Burkholderia cepacia Complex Vaccines: Where Do We Go from here?
Abstract
:1. Introduction
2. Bcc Virulence Factors
3. Bcc Animal Models
4. Immune Response
5. Vaccines
5.1. Subunit Vaccines
5.2. Complex Carbohydrates or Polysaccharides as Vaccine Components
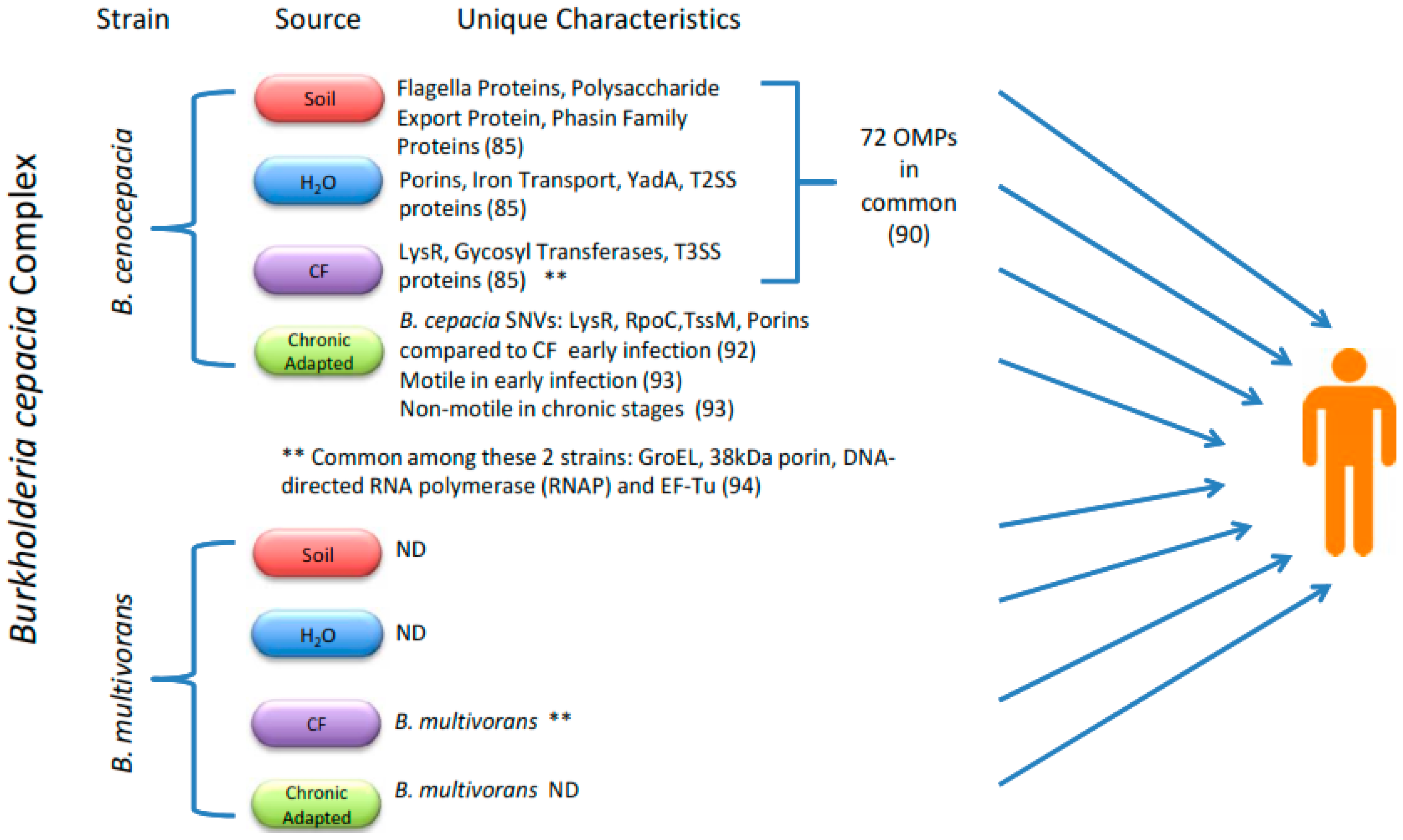
5.3. Proteomics
6. Vaccine Delivery Complications
7. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Bcc | Burkholderia cepacia Complex |
| CF | Cystic Fibrosis |
| EPS | Exopolysaccharides |
| SNVs | Single nucleotide variants |
| OMP | Outer membrane proteins |
References
- Master, E.R.; Mohn, W.W. Psychrotolerant bacteria isolated from arctic soil that degrade polychlorinated biphenyls at low temperatures. Appl. Environ. Microbiol. 1998, 64, 4823–4829. [Google Scholar] [PubMed]
- De Smet, B.; Mayo, M.; Peeters, C.; Zlosnik, J.E.; Spilker, T.; Hird, T.J.; LiPuma, J.J.; Kidd, T.J.; Kaestli, M.; Ginther, J.L.; et al. Burkholderia stagnalis sp. nov. and Burkholderia territorii sp. nov., two novel Burkholderia cepacia complex species from environmental and human sources. Int. J. Syst. Evol. Microbiol. 2015, 65, 2265–2271. [Google Scholar] [PubMed]
- Vandamme, P.; Dawyndt, P. Classification and identification of the Burkholderia cepacia complex: Past, present and future. Syst. Appl. Microbiol. 2011, 34, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Vandamme, P. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 2003, 5, 719–729. [Google Scholar] [CrossRef] [PubMed]
- LiPuma, J.J. Assessing Airway Microbiota in Cystic Fibrosis: What More Should Be Done? J. Clin. Microbiol. 2015, 53, 2006–2007. [Google Scholar] [CrossRef] [PubMed]
- CFF. Cystic Fibrosis Foundation Patient Registry Annual Data Report, 2012. Available online: http://www.cysticfibrosisdata.org/LiteratureRetrieve.aspx?ID=149756 (accessed on 8 December 2015).
- UKCFR. UK Cystic Fibrosis Registry Annual Data Report, 2013. Available online: https://www.cysticfibrosis.org.uk/~/media/documents/the-work-we-do/uk-cf-registry/2013-registry-annual-data-report.ashx (accessed on c).
- Drevinek, P.; Mahenthiralingam, E. Burkholderia cenocepacia in cystic fibrosis: Epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 2010, 16, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Courtney, J.M.; Dunbar, K.E.; McDowell, A.; Moore, J.E.; Warke, T.J.; Stevenson, M.; Elborn, J.S. Clinical outcome of Burkholderia cepacia complex infection in cystic fibrosis adults. J. Cyst. Fibros. 2004, 3, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Loutet, S.A.; Valvano, M.A. A decade of Burkholderia cenocepacia virulence determinant research. Infect. Immun. 2010, 78, 4088–4100. [Google Scholar] [CrossRef] [PubMed]
- Gautam, V.; Singhal, L.; Ray, P. Burkholderia cepacia complex: Beyond pseudomonas and acinetobacter. Indian J. Med. Microbiol. 2011, 29, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Nannini, E.C.; Ponessa, A.; Muratori, R.; Marchiaro, P.; Ballerini, V.; Flynn, L.; Limansky, A.S. Polyclonal outbreak of bacteremia caused by Burkholderia cepacia complex and the presumptive role of ultrasound gel. Braz. J. Infect. Dis. 2015, 19, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Souza Dias, M.B.; Cavassin, L.G.; Stempliuk, V.; Xavier, L.S.; Lobo, R.D.; Sampaio, J.L.; Pignatari, A.C.; Borrasca, V.L.; Bierrenbach, A.L.; Toscano, C.M. Multi-institutional outbreak of Burkholderia cepacia complex associated with contaminated mannitol solution prepared in compounding pharmacy. Am. J. Infect. Control. 2013, 41, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Vonberg, R.P.; Gastmeier, P. Hospital-acquired infections related to contaminated substances. J. Hosp. Infect. 2007, 65, 15–23. [Google Scholar]
- Ganesan, S.; Sajjan, U.S. Host evasion by Burkholderia cenocepacia. Front. Cell. Infect. Microbiol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Saldias, M.S.; Valvano, M.A. Interactions of Burkholderia cenocepacia and other Burkholderia cepacia complex bacteria with epithelial and phagocytic cells. Microbiology 2009, 155, 2809–2817. [Google Scholar] [CrossRef] [PubMed]
- Bazzini, S.; Udine, C.; Riccardi, G. Molecular approaches to pathogenesis study of Burkholderia cenocepacia, an important cystic fibrosis opportunistic bacterium. Appl. Microbiol. Biotechnol. 2011, 92, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Sokol, P.A.; Sajjan, U.; Visser, M.B.; Gingues, S.; Forstner, J.; Kooi, C. The CepIR quorum-sensing system contributes to the virulence of Burkholderia cenocepacia respiratory infections. Microbiology 2003, 149, 3649–3658. [Google Scholar] [CrossRef] [PubMed]
- Lewenza, S.; Visser, M.B.; Sokol, P.A. Interspecies communication between Burkholderia cepacia and Pseudomonas aeruginosa. Can. J. Microbiol. 2002, 48, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Tomlin, K.L.; Malott, R.J.; Ramage, G.; Storey, D.G.; Sokol, P.A.; Ceri, H. Quorum-sensing mutations affect attachment and stability of Burkholderia cenocepacia biofilms. Appl. Environ. Microbiol. 2005, 71, 5208–5218. [Google Scholar] [CrossRef] [PubMed]
- Kooi, C.; Subsin, B.; Chen, R.; Pohorelic, B.; Sokol, P.A. Burkholderia cenocepacia ZmpB is a broad-specificity zinc metalloprotease involved in virulence. Infect. Immun. 2006, 74, 4083–4093. [Google Scholar] [CrossRef] [PubMed]
- Malott, R.J.; Baldwin, A.; Mahenthiralingam, E.; Sokol, P.A. Characterization of the cciIR quorum-sensing system in Burkholderia cenocepacia. Infect. Immun. 2005, 73, 4982–4992. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, R.; Berry, V. Bacterial biofilm formation, pathogenicity, diagnostics and control: An overview. Indian J. Med. Sci. 2009, 63, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Saldias, M.S.; Lamothe, J.; Wu, R.; Valvano, M.A. Burkholderia cenocepacia requires the RpoN sigma factor for biofilm formation and intracellular trafficking within macrophages. Infect. Immun. 2008, 76, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.V.; Sousa, S.A.; Leitão, J.H.; Moreira, L.M.; Videira, P.A.; Sá-Correia, I. Studies on the involvement of the exopolysaccharide produced by cystic fibrosis-associated isolates of the Burkholderia cepacia complex in biofilm formation and in persistence of respiratory infections. J. Clin. Microbiol. 2004, 42, 3052–3058. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Morea, C.; Battistoni, A.; Sarli, S.; Cipriani, P.; Superti, F.; Ammendolia, M.G.; Valenti, P. Iron availability influences aggregation, biofilm, adhesion and invasion of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int. J. Immunopathol. Pharmacol. 2005, 18, 661–670. [Google Scholar] [PubMed]
- Riedel, K.; Hentzer, M.; Geisenberger, O.; Huber, B.; Steidle, A.; Wu, H.; Høiby, N.; Givskov, M.; Molin, S.; Eberl, L. N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 2001, 147, 3249–3262. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Abdullah, L.H.; Perlmutt, O.S.; Albert, D.; Davis, C.W.; Arnold, R.R.; Yankaskas, J.R.; Gilligan, P.; Neubauer, H.; Randell, S.H.; et al. Localization of Burkholderia cepacia complex bacteria in cystic fibrosis lungs and interactions with Pseudomonas aeruginosa in hypoxic mucus. Infect. Immun. 2014, 82, 4729–4745. [Google Scholar] [CrossRef] [PubMed]
- Aubert, D.F.; Flannagan, R.S.; Valvano, M.A. A novel sensor kinase-response regulator hybrid controls biofilm formation and type VI secretion system activity in Burkholderia cenocepacia. Infect. Immun. 2008, 76, 1979–1791. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Reyes, R.; Aubert, D.F.; Tolman, J.S.; Amer, A.O.; Valvano, M.A. Burkholderia cenocepacia type VI secretion system mediates escape of type II secreted proteins into the cytoplasm of infected macrophages. PLoS ONE 2012, 7, e41726. [Google Scholar] [CrossRef] [PubMed]
- Tomich, M.; Griffith, A.; Herfst, C.A.; Burns, J.L.; Mohr, C.D. Attenuated virulence of a Burkholderia cepacia type III secretion mutant in a murine model of infection. Infect. Immun. 2003, 71, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, S.U.; Carmody, L.A.; Gonzalez, C.F.; LiPuma, J.J. A type IV secretion system contributes to intracellular survival and replication of Burkholderia cenocepacia. Infect. Immun. 2008, 76, 5447–5455. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.A.; Kooi, C.; Sokol, P.A.; Valvano, M.A. Identification of Burkholderia cenocepacia genes required for bacterial survival in vivo. Infect. Immun. 2004, 72, 4010–4022. [Google Scholar] [CrossRef] [PubMed]
- Coburn, B.; Sekirov, I.; Finlay, B.B. Type III secretion systems and disease. Clin. Microbiol. Rev. 2007, 20, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Pati, N.B.; Vishwakarma, V.; Selvaraj, S.K.; Dash, S.; Saha, B.; Singh, N.; Suar, M. Salmonella Typhimurium TTSS-2 deficient mig-14 mutant shows attenuation in immunocompromised mice and offers protection against wild-type Salmonella Typhimurium infection. BMC Microbiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Nadal-Jimenez, P.; Cool, R.H.; Quax, W.J. Assessing Pseudomonas virulence with nonmammalian host: Galleria mellonella. Methods Mol. Biol. 2014, 1149, 681–688. [Google Scholar] [PubMed]
- Tsai, C.; Loh, J.M.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Benthall, G.; Touzel, R.E.; Hind, C.K.; Titball, R.W.; Sutton, J.M.; Thomas, R.J.; Wand, M.E. Evaluation of antibiotic efficacy against infections caused by planktonic or biofilm cultures of Pseudomonas aeruginosa and Klebsiella pneumoniae in Galleria mellonella. Int. J. Antimicrob. Agents 2015, 46, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S. Drosophila and Galleria insect model hosts: New tools for the study of fungal virulence, pharmacology and immunology. Virulence 2011, 2, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Castonguay-Vanier, J.; Vial, L.; Tremblay, J.; Déziel, E. Drosophila melanogaster as a model host for the Burkholderia cepacia complex. PLoS ONE 2010, 5, e11467. [Google Scholar] [CrossRef] [PubMed]
- Schwager, S.; Agnoli, K.; Köthe, M.; Feldmann, F.; Givskov, M.; Carlier, A.; Eberl, L. Identification of Burkholderia cenocepacia strain H111 virulence factors using nonmammalian infection hosts. Infect. Immun. 2013, 81, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Golshahi, L.; Lynch, K.H.; Dennis, J.J.; Finlay, W.H. In vitro lung delivery of bacteriophages KS4-M and PhiKZ using dry powder inhalers for treatment of Burkholderia cepacia complex and Pseudomonas aeruginosa infections in cystic fibrosis. J. Appl. Microbiol. 2011, 110, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Kamal, F.; Dennis, J.J. Burkholderia cepacia complex Phage-Antibiotic Synergy (PAS): Antibiotics stimulate lytic phage activity. Appl. Environ. Microbiol. 2015, 81, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Speert, D.P.; Steen, B.; Halsey, K.; Kwan, E. A murine model for infection with Burkholderia cepacia with sustained persistence in the spleen. Infect. Immun. 1999, 67, 4027–4032. [Google Scholar] [PubMed]
- Sousa, S.A.; Ulrich, M.; Bragonzi, A.; Burke, M.; Worlitzsch, D.; Leitão, J.H.; Meisner, C.; Eberl, L.; Sá-Correia, I.; Döring, G. Virulence of Burkholderia cepacia complex strains in gp91phox−/− mice. Cell. Microbiol. 2007, 9, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Bragonzi, A.; Farulla, I.; Paroni, M.; Twomey, K.B.; Pirone, L.; Lorè, N.I.; Bianconi, I.; Bevivino, A. Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response. PLoS ONE 2012, 7, e52330. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, U.; Thanassoulis, G.; Cherapanov, V.; Lu, A.; Sjolin, C.; Steer, B.; Wu, Y.J.; Rotstein, O.D.; Kent, G.; McKerlie, C.; et al. Enhanced susceptibility to pulmonary infection with Burkholderia cepacia in Cftr(−/−) mice. Infect. Immun. 2001, 69, 5138–5150. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, U.; Wu, Y.; Kent, G.; Forstner, J. Preferential adherence of cable-piliated Burkholderia cepacia to respiratory epithelia of CF knockout mice and human cystic fibrosis lung explants. J. Med. Microbiol. 2000, 49, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Koehler, D.R.; Sajjan, U.; Chow, Y.H.; Martin, B.; Kent, G.; Tanswell, A.K.; McKerlie, C.; Forstner, J.F.; Hu, J. Protection of Cftr knockout mice from acute lung infection by a helper-dependent adenoviral vector expressing Cftr in airway epithelia. Proc. Natl. Acad. Sci. USA 2003, 100, 15364–15369. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Delaney, C.A.; Orosz, S.E. Ferret respiratory system: Clinical anatomy, physiology, and disease. Vet. Clin. N. Am. Exot. Anim. Pract. 2011, 14, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sui, H.; Fisher, J.T.; Yan, Z.; Liu, X.; Cho, H.J.; Joo, N.S.; Zhang, Y.; Zhou, W.; Yi, Y.; et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Investig. 2010, 120, 3149–3160. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.A.; Katz, J.M.; Tumpey, T.M. The ferret as a model organism to study influenza A virus infection. Dis. Model. Mech. 2011, 4, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Ostedgaard, L.S.; Meyerholz, D.K.; Chen, J.H.; Pezzulo, A.A.; Karp, P.H.; Rokhlina, T.; Ernst, S.E.; Hanfland, R.A.; Reznikov, L.R.; Ludwig, P.S.; et al. The DeltaF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci. Transl. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Stoltz, D.A.; Meyerholz, D.K.; Pezzulo, A.A.; Ramachandran, S.; Rogan, M.P.; Davis, G.J.; Hanfland, R.A.; Wohlford-Lenane, C.; Dohrn, C.L.; Bartlett, J.A.; et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci. Transl. Med. 2010. [Google Scholar] [CrossRef] [PubMed]
- Kiros, T.G.; van Kessel, J.; Babiuk, L.A.; Gerdts, V. Induction, regulation and physiological role of IL-17 secreting helper T-cells isolated from PBMC, thymus, and lung lymphocytes of young pigs. Vet. Immunol. Immunopathol. 2011, 144, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.; Gerdts, V. Mouse and pig models for studies of natural and vaccine-induced immunity to Bordetella pertussis. J. Infect. Dis. 2014, 209, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.; Poxton, I.R.; Govan, J.R. Biological activity of Burkholderia (Pseudomonas) cepacia lipopolysaccharide. FEMS Immunol. Med. Microbiol. 1995, 11, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Urban, T.A.; Griffith, A.; Torok, A.M.; Smolkin, M.E.; Burns, J.L.; Goldberg, J.B. Contribution of Burkholderia cenocepacia flagella to infectivity and inflammation. Infect. Immun. 2004, 72, 5126–5134. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, U.S.; Hershenson, M.B.; Forstner, J.F.; LiPuma, J.J. Burkholderia cenocepacia ET12 strain activates TNFR1 signalling in cystic fibrosis airway epithelial cells. Cell. Microbiol. 2008, 10, 188–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sajjan, U.S.; Yang, J.H.; Hershenson, M.B.; LiPuma, J.J. Intracellular trafficking and replication of Burkholderia cenocepacia in human cystic fibrosis airway epithelial cells. Cell. Microbiol. 2006, 8, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Kaza, S.K.; McClean, S.; Callaghan, M. IL-8 released from human lung epithelial cells induced by cystic fibrosis pathogens Burkholderia cepacia complex affects the growth and intracellular survival of bacteria. Int. J. Med. Microbiol. 2011, 301, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.A.; Cristina de Assis, M.; Ventura, G.C.; Saliba, A.M.; Gonzaga, L., Jr.; Si-Tahar, M.; Marques Ede, A.; Plotkowski, M.C. Differential interaction of bacterial species from the Burkholderia cepacia complex with human airway epithelial cells. Microbes Infect. 2008, 10, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Duff, C.; Murphy, P.G.; Callaghan, M.; McClean, S. Differences in invasion and translocation of Burkholderia cepacia complex species in polarised lung epithelial cells in vitro. Microb. Pathog. 2006, 41, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, J.; Huynh, K.K.; Grinstein, S.; Valvano, M.A. Intracellular survival of Burkholderia cenocepacia in macrophages is associated with a delay in the maturation of bacteria-containing vacuoles. Cell. Microbiol. 2007, 9, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.W.; Mohr, C.D. Invasion and intracellular survival of Burkholderia cepacia. Infect. Immun. 2000, 68, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Leigh, M.; Ribeiro, C.; Yankaskas, J.; Burns, K.; Gilligan, P.; Sokol, P.; Boucher, R. Patterns of epithelial cell invasion by different species of the Burkholderia cepacia complex in well-differentiated human airway epithelia. Infect. Immun. 2002, 70, 4547–4555. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology 2008, 123, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Schunk, M.K.; Macallum, G.E. Applications and optimization of immunization procedures. ILAR J. 2005, 46, 241–257. [Google Scholar] [CrossRef] [PubMed]
- McClean, S.; Healy, M.E.; Collins, C.; Carberry, S.; O'Shaughnessy, L.; Dennehy, R.; Adams, Á.; Kennelly, H.; Corbett, J.M.; Carty, F.; et al. Linocin and OmpW are involved in attachment of the cystic fibrosis associated pathogen Burkholderia cepacia complex to lung epithelial cells and protect mice against infection. Infect. Immun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Makidon, P.E.; Knowlton, J.; Groom, J.V.; Blanco, L.P.; LiPuma, J.J.; Bielinska, A.U.; Baker, J.R., Jr. Induction of immune response to the 17 kDa OMPA Burkholderia cenocepacia polypeptide and protection against pulmonary infection in mice after nasal vaccination with an OMP nanoemulsion-based vaccine. Med. Microbiol. Immunol. 2010, 199, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Bertot, G.M.; Restelli, M.A.; Galanternik, L.; Aranibar Urey, R.C.; Valvano, M.A.; Grinstein, S. Nasal immunization with Burkholderia multivorans outer membrane proteins and the mucosal adjuvant adamantylamide dipeptide confers efficient protection against experimental lung infections with B. multivorans and B. cenocepacia. Infect. Immun. 2007, 75, 2740–2752. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.B.; Ganesan, S.; Comstock, A.T.; Zhao, Y.; Sajjan, U.S. Cable pili and the associated 22 kDa adhesin contribute to Burkholderia cenocepacia persistence in vivo. PLoS ONE 2011, 6, e22435. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D. Immunological mechanisms behind the cystic fibrosis-ABPA link. Med. Mycol. 2009, 47, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Kjaergaard, S.; Pressler, T.; Kharazmi, A.; Koch, C.; Høiby, N. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. APMIS 2000, 108, 329–335. [Google Scholar] [CrossRef] [PubMed]
- O'Connor, R.A.; Taams, L.S.; Anderton, S.M. Translational mini-review series on Th17 cells: CD4 T helper cells: functional plasticity and differential sensitivity to regulatory T cell-mediated regulation. Clin. Exp. Immunol. 2010, 159, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; McAleer, J.P.; Lin, Y.; Paterson, D.L.; Zheng, M.; Alcorn, J.F.; Weaver, C.T.; Kolls, J.K. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity 2011, 35, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Kummer, L.W.; Szaba, F.M.; Smiley, S.T. IL-17 contributes to cell-mediated defense against pulmonary Yersinia pestis infection. J. Immunol. 2011, 186, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Khader, S.A.; Bell, G.K.; Pearl, J.E.; Fountain, J.J.; Rangel-Moreno, J.; Cilley, G.E.; Shen, F.; Eaton, S.M.; Gaffen, S.L.; Swain, S.L.; et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007, 8, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Priebe, G.P.; Walsh, R.L.; Cederroth, T.A.; Kamei, A.; Coutinho-Sledge, Y.S.; Goldberg, J.B.; Pier, G.B. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J. Immunol. 2008, 181, 4965–4975. [Google Scholar] [CrossRef] [PubMed]
- Heijerman, H. Infection and inflammation in cystic fibrosis: A short review. J. Cyst. Fibros. 2005, 4, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Dubin, P.J.; McAllister, F.; Kolls, J.K. Is cystic fibrosis a TH17 disease? Inflamm. Res. 2007, 56, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; Hafler, D.A.; et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.K.; Tippayawat, P.; Walker, N.J.; Harding, S.V.; Atkins, H.S.; Maillere, B.; Bancroft, G.J.; Lertmemongkolchai, G.; Altmann, D.M. CD4+ T-cell immunity to the Burkholderia pseudomallei ABC transporter LolC in melioidosis. Eur. J. Immunol. 2011, 41, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Musson, J.A.; Reynolds, C.J.; Rinchai, D.; Nithichanon, A.; Khaenam, P.; Favry, E.; Spink, N.; Chu, K.K.; De Soyza, A.; Bancroft, G.J.; et al. CD4+ T cell epitopes of FliC conserved between strains of Burkholderia: implications for vaccines against melioidosis and cepacia complex in cystic fibrosis. J. Immunol. 2014, 193, 6041–6049. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, G.C.; Deeraksa, A.; Qazi, O.; Judy, B.M.; Taylor, K.; Propst, K.L.; Duffy, A.J.; Johnson, K.; Kitto, G.B.; Brown, K.A.; et al. Protective response to subunit vaccination against intranasal Burkholderia mallei and B. pseudomallei challenge. Procedia Vaccinol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Sokol, P.A.; Kooi, C.; Hodges, R.S.; Cachia, P.; Woods, D.E. Immunization with a Pseudomonas aeruginosa elastase peptide reduces severity of experimental lung infections due to P. aeruginosa or Burkholderia cepacia. J. Infect. Dis. 2000, 181, 1682–1692. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, C.L.; Muruato, L.A.; Torres, A.G. Recent Advances in Burkholderia mallei and B. pseudomallei Research. Curr. Trop. Med. Rep. 2015, 2, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, I.; Rohde, M.; Brenneke, B. Purification and characterization of an exopolysaccharide of Burkholderia (Pseudomonas) pseudomallei. Infect. Immun. 1995, 63, 3959–3965. [Google Scholar] [PubMed]
- Vella, M.; Pace, D. Glycoconjugate vaccines: An update. Expert. Opin. Biol. Ther. 2015, 15, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; Adamo, R. Recent mechanistic insights on glycoconjugate vaccines and future perspectives. ACS Chem. Biol. 2013, 8, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Avery, O.T.; Goebel, W.F. Chemo-immunological studies on conjugated carbohydrate-proteins : II. Immunological specificity of synthetic sugar-protein antigens. J. Exp. Med. 1929, 50, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Laroussarie, A.; Barycza, B.; Andriamboavonjy, H.; Tamigney Kenfack, M.; Blériot, Y.; Gauthier, C. Synthesis of the Tetrasaccharide Repeating Unit of the beta-Kdo-Containing Exopolysaccharide from Burkholderia pseudomallei and B. cepacia Complex. J. Org. Chem. 2015, 80, 10386–10396. [Google Scholar] [CrossRef] [PubMed]
- Ziaco, M.; De Castro, C.; Silipo, A.; Corsaro, M.M.; Molinaro, A.; Iadonisi, A.; Lanzetta, R.; Parrilli, M.; Bedini, E. Synthesis of the tetrasaccharide outer core fragment of Burkholderia multivorans lipooligosaccharide. Carbohydr. Res. 2015, 403, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ibrahim, M.; Qiu, H.; Kausar, S.; Ilyas, M.; Cui, Z.; Hussain, A.; Li, B.; Waheed, A.; Zhu, B.; et al. Protein profiling analyses of the outer membrane of Burkholderia cenocepacia reveal a niche-specific proteome. Microb. Ecol. 2015, 69, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vial, L.; Chapalain, A.; Groleau, M.C.; Déziel, E. The various lifestyles of the Burkholderia cepacia complex species: A tribute to adaptation. Environ. Microbiol. 2011, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.R.; Hird, T.J.; Tang, P.; Zlosnik, J.E. Whole-genome sequencing of three clonal clinical isolates of B. cenocepacia from a patient with cystic fibrosis. PLoS ONE 2015, 10, e0143472. [Google Scholar] [CrossRef] [PubMed]
- Kalferstova, L.; Kolar, M.; Fila, L.; Vavrova, J.; Drevinek, P. Gene expression profiling of Burkholderia cenocepacia at the time of cepacia syndrome: loss of motility as a marker of poor prognosis? J. Clin. Microbiol. 2015, 53, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Shinoy, M.; Dennehy, R.; Coleman, L.; Carberry, S.; Schaffer, K.; Callaghan, M.; Doyle, S.; McClean, S. Immunoproteomic analysis of proteins expressed by two related pathogens, Burkholderia multivorans and Burkholderia cenocepacia, during human infection. PLoS ONE 2013, 8, e80796. [Google Scholar] [CrossRef] [PubMed]
- Riedel, K.; Carranza, P.; Gehrig, P.; Potthast, F.; Eberl, L. Towards the proteome of Burkholderia cenocepacia H111: Setting up a 2-DE reference map. Proteomics 2006, 6, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Nie, X.; Zou, M.; Shi, Y.; Cheng, G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011, 64, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Zabner, J.; Smith, J.J.; Karp, P.H.; Widdicombe, J.H.; Welsh, M.J. Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol. Cell 1998, 2, 397–403. [Google Scholar] [CrossRef]
- Bylund, J.; Burgess, L.A.; Cescutti, P.; Ernst, R.K.; Speert, D.P. Exopolysaccharides from Burkholderia cenocepacia inhibit neutrophil chemotaxis and scavenge reactive oxygen species. J. Biol. Chem. 2006, 281, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Caraher, E. Residence in biofilms allows Burkholderia cepacia complex (Bcc) bacteria to evade the antimicrobial activities of neutrophil-like dHL60 cells. Pathog. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Suppiger, A.; Schmid, N.; Aguilar, C.; Pessi, G.; Eberl, L. Two quorum sensing systems control biofilm formation and virulence in members of the Burkholderia cepacia complex. Virulence 2013, 4, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Watanabe, M.; Matsuura, M.; Nishijima, M.; Kawahara, K. The partially degraded lipopolysaccharide of Burkholderia cepacia and ornithine-containing lipids derived from some Gram-negative bacteria are useful complex lipid adjuvants. FEMS Immunol. Med. Microbiol. 2002, 34, 173–179. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradenas, G.A.; Ross, B.N.; Torres, A.G. Burkholderia cepacia Complex Vaccines: Where Do We Go from here? Vaccines 2016, 4, 10. https://doi.org/10.3390/vaccines4020010
Pradenas GA, Ross BN, Torres AG. Burkholderia cepacia Complex Vaccines: Where Do We Go from here? Vaccines. 2016; 4(2):10. https://doi.org/10.3390/vaccines4020010
Chicago/Turabian StylePradenas, Gonzalo A., Brittany N. Ross, and Alfredo G. Torres. 2016. "Burkholderia cepacia Complex Vaccines: Where Do We Go from here?" Vaccines 4, no. 2: 10. https://doi.org/10.3390/vaccines4020010
APA StylePradenas, G. A., Ross, B. N., & Torres, A. G. (2016). Burkholderia cepacia Complex Vaccines: Where Do We Go from here? Vaccines, 4(2), 10. https://doi.org/10.3390/vaccines4020010





