A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine 2008, 26, 5046–5057. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; Tacken, P.J.; Fokkink, R.; Figdor, C.G. The influence of PEG chain length and targeting moiety on antibody-mediated delivery of nanoparticle vaccines to human dendritic cells. Biomaterials 2011, 32, 6791–6803. [Google Scholar] [CrossRef] [PubMed]
- Bivas-Benita, M.; Lin, M.Y.; Bal, S.M.; van Meijgaarden, K.E.; Franken, K.L.; Friggen, A.H.; Junginger, H.E.; Borchard, G.; Klein, M.R.; Ottenhoff, T.H. Pulmonary delivery of DNA encoding Mycobacterium tuberculosis latency antigen Rv1733c associated to PLGA–PEI nanoparticles enhances T cell responses in a DNA prime/protein boost vaccination regimen in mice. Vaccine 2009, 27, 4010–4017. [Google Scholar] [CrossRef] [PubMed]
- Prego, C.; Paolicelli, P.; Díaz, B.; Vicente, S.; Sánchez, A.; González-Fernández, Á.; Alonso, M.J. Chitosan-based nanoparticles for improving immunization against hepatitis B infection. Vaccine 2010, 28, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Barzegar-Jalali, M. Nanovaccine for leishmaniasis: Preparation of chitosan nanoparticles containing Leishmania superoxide dismutase and evaluation of its immunogenicity in BALB/c mice. Int. J. Nanomedicine 2011, 6, 835–842. [Google Scholar]
- Vila, A.; Sánchez, A.; Janes, K.; Behrens, I.; Kissel, T.; Jato, J.L.; Alonso, M.J. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur. J. Pharm. Biopharm. 2004, 57, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Correia-Pinto, J.F.; Csaba, N.; Schiller, J.T.; Alonso, M.J. Chitosan-poly (I: C)-PADRE based nanoparticles as delivery vehicles for synthetic peptide vaccines. Vaccines 2015, 3, 730–750. [Google Scholar] [CrossRef] [PubMed]
- Sarti, F.; Perera, G.; Hintzen, F.; Kotti, K.; Karageorgiou, V.; Kammona, O.; Kiparissides, C.; Bernkop-Schnürch, A. In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A. Biomaterials 2011, 32, 4052–4057. [Google Scholar] [CrossRef] [PubMed]
- Ataman-Önal, Y.; Munier, S.; Ganée, A.; Terrat, C.; Durand, P.Y.; Battail, N.; Martinon, F.; Le Grand, R.; Charles, M.H.; Delair, T. Surfactant-free anionic PLA nanoparticles coated with HIV-1 p24 protein induced enhanced cellular and humoral immune responses in various animal models. J. Control. Release 2006, 112, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Yoshii, H.; Akagi, T.; Akashi, M.; Ishikawa, T.; Okuno, Y.; Takahashi, M.; Yamanishi, K.; Mori, Y. Influenza hemagglutinin vaccine with poly (γ-glutamic acid) nanoparticles enhances the protection against influenza virus infection through both humoral and cell-mediated immunity. Vaccine 2007, 25, 8270–8278. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Pandit, S.; Bramwell, V.W.; Alpar, H.O. Diphtheria toxoid loaded poly-(ε-caprolactone) nanoparticles as mucosal vaccine delivery systems. Methods 2006, 38, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Hirosue, S.; Kourtis, I.C.; van der Vlies, A.J.; Hubbell, J.A.; Swartz, M.A. Antigen delivery to dendritic cells by poly (propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and T cell activation. Vaccine 2010, 28, 7897–7906. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Rawat, A.; Hope-Weeks, L.; Ahsan, F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharm. 2011, 8, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Marrache, S.; Dhar, S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc. Natl. Acad. Sci. USA 2012, 109, 16288–16293. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Goyal, A.K.; Mishra, N.; Vaidya, B.; Mangal, S.; Vyas, S.P. PEG-PLA-PEG block copolymeric nanoparticles for oral immunization against hepatitis B. Int. J. Pharm. 2010, 387, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Dai, M.; Li, X.; Yang, L.; Huang, M.; Wang, Y.; Kan, B.; Lu, Y.; Wei, Y.; Qian, Z. Preparation of mannan modified anionic PCL–PEG–PCL nanoparticles at one-step for bFGF antigen delivery to improve humoral immunity. Colloids Surf. B: Biointerfaces 2008, 64, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Slütter, B.; Bal, S.; Keijzer, C.; Mallants, R.; Hagenaars, N.; Que, I.; Kaijzel, E.; van Eden, W.; Augustijns, P.; Löwik, C. Nasal vaccination with N-trimethyl chitosan and PLGA based nanoparticles: Nanoparticle characteristics determine quality and strength of the antibody response in mice against the encapsulated antigen. Vaccine 2010, 28, 6282–6291. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ellens, H.; Bentz, J.; Szoka, F.C. Fusion of phosphatidylethanolamine-containing liposomes and mechanism of L. alpha.-HII phase transition. Biochemistry 1986, 25, 4141–4147. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Mumper, R.J. The effect of co-administration of adjuvants with a nanoparticle-based genetic vaccine delivery system on the resulting immune responses. Eur. J. Pharm. Biopharm. 2003, 55, 11–18. [Google Scholar] [CrossRef]
- Krishnamachari, Y.; Geary, S.M.; Lemke, C.D.; Salem, A.K. Nanoparticle delivery systems in cancer vaccines. Pharm. Res. 2011, 28, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Diebold, Y.; Jarrín, M.; Sáez, V.; Carvalho, E.L.; Orea, M.; Calonge, M.; Seijo, B.; Alonso, M.J. Ocular drug delivery by liposome–chitosan nanoparticle complexes (LCS-NP). Biomaterials 2007, 28, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.M.; Chung, Y.C.; Hwang, J.H. Enhanced adjuvantic property of polymerized liposome as compared to a phospholipid liposome. J. Biotechnol. 2002, 94, 255–263. [Google Scholar] [CrossRef]
- Chen, H.; Torchilin, V.; Langer, R. Polymerized liposomes as potential oral vaccine carriers: Stability and bioavailability. J. Control. Release 1996, 42, 263–272. [Google Scholar] [CrossRef]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Mishra, D.; Khan, S.; Varshney, S.K.; Banerjee, S.; Mishra, P.K. Assessment of tumor antigen-loaded solid lipid nanoparticles as an efficient delivery system for dendritic cell engineering. Nanomedicine 2013, 8, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Meidenbauer, N.; Harris, D.; Spitler, L.; Whiteside, T. Generation of PSA-reactive effector cells after vaccination with a PSA-based vaccine in patients with prostate cancer. Prostate 2000, 43, 88–100. [Google Scholar] [CrossRef]
- North, S.A.; Graham, K.; Bodnar, D.; Venner, P. A pilot study of the liposomal MUC1 vaccine BLP25 in prostate specific antigen failures after radical prostatectomy. J. Urol. 2006, 176, 91–95. [Google Scholar] [CrossRef]
- Butts, C.; Socinski, M.A.; Mitchell, P.L.; Thatcher, N.; Havel, L.; Krzakowski, M.; Nawrocki, S.; Ciuleanu, T.E.; Bosquée, L.; Trigo, J.M.; et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 59–68. [Google Scholar] [CrossRef]
- Villa, C.H.; Dao, T.; Ahearn, I.; Fehrenbacher, N.; Casey, E.; Rey, D.A.; Korontsvit, T.; Zakhaleva, V.; Batt, C.A.; Philips, M.R. Single-walled carbon nanotubes deliver peptide antigen into dendritic cells and enhance IgG responses to tumor-associated antigens. ACS Nano 2011, 5, 5300–5311. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhao, L.; Zhang, S.; Zhang, F.; Dong, M.; Xu, S. Morphologies, preparations and applications of layered double hydroxide micro-/nanostructures. Materials 2010, 3, 5220–5235. [Google Scholar] [CrossRef]
- Xu, Z.P.; Niebert, M.; Porazik, K.; Walker, T.L.; Cooper, H.M.; Middelberg, A.P.; Gray, P.P.; Bartlett, P.F.; Lu, G.Q. Subcellular compartment targeting of layered double hydroxide nanoparticles. J. Control. Release 2008, 130, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Rolfe, B.E.; Zhang, B.; Mohammed, Y.H.; Gu, W.; Xu, Z.P. Polarized immune responses modulated by layered double hydroxides nanoparticle conjugated with CpG. Biomaterials 2014, 35, 9508–9516. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, R.; Gao, B.; Wu, B.; Li, K.; Sun, X.; Liu, H.; Wang, S. The enhanced immune response of hepatitis B virus DNA vaccine using SiO2@LDH nanoparticles as an adjuvant. Biomaterials 2014, 35, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Christopher, M.E.; Nagata, L.P.; Zabielski, M.A.; Li, H.; Wong, J.P.; Samuel, J. Intranasal immunization with liposome-encapsulated plasmid DNA encoding influenza virus hemagglutinin elicits mucosal, cellular and humoral immune responses. J. Clin. Virol. 2004, 31, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Matsuoka, F.; Honda, H.; Kobayashi, T. Antitumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Immunol. Immunother. 2004, 53, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Echechipia, S.; Garcia, B.; Tabar, A.; Martin, S.; Rico, P.; Olaguibel, J. Liposome-entrapped D. pteronyssinus vaccination in mild asthma patients: Effect of 1-year double-blind, placebo-controlled trial on inflammation, bronchial hyper-responsiveness and immediate and late bronchial responses to the allergen. Clin. Exp. Allergy 2002, 32, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Rosada, R.S.; de la Torre, L.G.; Frantz, F.G.; Trombone, A.P.; Zárate-Bladés, C.R.; Fonseca, D.M.; Souza, P.R.; Brandão, I.T.; Masson, A.P.; Soares, É.G.; et al. Protection against tuberculosis by a single intranasal administration of DNA-hsp65 vaccine complexed with cationic liposomes. BMC Immunol. 2008, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Just, M.; Berger, R.; Drechsler, H.; Brantschen, S.; Glück, R. A single vaccination with an inactivated hepatitis A liposome vaccine induces protective antibodies after only two weeks. Vaccine 1992, 10, 737–739. [Google Scholar] [CrossRef]
- Zheng, L.; Huang, X.L.; Fan, Z.; Borowski, L.; Wilson, C.C.; Rinaldo, C.R. Delivery of liposome-encapsulated HIV type 1 proteins to human dendritic cells for stimulation of HIV type 1-specific memory cytotoxic T lymphocyte responses. AIDS Res. Hum. Retroviruses 1999, 15, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.D.; de Jong, S.D.; Tam, Y.K. Lipid-based delivery of CpG oligonucleotides enhances immunotherapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Huang, L. Liposome-polycation-DNA (LPD) particle as a carrier and adjuvant for protein-based vaccines: Therapeutic effect against cervical cancer. Cancer Immunol. Immunother. 2005, 54, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kolar, S.S.; Zao, M.; McDermott, A.M.; Cai, C. Localization of antimicrobial peptides on polymerized liposomes leading to their enhanced efficacy against Pseudomonas aeruginosa. Mol. BioSyst. 2011, 7, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Kossovsky, N.; Gelman, A.; Hnatyszyn, H.J.; Rajguru, S.; Garrell, R.L.; Torbati, S.; Freitas, S.S.; Chow, G.M. Surface-modified diamond nanoparticles as antigen delivery vehicles. Bioconjugate Chem. 1995, 6, 507–511. [Google Scholar] [CrossRef]
- Mendonça, E.; Diniz, M.; Silva, L.; Peres, I.; Castro, L.; Correia, J.B.; Picado, A. Effects of diamond nanoparticle exposure on the internal structure and reproduction of Daphnia magna. J. Hazard Mater. 2011, 186, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Safari, D.; Marradi, M.; Chiodo, F.; Th Dekker, H.A.; Shan, Y.; Adamo, R.; Oscarson, S.; Rijkers, G.T.; Lahmann, M.; Kamerling, J.P. Gold nanoparticles as carriers for a synthetic Streptococcus pneumoniae type 14 conjugate vaccine. Nanomedicine 2012, 7, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Kwon, H.K.; An, S.; Kim, D.; Kim, S.; Yu, M.K.; Lee, J.H.; Lee, T.S.; Im, S.H.; Jon, S. Imageable Antigen-Presenting Gold Nanoparticle Vaccines for Effective Cancer Immunotherapy In Vivo. Angew. Chem. 2012, 124, 8930–8935. [Google Scholar] [CrossRef]
- Rogers, J.V.; Parkinson, C.V.; Choi, Y.W.; Speshock, J.L.; Hussain, S.M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 2008, 3, 129–133. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Neutra, M.R.; Robey, F.A. Peptomer aluminum oxide nanoparticle conjugates as systemic and mucosal vaccine candidates: Synthesis and characterization of a conjugate derived from the C4 domain of HIV-1MN gp120. Bioconjugate Chem. 1997, 8, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Akagi, T.; Toyama, M.; Nishi, Y.; Shima, F.; Akashi, M.; Baba, M. Comparative activity of biodegradable nanoparticles with aluminum adjuvants: Antigen uptake by dendritic cells and induction of immune response in mice. Immunol. Lett. 2011, 140, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Suh, H.; Bershteyn, A.; Stephan, M.T.; Liu, H.; Huang, B.; Sohail, M.; Luo, S.; Um, S.H.; Khant, H. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011, 10, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Plummer, E.M.; Manchester, M. Viral nanoparticles and virus-like particles: Platforms for contemporary vaccine design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 174–196. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Laliberté-Gagné, M.È.; Babin, C.; Bolduc, M.; Guérin, A.; Drouin, K.; Forget, M.A.; Majeau, N.; Lapointe, R.; Leclerc, D. Improvement of the PapMV nanoparticle adjuvant property through an increased of its avidity for the antigen [influenza NP]. Vaccine 2012, 30, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Okuno, J.; Maehashi, K.; Kerman, K.; Takamura, Y.; Matsumoto, K.; Tamiya, E. Label-free immunosensor for prostate-specific antigen based on single-walled carbon nanotube array-modified microelectrodes. Biosens. Bioelectron. 2007, 22, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, H.; Zhao, Q.; Wang, S.; Zou, M.; Cheng, G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: Effect of silica architecture on immunological properties. Int. J. Pharm. 2012, 436, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Kato, H.; Maeyama, J.I.; Eto, K.; Yoshihara, S. Studies on the toxicities of aluminium hydroxide and calcium phosphate as immunological adjuvants for vaccines. Vaccine 1993, 11, 914–918. [Google Scholar] [CrossRef]
- Aggerbeck, H.; Fenger, C.; Heron, I. Booster vaccination against diphtheria and tetanus in man. Comparison of calcium phosphate and aluminium hydroxide as adjuvants—II. Vaccine 1995, 13, 1366–1374. [Google Scholar] [CrossRef]
- He, Q.; Mitchell, A.; Morcol, T.; Bell, S.J. Calcium phosphate nanoparticles induce mucosal immunity and protection against herpes simplex virus type 2. Clin. Diagn. Lab. Immunol. 2002, 9, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.S.; Peek, L.J.; Power, J.; Markham, A.; Yazzie, B.; Middaugh, C.R. Effects of adsorption to aluminum salt adjuvants on the structure and stability of model protein antigens. J. Biol. Chem. 2005, 280, 13406–13414. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Fisher, K.; Smith, J.G.; Chen, F.; Tobery, T.W.; Ulmer, J.B.; Evans, R.K.; Caulfield, M.J. Enhanced type I immune response to a hepatitis B DNA vaccine by formulation with calcium- or aluminum phosphate. Vaccine 2000, 18, 1227–1235. [Google Scholar] [CrossRef]
- Ludwig, C.; Wagner, R. Virus-like particles—universal molecular toolboxes. Curr. Opin. Biotechnol. 2007, 18, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Labbe, M.; Cohen, J.; Burroughs, M.H.; Zhou, Y.J.; Estes, M.K. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J. Virol. 1994, 68, 5945–5952. [Google Scholar] [PubMed]
- Storni, T.; Ruedl, C.; Schwarz, K.; Schwendener, R.A.; Renner, W.A.; Bachmann, M.F. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J. Immunol. 2004, 172, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Gahéry-Ségard, H.; Pialoux, G.; Charmeteau, B.; Sermet, S.; Poncelet, H.; Raux, M.; Tartar, A.; Lévy, J.P.; Gras-Masse, H.; Guillet, J.G. Multiepitopic B-and T-cell responses induced in humans by a human immunodeficiency virus type 1 lipopeptide vaccine. J. Virol. 2000, 74, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Klinguer, C.; David, D.; Kouach, M.; Wieruszeski, J.M.; Tartar, A.; Marzin, D.; Levy, J.P.; Gras-Masse, H. Characterization of a multi-lipopeptides mixture used as an HIV-1 vaccine candidate. Vaccine 1999, 18, 259–267. [Google Scholar] [CrossRef]
- BenMohamed, L.; Wechsler, S.L.; Nesburn, A.B. Lipopeptide vaccines—yesterday, today, and tomorrow. Lancet Infect. Dis. 2002, 2, 425–431. [Google Scholar] [CrossRef]
- Minigo, G.; Scholzen, A.; Tang, C.K.; Hanley, J.C.; Kalkanidis, M.; Pietersz, G.A.; Apostolopoulos, V.; Plebanski, M. Poly-l-lysine-coated nanoparticles: A potent delivery system to enhance DNA vaccine efficacy. Vaccine 2007, 25, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Locher, C.P.; Putnam, D.; Langer, R.; Witt, S.A.; Ashlock, B.M.; Levy, J.A. Enhancement of a human immunodeficiency virus env DNA vaccine using a novel polycationic nanoparticle formulation. Immunol. Lett. 2003, 90, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Qian, F.; He, X.; Wang, F.; Ren, D.; He, Y.; Li, K.; Sun, S.; Yin, C. Novel chitosan derivative nanoparticles enhance the immunogenicity of a DNA vaccine encoding hepatitis B virus core antigen in mice. J. Gene Med. 2007, 9, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Gómez, S.; Gamazo, C.; San Roman, B.; Ferrer, M.; Sanz, M.L.; Espuelas, S.; Irache, J.M. Allergen immunotherapy with nanoparticles containing lipopolysaccharide from Brucella ovis. Eur. J. Pharm. Biopharm. 2008, 70, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Persing, D.H.; Coler, R.N.; Lacy, M.J.; Johnson, D.A.; Baldridge, J.R.; Hershberg, R.M.; Reed, S.G. Taking toll: Lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 2002, 10, 32–37. [Google Scholar] [CrossRef]
- Li, A.; Qin, L.; Wang, W.; Zhu, R.; Yu, Y.; Liu, H.; Wang, S. The use of layered double hydroxides as DNA vaccine delivery vector for enhancement of anti-melanoma immune response. Biomaterials 2011, 32, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Marrache, S.; Harn, D.A.; Dhar, S. Mito-DCA: A mitochondria targeted molecular scaffold for efficacious delivery of metabolic modulator dichloroacetate. ACS Chem. Biol. 2014, 9, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Dodwadkar, N.S.; Sawant, R.R.; Koshkaryev, A.; Torchilin, V.P. Surface modification of liposomes with rhodamine-123-conjugated polymer results in enhanced mitochondrial targeting. J. Drug Target. 2011, 19, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Midgley, M.; Thompson, C. The role of mitochondria in the uptake of methyltriphenylphosphonium ion by Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1985, 26, 311–315. [Google Scholar] [CrossRef]
- Murphy, M.P. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta 2008, 1777, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xing, D.; Wu, B.; Wu, S.; Ou, Z.; Chen, W.R. New insights of transmembranal mechanism and subcellular localization of noncovalently modified single-walled carbon nanotubes. Nano Lett. 2010, 10, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.B. Mitochondrial membrane potential in living cells. Annu. Rev. Cell Biol. 1988, 4, 155–181. [Google Scholar] [CrossRef] [PubMed]
- Appleby, R.D.; Porteous, W.K.; Hughes, G.; James, A.M.; Shannon, D.; Wei, Y.H.; Murphy, M.P. Quantitation and origin of the mitochondrial membrane potential in human cells lacking mitochondrial DNA. Eur. J. Biochem. 1999, 262, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Schneider Berlin, K.R.; Ammini, C.V.; Rowe, T.C. Dequalinium induces a selective depletion of mitochondrial DNA from HeLa human cervical carcinoma cells. Exp. Cell Res. 1998, 245, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Lizano, C.; Torchilin, V.P. Micellar delivery system for dequalinium-A lipophilic cationic drug with anticarcinoma activity. J. Liposome Res. 1998, 8, 391–400. [Google Scholar] [CrossRef]
- Wang, X.X.; Li, Y.B.; Yao, H.J.; Ju, R.J.; Zhang, Y.; Li, R.J.; Yu, Y.; Zhang, L.; Lu, W.L. The use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cells. Biomaterials 2011, 32, 5673–5687. [Google Scholar] [CrossRef] [PubMed]
- Deocaris, C.C.; Widodo, N.; Shrestha, B.G.; Kaur, K.; Ohtaka, M.; Yamasaki, K.; Kaul, S.C.; Wadhwa, R. Mortalin sensitizes human cancer cells to MKT-077-induced senescence. Cancer Lett. 2007, 252, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Fantin, V.R.; Leder, P. F16, a mitochondriotoxic compound, triggers apoptosis or necrosis depending on the genetic background of the target carcinoma cell. Cancer Res. 2004, 64, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Li, D.W.; Qi, Z.D.; Jiang, F.L.; Ge, Y.S.; Liu, Y. Synthesis of F16 conjugated with 5-fluorouracil and biophysical investigation of its interaction with bovine serum albumin by a spectroscopic and molecular modeling approach. Luminescence 2013, 28, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.L.; Ruhayel, R.A.; Barnard, P.J.; Baker, M.V.; Berners-Price, S.J.; Filipovska, A. Mitochondria-targeted chemotherapeutics: The rational design of gold (I) N-heterocyclic carbene complexes that are selectively toxic to cancer cells and target protein selenols in preference to thiols. J. Am. Chem. Soc. 2008, 130, 12570–12571. [Google Scholar] [CrossRef] [PubMed]
- Marrache, S.; Pathak, R.K.; Dhar, S. Formulation and optimization of mitochondria-targeted polymeric nanoparticles. Methods Mol. Biol. 2015, 2, 103–112. [Google Scholar]
- Marrache, S.; Pathak, R.K.; Dhar, S. Detouring of cisplatin to access mitochondrial genome for overcoming resistance. Proc. Natl. Acad. Sci. USA 2014, 111, 10444–10449. [Google Scholar] [CrossRef] [PubMed]
- Feldhaeusser, B.; Platt, S.R.; Marrache, S.; Kolishetti, N.; Pathak, R.K.; Montgomery, D.J.; Reno, L.R.; Howerth, E.; Dhar, S. Evaluation of nanoparticle delivered cisplatin in beagles. Nanoscale 2015, 7, 13822–13830. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Dhar, S. A Nanoparticle Cocktail: Temporal Release of Predefined Drug Combinations. J. Am. Chem. Soc. 2015, 137, 8324–8327. [Google Scholar] [CrossRef] [PubMed]
- Kalathil, A.A.; Kumar, A.; Banik, B.; Ruiter, T.A.; Pathak, R.K.; Dhar, S. New formulation of old aspirin for better delivery. Chem. Commun. 2016, 52, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Marrache, S.; Dhar, S. The energy blocker inside the power house: Mitochondria targeted delivery of 3-bromopyruvate. Chem. Sci. 2015, 6, 1832–1845. [Google Scholar] [CrossRef] [PubMed]
- Marrache, S.; Dhar, S. Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc. Natl. Acad. Sci. USA 2013, 110, 9445–9450. [Google Scholar] [CrossRef] [PubMed]
- Omura, T. Mitochondria-targeting sequence, a multi-role sorting sequence recognized at all steps of protein import into mitochondria. J. Biochem. 1998, 123, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Neupert, W. Protein import into mitochondria. Annu. Rev. Biochem. 1997, 66, 863–917. [Google Scholar] [CrossRef] [PubMed]
- Kalafut, D.; Anderson, T.N.; Chmielewski, J. Mitochondrial targeting of a cationic amphiphilic polyproline helix. Bioorg. Med. Chem. Lett. 2012, 22, 561–563. [Google Scholar] [CrossRef] [PubMed]
- von Heijne, G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986, 5, 1335–1342. [Google Scholar] [PubMed]
- Yousif, L.F.; Stewart, K.M.; Kelley, S.O. Targeting Mitochondria with Organelle-Specific Compounds: Strategies and Applications. ChemBioChem 2009, 10, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Metón, I.; Egea, M.; Fernández, F.; Eraso, M.A.; Baanante, I.V. The N-terminal sequence directs import of mitochondrial alanine aminotransferase into mitochondria. FEBS Lett. 2004, 566, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Shokolenko, I.N.; Alexeyev, M.F.; LeDoux, S.P.; Wilson, G.L. TAT-mediated protein transduction and targeted delivery of fusion proteins into mitochondria of breast cancer cells. DNA Repair 2005, 4, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Takaya, K.; Higuchi, Y.; Kitamoto, K.; Arioka, M. A cytosolic phospholipase A2-like protein in the filamentous fungus Aspergillus oryzae localizes to the intramembrane space of the mitochondria. FEMS Microbiol. Lett. 2009, 301, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, N.D.; Zaika, A.; Moll, U.M. Death signal-induced localization of p53 protein to mitochondria a potential role in apoptotic signaling. J. Biol. Chem. 2000, 275, 16202–16212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhao, G.M.; Wu, D.; Soong, Y.; Birk, A.V.; Schiller, P.W.; Szeto, H.H. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J. Biol. Chem. 2004, 279, 34682–34690. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; Stauffer, C.; Zhao, K.; Yang, H.; Sharma, V.K.; Szeto, H.H.; Suthanthiran, M. Mitochondrial targeting with antioxidant peptide SS-31 prevents mitochondrial depolarization, reduces islet cell apoptosis, increases islet cell yield, and improves posttransplantation function. J. Am. Soc. Nephrol. 2007, 18, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Xun, Z.; Rivera-Sánchez, S.; Ayala-Peña, S.; Lim, J.; Budworth, H.; Skoda, E.M.; Robbins, P.D.; Niedernhofer, L.J.; Wipf, P.; McMurray, C.T. Targeting of XJB-5-131 to mitochondria suppresses oxidative DNA damage and motor decline in a mouse model of huntington’s disease. Cell Rep. 2012, 2, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Wipf, P.; Xiao, J.; Jiang, J.; Belikova, N.A.; Tyurin, V.A.; Fink, M.P.; Kagan, V.E. Mitochondrial targeting of selective electron scavengers: Synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J. Am. Chem. Soc. 2005, 127, 12460–12461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Belikova, N.A.; Hoye, A.T.; Zhao, Q.; Epperly, M.W.; Greenberger, J.S.; Wipf, P.; Kagan, V.E. A mitochondria-targeted nitroxide/hemigramicidin S conjugate protects mouse embryonic cells against gamma irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Robin, M.A.; Anandatheerthavarada, H.K.; Fang, J.K.; Cudic, M.; Otvos, L.; Avadhani, N.G. Mitochondrial targeted cytochrome P450 2E1 (P450 MT5) contains an intact N terminus and requires mitochondrial specific electron transfer proteins for activity. J. Biol. Chem. 2001, 276, 24680–24689. [Google Scholar] [CrossRef] [PubMed]
- Nargund, A.M.; Pellegrino, M.W.; Fiorese, C.J.; Baker, B.M.; Haynes, C.M. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 2012, 337, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, L.; He, X.; Yi, Q.; He, B.; Cao, J.; Pan, W.; Gu, Z. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-response. Biomaterials 2015, 52, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Okumura, S.; Katayama, S.; Hirose, H.; Pujals, S.; Yamaguchi, H.; Arakawa, S.; Shimizu, S.; Futaki, S. Transformation of an antimicrobial peptide into a plasma membrane-permeable, mitochondria-targeted peptide via the substitution of lysine with arginine. Chem. Commun. 2012, 48, 11097–11099. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Flierl, A.; Jackson, C.; Cottrell, B.; Murdock, D.; Seibel, P.; Wallace, D.C. Targeted delivery of DNA to the mitochondrial compartment via import sequence-conjugated peptide nucleic acid. Mol. Ther. 2003, 7, 550–557. [Google Scholar] [CrossRef]
- Horton, K.L.; Stewart, K.M.; Fonseca, S.B.; Guo, Q.; Kelley, S.O. Mitochondria-penetrating peptides. Chem. Biol. 2008, 15, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, E.; Yamada, Y.; Yasuzaki, Y.; Hyodo, M.; Harashima, H. Intracellular observation of nanocarriers modified with a mitochondrial targeting signal peptide. J. Biosci. Bioeng. 2013, 116, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Harashima, H. Enhancement in selective mitochondrial association by direct modification of a mitochondrial targeting signal peptide on a liposomal based nanocarrier. Mitochondrion 2013, 13, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Shedlock, D.J.; Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003, 300, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Brossart, P.; Wirths, S.; Brugger, W.; Kanz, L. Dendritic cells in cancer vaccines. Exp. Hematol. 2001, 29, 1247–1255. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Valitutti, S.; Müller, S.; Cella, M.; Padovan, E.; Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide MHC complexes. Nature 1995, 375, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gordon, J.R.; Xiang, J. Advances in dendritic cell-based vaccine of cancer. Cancer Biother. Radiopharm. 2002, 17, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, L.H. Dendritic cells in cancer immunotherapy clinical trials: Are we making progress? Front. Immunol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, M.; Ligeza-Poisson, C.; Juge-Morineau, N.; Spisek, R. Anti-cancer therapy using dendritic cells and apoptotic tumour cells: Pre-clinical data in human mesothelioma and acute myeloid leukaemia. Vaccine 2003, 21, 791–794. [Google Scholar] [CrossRef]
- Figdor, C.G.; de Vries, I.J.; Lesterhuis, W.J.; Melief, C.J. Dendritic cell immunotherapy: Mapping the way. Nat. Med. 2004, 10, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Lanzavecchia, A.; Sallusto, F. Regulation of T cell immunity by dendritic cells. Cell 2001, 106, 263–266. [Google Scholar] [CrossRef]
- Mayordomo, J.; Zorina, T.; Storkus, W.; Zitvogel, L.; Celluzzi, C.; Falo, L.; Melief, C.; Ildstad, S.; Kast, W.M.; Deleo, A. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat. Med. 1995, 1, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Herrin, V.E.; Ibrahim, R.A.; Toubaji, A.; Bernstein, S.; Dakheel, O.; Steinberg, S.M.; Abu, E.R.; Mkrtichyan, M.; Berzofsky, J.A. Pre-immature dendritic cells (PIDC) pulsed with HPV16 E6 or E7 peptide are capable of eliciting specific immune response in patients with advanced cervical cancer. J. Transl. Med. 2014, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Qin, X.; Jin, D.; Lou, W.; Wu, L.; Wang, D.; Wu, W.; Ni, X.; Mao, Z.; Kuang, T. A phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer. Clin. Exp. Med. 2012, 12, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Phuphanich, S.; Wheeler, C.J.; Rudnick, J.D.; Mazer, M.; Wang, H.; Nuno, M.A.; Richardson, J.E.; Fan, X.; Ji, J.; Chu, R.M. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol. Immunother. 2013, 62, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, I.; Irache, J.M.; Mansilla, C.; Ochoa-Repáraz, J.; Lasarte, J.J.; Gamazo, C. Poly (anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin. Vaccine Immunol. 2010, 17, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Clements, C.; Griffiths, E. The global impact of vaccines containing aluminium adjuvants. Vaccine 2002, 20, 24–33. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Jiao, J.; Hu, H.M. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat. Nanotechnol. 2011, 6, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Juarranz, Á.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Onco. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.; Stewart, F.A. Photodynamic therapy in oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, L.A.; Maciaszek, J.W.; Rock, K.L. Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J. Immunol. 2005, 175, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Plapied, L.; des Rieux, A.; Pourcelle, V.; Freichels, H.; Wascotte, V.; Vanderhaeghen, M.L.; Jerôme, C.; Vanderplasschen, A.; Marchand-Brynaert, J. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination. Eur. J. Pharm. Biopharm. 2009, 73, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Kawasaki, N.; Nycholat, C.M.; Han, S.; Pilotte, J.; Crocker, P.R.; Paulson, J.C. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS ONE 2012, 7, e39039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, K.; Li, W.; Yang, N.; Liu, Y.; Chen, C.; Wei, T. The interactions between pristine graphene and macrophages and the production of cytokines/chemokines via TLR-and NF-κB-related signaling pathways. Biomaterials 2012, 33, 6933–6942. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Thurber, A.; Hanna, C.; Punnoose, A.; Zhang, J.; Wingett, D. The Influences of Cell Type and ZnO Nanoparticle Size on Immune Cell Cytotoxicity and Cytokine Induction. Nanoscale Res. Lett. 2009, 4, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tripathi, A.; Das, M.; Dwivedi, P.D. Cytotoxicity and uptake of zinc oxide nanoparticles leading to enhanced inflammatory cytokines levels in murine macrophages: Comparison with bulk zinc oxide. J. Biomed. Nanotechnol. 2011, 7, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiao, F.; Qiu, Y.; Li, W.; Lao, F.; Zhou, G.; Sun, B.; Xing, G.; Dong, J.; Zhao, Y.; et al. The effect of Gd@C82(OH)22 nanoparticles on the release of Th1/Th2 cytokines and induction of TNF-α mediated cellular immunity. Biomaterials 2009, 30, 3934–3945. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Balachandran, Y.L.; Li, D.; Shao, Y.; Jiang, X. Polyvinylpyrrolidone-poly (ethylene glycol) modified silver nanorods can be a safe, noncarrier adjuvant for HIV vaccine. ACS Nano 2016, 10, 3589–3596. [Google Scholar] [CrossRef] [PubMed]
- Parry, A.L.; Clemson, N.A.; Ellis, J.; Bernhard, S.S.; Davis, B.G.; Cameron, N.R. Multicopy multivalent’ glycopolymer-stabilized gold nanoparticles as potential synthetic cancer vaccines. J. Am. Chem. Soc. 2013, 135, 9362–9365. [Google Scholar] [CrossRef] [PubMed]
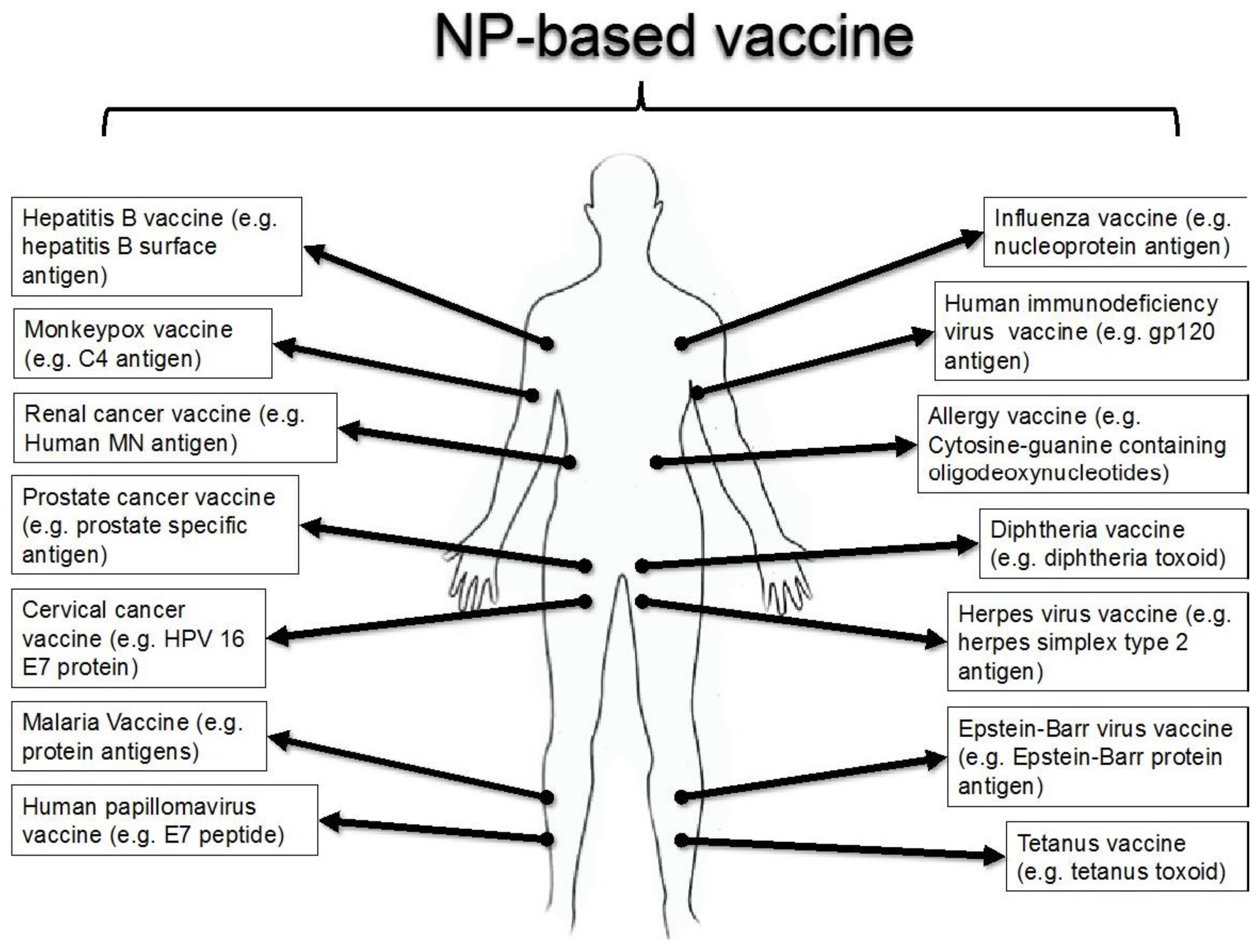
| Cell type | Possible targets | Immune response | Possible Application | Ref. |
|---|---|---|---|---|
| Dendritic cell (DC) | Mitochondrial DNA (mtDNA) | Induces CD8+, IFN-γ, T cell response specific for tumor-associated mitochondrial antigens | Cancer | [19] |
| Cytolytic T lymphocytes | mtDNA | Controls the expression of maternally transmitted antigens | Hearing impairment | [23,24] |
| Pyruvate dehydrogenase complexes | Increases CD8+ T cells for immune-pathogenesis of PBC. | Primary biliary cirrhosis | [25] | |
| B cells | Mitochondrial permeability transition pore (MPTP) | Connects the B cell antigen receptor to the effector caspases of apoptotic cell death | acute cerebral ischemia | [26] |
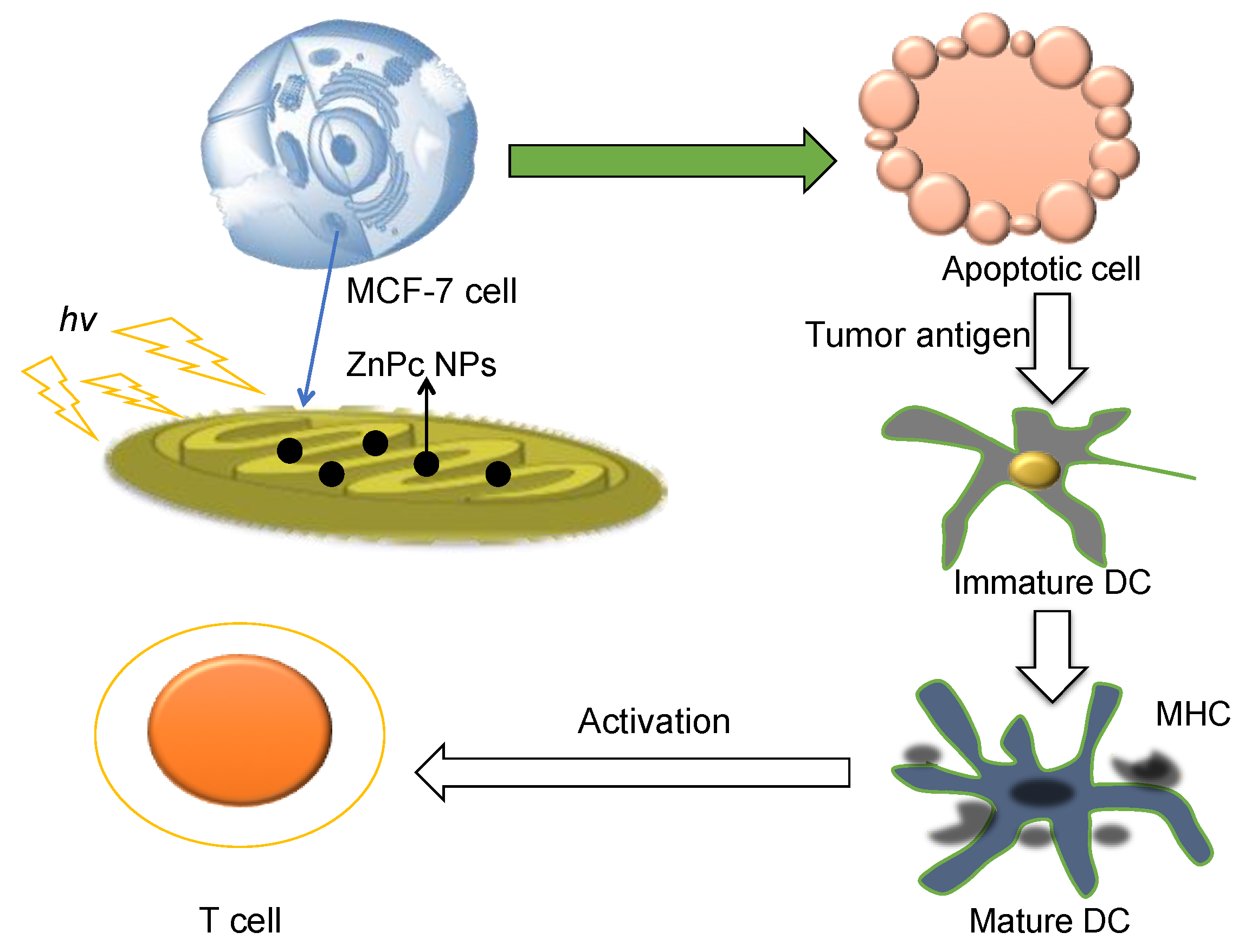
| Breast cancer cell (MCF-7) and DCs | Mitochondrial matrix (MM) | Generates the apoptotic cancer cells providing tumor antigens for immune response | Cancer | [21,27] |
| 4T1 cell | MM | Increases pro-inflammatory IL-2, IL-6, IL-12, TNF-α cytokines | Cancer | [28] |
| T cells | Bcl-xL/Bcl-2 proteins in outer mitochondrial membrane (OMM) | SARM causes T cell death by inhibiting Bcl-xL and down regulating signal-regulated kinase phosphorylation for immune homeostasis | Influenza | [29] |
| 2-oxo-dehydrogenase enzymes in inner mitochondria membrane (IMM) | Up regulates the expression of MHC class II, produces IL-2 cytokine in response to PDH-E2/BCKD-E2 | Primary biliary cirrhosis | [30] | |
| Electron transport chain (ETC) | Generates ROS for the nuclear factor of activated T cells (NFAT) and IL-2 induction | Cancer | [31] | |
| Cytolytic T lymphocytes | Pyruvate dehydrogenase complexes | Increases CD8+ T cells for immune-pathogenesis of PBC | Primary biliary cirrhosis | [25] |
| Polymer System | Preparation/Diameter (nm) | Activity/Outcome | Delivery route | Comments | Ref. |
|---|---|---|---|---|---|
| PLGA | Double emulsion method/320 nm | OVA and MPLA dual loading PLGA NPs show enhanced mucosal immune response with higher IgA titers production than individually loaded NPs. | Oral | FDA approved delivery system, (OVA +MPLA) PLGA NPs were stable up to one month. | [50] |
| PLA | Dialysis method/300–600 nm | HIV-1 p24 PLA NPs show the best CTL results, antibody production, cytokine secretion (IL-2, 4, 6, 10, INF-γ) within the controls. | Subcutaneous injection | PLA NPs were stable for months | [51] |
| PGA | Dialysis method/200 nm | The hemagglutinin (HA) loaded PGA-NPs show enhanced CTL activity and greater production of IFN-γ, IL-4, and IL-6 in vitro. NPs vaccination shows better defense to influenza virus infection in vivo than controls. | Subcutaneous injection | Low cost, safe, relatively abundance, water-soluble, biodegradable | [52] |
| PMMA | Reflux-filtration methods | HIV-1 Tat Protein loaded PMMA NPs show efficient cellular uptake, well-patterned antigen release properties, and enhanced immune responses with greater proliferation index and cytokine level (INF-γ, IL-2) compared to Tat alone. | Intramuscular | Core-shell NPs were prepared. Tat was protected from oxidation. No severe damage was observed for Tat PMMA NPs. | [53] |
| PPS | Emulsion-incubation/size was not specified | OVA loaded PPS NPs with longer peptide showed greater cellular uptake, enhanced IFN-γ secretion, and T cell activation both in vitro and in vivo. | Tail vein injection | Surfactant pluronic F127 was used to stabilize NPs, PPS NPs internalized into cell via miscellaneous pathways. | [54] |
| PLA-PLGA | Double Emulsion-solvent evaporation method/450–800 nm | HBsAg co-polymeric NPs show increased immune responses with enhanced sIgA levels and greater production of cytokines (IL-2, IFN-γ) in vivo. | Intramuscular injection via pulmonary route | To deliver hepatitis B vaccine; Certain toxicity to pulmonary epithelium still exists. Limited for oral vaccine delivery | [55] |
| PLGA-PEG-TPP | Nano-precipitation method | ZnPc loaded co-polymeric NPs showed greatly enhanced T cell activation with combination of photodynamic therapy. | Ex vivo | Copolymer is of non-immunogenic and nontoxic, and designed for mitochondria targeting delivery. | [21,56] |
| PEG-PLA-PEG | Double emulsion & solvent evaporation/215 nm | The co-polymeric NPs showed elevated immune response in vivo. Cytokine levels (IFN-γ and IL-2) were greatly enhanced. | Oral | The NPs was stable in gastric and intestinal fluids. 90% of hepatitis B antigen was encapsulated. | [57] |
| PCL–PEG–PCL | Emulsion-solvent evaporation method/137 nm | The co-polymeric NPs delivery of bFGF antigen induces better antibody production for immune response in vivo than antigen alone. | Subcutaneous injection | A few studies have been made on this co-polymeric system. | [58] |
| Chitosan | Ionotropic gelation technique/160–200 nm | rHBsAg loaded chitosan NPs induced pretty delay immune response but much greater production of IgG than conventional alum vaccines in vivo. | Intramuscular or intranasal | NPs could be damaged by centrifugation-resuspension cycles. NPs could release antigen in a well-controlled pattern. | [46] |
| Chitoson-PLGA | Emulsification-solvent extraction/448 nm | Chitoson/PLGA NPs show gradual release of OVA up to 100% in 15 days, effective cellular uptake by crossing nasal epithelium, efficient T cell proliferation and stimulation in vivo. | Nasal | NPs charge, size, and antige release properties are critical factors for vaccination. | [59] |
| Liposome Type | Example | Advantage | Disadvantage |
|---|---|---|---|
| Liposomal NP | E7 Peptide vaccinates against HPV [14] | No hypersensitivity reactions | Vulnerable to deoxyribonulease |
| Plasmid DNA vaccinates against influenza [77] | |||
| HSP70 targets tumors [78] | |||
| D. pteronyssinus vaccination againsts asthma [79] | Do not create antibodies against the phospholipid components | Do not target antigen-presenting cells well | |
| DNA-hsp65 vaccinates against tuberculosis [80] | Can release antigens over long period of time | Short systemic half life | |
| Hepatitis A virus vaccinates against Hepatitis A [81] | Potential to cross epithelial barriers | Difficulty keeping certain molecules encapsulated | |
| HIV type 1 vaccinates against AIDS [82] | Low toxicity | ||
| Solid Lipid NP | Cystosine-guanine containing oligodeoxynucleotides (CpG ODN) antigen treats allergies and inflammatory disease [83] | Stimulate a more effective immune response due to a good pharmacokinetic profile | Poor stability and biodistribution |
| Capable of reversible denaturation | Low loading capacity | ||
| Protein antigen vaccinates against hepatitis B and malaria [84] | Quick production time | Colloidal structures are present | |
| Liposome-polycation-DNA (LPD) | HPV 16 E7 protein used to vaccinate against cervical cancer and HPV [85] | Safe toxicity profile | Most effective targeting is with proteins |
| The plasmid DNA and cationic liposomes are immunostimulatory | |||
| Polymerized Liposomes | Cationic antimicrobial peptides (AMPs) vaccinates against Pseudomonas aeruginosa [86] | Stable in the GI tract | Inconsistent targeting |
| NPs | Example | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|
| Surface-Modified Diamond NPs | Mussel Adhesive Protein (MAP) antigen | Strong and specific antibody response | Studies show that the NPs may adhere to the GI tract and block gut cells | [87,88] |
| NPs have efficient surface exposure | ||||
| Gold NP | T-helper ovalbumin323–339 peptide (OVA323–339), CpG1668 oligodeoxynucleotide | Able to deliver fully synthetic carbohydrate-antigens, larger accumulation in a local lymph node | They are highly polarizable and are prone to aggregation | [89,90] |
| Silver NP | CD4 and gp120 for HIV and monkey pox | Exhibit antiviral tendencies | Tests show that these NPs aggregate in the presence of cations | [91,92] |
| Has electrostatic double layer repulsion which stabilizes dispersion | ||||
| Aluminum Oxide NP | HIV gp120 C4 antigen for HIV | Less inhibited by pinocytosis and phagocytosis once in the body | Tend to aggregate when the pH changes | [93,94] |
| Surface charge is not particularly stable | ||||
| Interbilayer-crosslinked multilamellar vesicles | VMP001- protein based malaria antigen for malaria | Elicit a powerful T-cell response | Rapid release when exposed to endolysosomal lipases | [95] |
| Papaya Mosaic Virus Capsid Protein NP (PapMV) | Nucleoprotein Antigen for influenza | Very stable NP | Only been used when working with influenza | [96,97] |
| Single Walled Carbon Nanotubes | Prostate-Specific Antigen for prostate cancer | High affinity for graphite structures | Poor survival times | [98] |
| High selectivity | ||||
| Active immune response | ||||
| Silica NP | Bovine Serum Albumin for HIV, influenza, and Hepatitis | Chemically stable, good biocompatibility, low toxicity | Ineffective for quick release | [99] |
| Calcium Phosphate Adjuvant | Mucosal delivery of herpes simplex virus type 2 antigen against the herpes virus | Very low toxicity | Tendency towards adverse reactions | [100,101,102] |
| Epstein-Barr virus proteins against Epstein-Barr virus | ||||
| Diphtheria Toxoid against Diphtheria | No detectable immunoglobulin E response | Relatively small binding capacity | ||
| Tetanus Toxoid against tetanus | ||||
| Aluminum Phosphate Adjuvant | Hepatitis B surface antigen against Hepatitis B | Enhance antibody responses in DNA vaccines | Thermal stability of the protein is reduced once absorbed | [103,104] |
| Proteins absorb well if oppositely charged | ||||
| Virus-like Particles | HPV-16/18 against human papilloma virus | Can be produced for mucosal delivery | Incapable of co-expression | [105,106,107] |
| Cheap production | ||||
| Hepatitis B core antigen against Hepatitis B | VLP size is favorable for being taken up by dendritic cells | Not readily taken up by cells other than DCs | ||
| Lipopeptides | Hepatitis B vaccine, Human immunodeficiency virus vaccine | Highly immunogenic | Require organic solvents or detergents | [108,109,110] |
| Do not need ad adjuvant | Poor stability over time | |||
| Bacterial DNA | Ovalbumin antigen against tumor growth | Activate natural killer cells | Low immunogenicity | [111,112,113] |
| Gp140 against human immunodeficiency virus | Cost efficient | DNA is subject to degradation | ||
| Hepatitis B core antigen against Hepatitis B | Non toxic | |||
| Lipopolysaccharide | Brucella against brucellosis | Biodegradable | High toxicity | [114,115] |
| Allergy vaccines | Good binding | High inflammatory response | ||
| Layered double hydroxide | Ovalbumin against tumor, DNA against melanoma | Low toxic, biocompatible, controllable antigen release | Toxic activity of LDHs still exists in in vitro and in vivo models | [75,116] |
| Targeting moiety | Examples | Outcome | Ref. |
|---|---|---|---|
| Phospholipid (PL)-PEG-NH2 | Single walled carbon nanotube functionalization (SWNT-PL-PEG) | To reduce nonspecific binding effect of SWNT surface. To improve the solubility of SWNTs in aqueous solutions. To accumulate in the mitochondria of normal and cancer cells | [121] |
| TPP+ | PLGA-PEG-TPP as carrier for ZnPc | To induce cytotoxicity in cancer cells under light irradiation, which is used to activate DCs | [21] |
| Rhodamine 123 | Liposomes-rhodamine-123-conjugated polymer | Least toxic among the liphophilic dye | [118,122] |
| Facilitate the cellular association and internalization, direct the trafficking of NPs to mitochondria, and substantial cell killing was observed as the drug cargo | |||
| Methyltriphenyl phosphonium | NA | Did not protect against cell death. | [119,123] |
| Δψm was selectively depolarized | |||
| Dequalinium (DQA) | DQA-PEG(5000)-DSPE | To cause cell death by inhibiting the mtDNA synthesis | [124,125,126] |
| DQA-PEG(2000)-DSPE) | |||
| MKT-007 | NA | A mitochondria localized cationic dye, causes selective death of cancer cells | [127] |
| F16 | F16 conjugated with 5-fluorouracil | F16 was used as a vehicle, selectively inhibits tumor cell proliferation and dissipates Δψm | [128,129] |
| N-Heterocyclic Carbene (NHC) | Gold(I)-NHC Complex | Au(I)-NHC complexes toxic to breast cancer cell (MDA-MB-231, MDA-MB-468), but not to normal cells | [130] |
| Name | Sequence | Targets | Comments | Ref. |
|---|---|---|---|---|
| Mitochondrial alanine aminotransferase (mALT) | MSATRMQLLSPRNVRLLSRGRSELFAGGSGGGPRVRSLISPPLSSSSPGRALSSVSATRRGLPKEKMTENGVSSRAKVLTIDT | Through interaction with translocases of the outer and inner mitochondrial membranes | Exhibits higher affinity for L-alanine | [143] |
| Amino acids 1–83 contains MTS | ||||
| MTS-ExoIII-TAT-fusion protein | MLSRAVCGTSRQLAPALGYLGSRQ | Mitochondrial matrix | More efficient in mtDNA damage and less repair to cancer cell | [144] |
| AoPlaA | MLSCTSPLLRGACHNMGAAKALRLRWTVPPAVLIALGSGALYTTSGQTLYYKNSVQQTD | Mitochondrial intermembrane space | It is a cytosolic phospholipase A2 (cPLA2) like protein | [145] |
| p53 Protein | MLFNLRILLNNAAFRNGHNFMVRNFRCGQPLQ | Localizes within the membrane compartment | Mitochondrial accumulation of p53 is rapid, and precedes the apoptotic cascade. | [146] |
| SS peptide | 2’,6’-dimethyltyrosine-D-Arg-Phe-Lys-NH2 | Inner mitochondrial membrane | Prevents mitochondrial depolarization | [147,148] |
| Phe-D-Arg-Phe-Lys-NH2 | ||||
| d-Arg-2’,6’-dimethyltyrosine-Lys-Phe-NH2 | ||||
| XJB-5-131 | 4-hydroxy-2,2,6,6-tetramethyl piperidine-1-oxyl conjugated to nitroxide-Leu-D-Phe-Pro-Val-Orn | Mitochondrial membrane | ROS/RNS scavenger | [149] |
| Gramicidin S | Boc-Leu-DPhe-Val-Orn(Cbz)-OMe | Mitochondrial membrane | Electron scavenger | [150] |
| Nitroxide/Hemigramicidin S Conjugate | Hemigramicidin S-4-amino-2,2,6,6-tetramethyl-piperidine-N-oxyl (hemi-GS-TEMPO) 5-125 | Accumulates at the interface of mitochondrial membrane | Acts as electron scavenger and provides the radioprotection of gamma | [151] |
| COX1291–306 | MFTVGLDVDTRTYFT | mtDNA | Stimulates the CD8+ IFN-γ+ T cell response specific for tumor-associated mitochondrial Ags | [19] |
| Cytochrome P450 2E1 (P450 MT5) | MAVLGITVALLGWMVILLFI | Mitochondrial out and inner membrane | Reacts with cytochrome P450 in mitochondria | [152] |
| Activating transcription factor associated with stress-1 (ATFS-1) | AAVAYREAARAE | Inner mitochondrial membrane | ATFS-1 is degraded in mitochondria, which helps to maintain the mitochondrial homeostasis | [153] |
| KLA peptide | D(KLAKLAK)2 | Mitochondrial membrane | KLA lysine units interact with the membranes for mitochondria uptake via hydrogen bonding and electrostatic attraction | [154] |
| RLA peptide | D[RLARLAR]2 | Mitochondrial outer membrane | The substitution of D-lysines in KLA with D-arginines improves the plasma membrane permeability and increases mitochondrial accumulation of RLA (as early as 6 min) | [155] |
| Mitochondrial open reading frame of the 12S rRNA-c (MOTS-c) | MRWQEMGYIFYPRKLR | mtDNA | 16-amino-acid peptide, which promotes metabolic homeostasis and prevents the obesity and insulin resistance | [156] |
| Y- or M-conjugate | NH2-MLSLRQSIRFFKPAT-o-o-N-TTCCTCGCTCACT-c (Y conjugate) | Matrix | Accesses into the matrix through outer and inner mitochondria protein import channels | [157] |
| NH-MALLRGVFIVAAKRTPF-o-o-N-GATTCTTCACCGT-C (M-conjugate) | ||||
| Mitochondria-penetrating peptides (MPPs) | FX-r-FX-K-FX-r-FX-K, F-r-F-K-F-r-F-K, F-r-FX-K-F-r-FX-K, F-r-Y-K-F-r-Y-K, FX-r-FX-K,F-r-F-K, F-r-FX-K, F-r-F2-K, F-r-Nap-K, F-r-Hex-K, F-r-YMe-K, F-r-FF-K, F-r-Y-K, Y-r-Y-K | Matrix | Systematic series of MPPs were studied, delivery of nonpolar species into mitochondria has been demonstrated to be successful | [158] |
| MTS-Cys peptide | NH2-MVSGSSGLAAARLLSRTFLLQQNGIRHGSYC | Mitochondrial outer membrane | MTS peptide can be enhanced slightly outer stearyl-R8 modification | [159,160] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, R.; Umeano, A.C.; Francis, L.; Sharma, N.; Tundup, S.; Dhar, S. Mitochondrion: A Promising Target for Nanoparticle-Based Vaccine Delivery Systems. Vaccines 2016, 4, 18. https://doi.org/10.3390/vaccines4020018
Wen R, Umeano AC, Francis L, Sharma N, Tundup S, Dhar S. Mitochondrion: A Promising Target for Nanoparticle-Based Vaccine Delivery Systems. Vaccines. 2016; 4(2):18. https://doi.org/10.3390/vaccines4020018
Chicago/Turabian StyleWen, Ru, Afoma C. Umeano, Lily Francis, Nivita Sharma, Smanla Tundup, and Shanta Dhar. 2016. "Mitochondrion: A Promising Target for Nanoparticle-Based Vaccine Delivery Systems" Vaccines 4, no. 2: 18. https://doi.org/10.3390/vaccines4020018
APA StyleWen, R., Umeano, A. C., Francis, L., Sharma, N., Tundup, S., & Dhar, S. (2016). Mitochondrion: A Promising Target for Nanoparticle-Based Vaccine Delivery Systems. Vaccines, 4(2), 18. https://doi.org/10.3390/vaccines4020018





