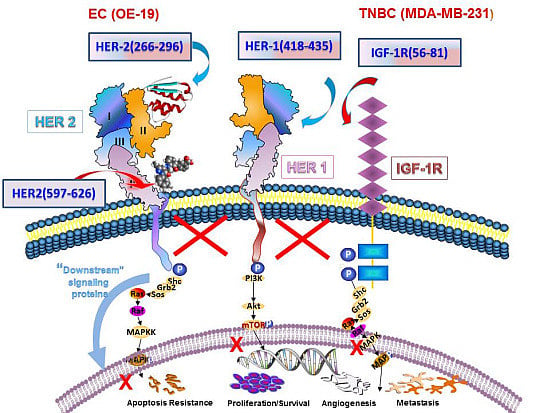
Anti-Tumor Effects of Peptide Therapeutic and Peptide Vaccine Antibody Co-targeting HER-1 and HER-2 in Esophageal Cancer (EC) and HER-1 and IGF-1R in Triple-Negative Breast Cancer (TNBC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Selection, Design and Peptide Synthesis
2.2. Cell Lines and Inhibitors
2.3. Rabbits
2.4. MTT Cell Growth Proliferation Assay.
2.5. Receptor Phosphorylation Assay
2.6. Caspase Activity Assay for Apoptosis
2.7. Antibody Dependent Cellular Cytotoxicity (ADCC)
2.8. Western Blotting
2.9. Statistical Analysis
3. Results and Discussion
3.1. Epitope Selection, Design, Synthesis and Characterization
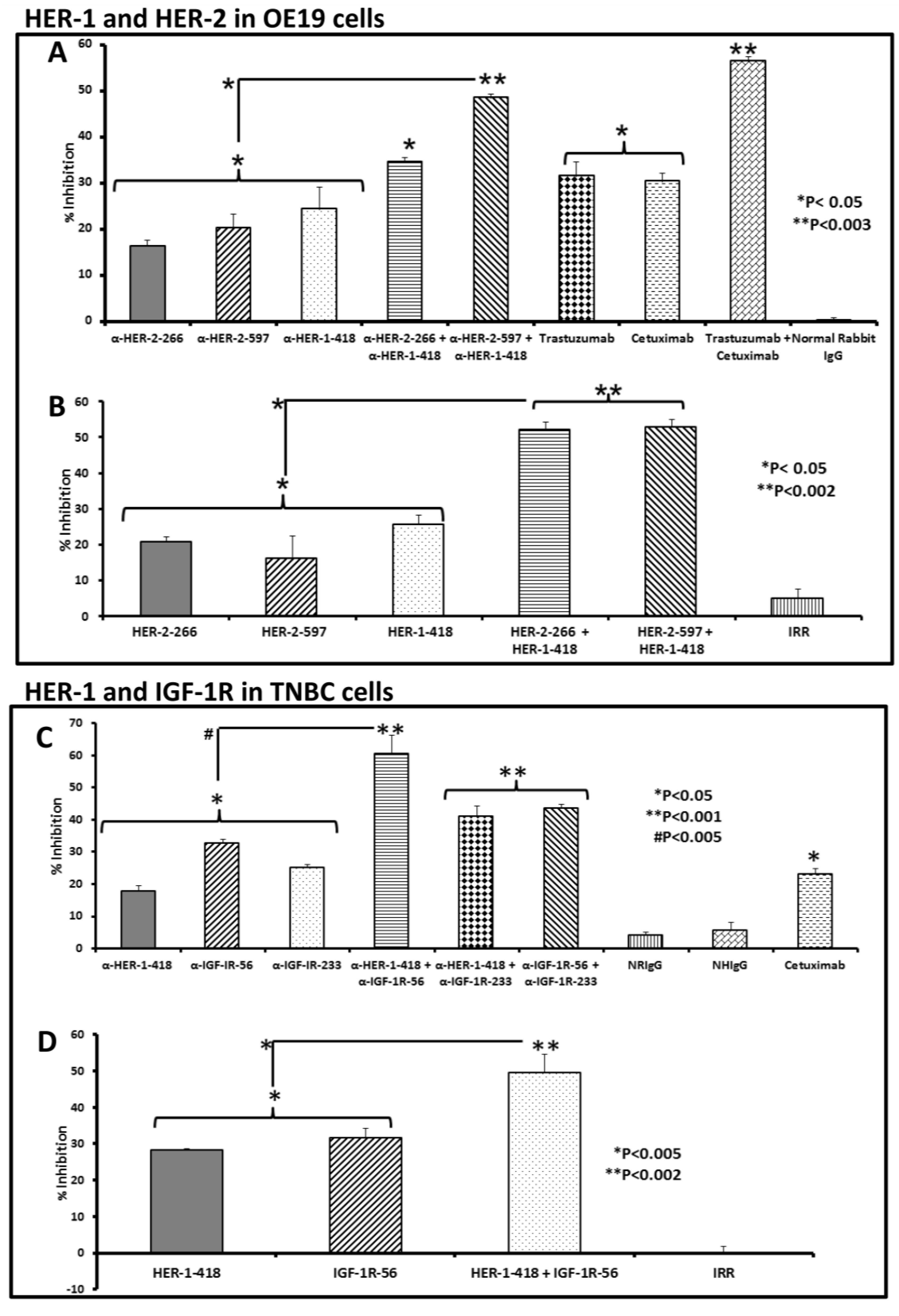
3.2. Single and Combination Treatment with Peptide Mimics or Peptide Vaccine Antibodies of HER-1 and HER-2 in EC or HER-1 and IGF-1R in TNBC Inhibits Proliferation of Cells in an MTT Assay
| Peptides | Amino Acid Sequence of HER-1,HER-2 and IGF-1R Peptides | Mol.Wt (Da) |
|---|---|---|
| HER-1 (418–435) | Ac-SLNITSLGLRSLKEISDG-OH | 1944 |
| HER-2 (266–296) | LHCPALVTYNTDTFESMPNPEGRYTFGASCV-OH | 3420 |
| HER-2 (597–626) | VARCPSGVKPDLSYMPIWKFPDEEGACQPL-OH | 3333 |
| IGF-1R (56–81) | Ac-LLFRVAGLESLGDLFPNLTVIRGWKL-NH2 | 2969 |
| IGF-1R (233–251) | Ac-ACPPNTYRFEGWRCVDRDF-NH2 | 2372 |
| MVF-HER-1 (418–435) | KLLSLIKGVIVHRLEGVE-GPSL-SLNITSLGLRSLKEISDG-OH | 4242 |
| MVF-HER-2 (266–296) | KLLSLIKGVIVHRLEGVE-GPSL-LHCPALVTYNTDTFESMPNPEGRYTFGASCV-OH | 5757 |
| MVF-HER-2 (597–626) | KLLSLIKGVIVHRLEGVE-GPSL-VARCPSGVKPDLSYMPIWKFPDEEGACQPL-OH | 5672 |
| MVF-IGF-1R (56–81) | KLLSLIKGVIVHRLEGVE-LSPG-LLFRVAGLESLGDLFPNLTVIRGWKL-NH2 | 5267 |
| MVF-IGF-1R (233–251) | Ac-KLLSLIKGVIVHRLEGVE-GPSL-Ac-ACPPNTYRFEGWRCVDRDF-NH2 | 4712 |
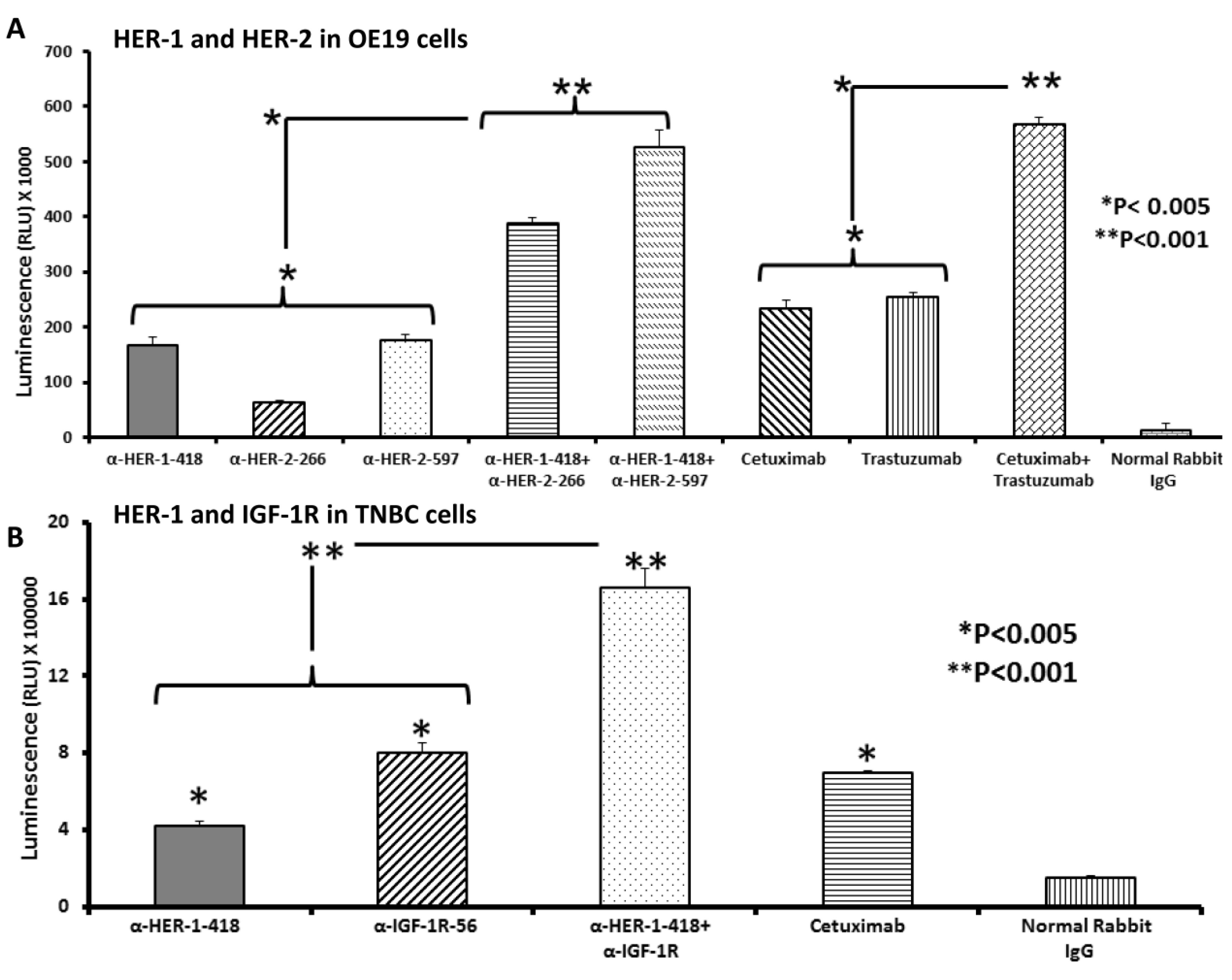
3.3. Peptide Vaccine Antibodies Induce ADCC of OE19 and TNBC Cells in Vitro

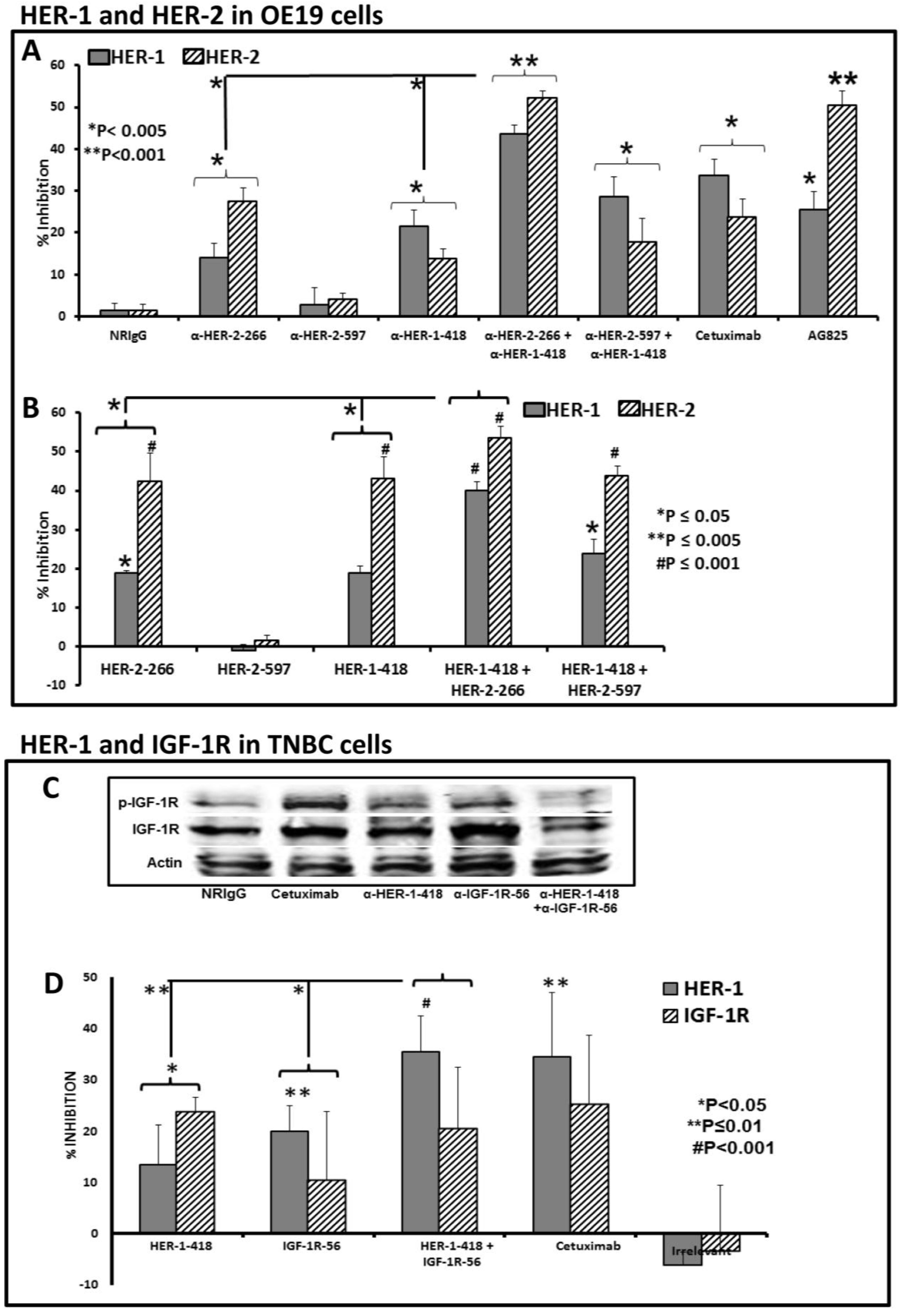
3.4. Single and Combination Treatment with Peptide Mimics or Peptide Vaccine Antibodies of HER-1 and HER-2 in EC or HER-1 and IGF-1R in TNBC down Regulates Receptor Phosphorylation in Vitro
3.5. Apoptosis Determination of OE19 and MDA-MB-231 Cancer Cells in Vitro by Caspase Activity Assay
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Dong, Z.; Tang, P.; Li, L.; Wang, G. The strategy for esophageal cancer control in high-risk areas of China. Jpn. J. Clin. Oncol. 2002, 32, S10–S12. [Google Scholar] [CrossRef]
- Twarock, S.; Freudenberger, T.; Poscher, E.; Dai, G.; Jannasch, K.; Dullin, C.; Alves, F.; Prenzel, K.; Knoefel, W.T.; Stoecklein, N.H.; et al. Inhibition of oesophageal squamous cell carcinoma progression by in vivo targeting of hyaluronan synthesis. Mol. Cancer 2011. [Google Scholar] [CrossRef]
- Heath, E.I.; Limburg, P.J.; Hawk, E.T.; Forastiere, A.A. Adenocarcinoma of the esophagus: Risk factors and prevention. Oncology (Williston Park) 2000, 14, 507–514; discussion 518–520, 522–503. [Google Scholar] [PubMed]
- Chow, W.H.; Finkle, W.D.; McLaughlin, J.K.; Frankl, H.; Ziel, H.K.; Fraumeni, J.F., Jr. The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA 1995, 274, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Starling, N.; Rao, S.; Iveson, T.; Nicolson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 2008, 358, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.; Eroles, P.; Zaragoza, R.; Vina, J.R.; Lluch, A. Triple-negative breast cancer: Molecular features, pathogenesis, treatment and current lines of research. Cancer Treat. Rev. 2010, 36, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.D.; Zhu, R.; Zhan, M.; Rodriguez, A.A.; Yang, W.; Wong, S.; Makris, A.; Lehmann, B.D.; Chen, X.; Mayer, I.; et al. Identification of prognosis-relevant subgroups in patients with chemoresistant triple-negative breast cancer. Clin. Cancer Res. 2013, 19, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Arteaga, C.L. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J. Clin. Oncol. 2005, 23, 2445–2459. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J. Targeting tyrosine kinases in cancer: The second wave. Science 2006, 312, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Duda, D.G.; Clark, J.W.; Loeffler, J.S. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat. Clin. Pract. Oncol. 2006, 3, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Houck, K.A.; Ferrara, N.; Winer, J.; Cachianes, G.; Li, B.; Leung, D.W. The vascular endothelial growth factor family: Identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 1991, 5, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Cobleigh, M.A.; Langmuir, V.K.; Sledge, G.W.; Miller, K.D.; Haney, L.; Novotny, W.F.; Reimann, J.D.; Vassel, A. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Sem. Oncol. 2003, 30, 117–124. [Google Scholar] [CrossRef]
- Hynes, N.E.; Lane, H.A. ErbB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer 2005, 5, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; van Oosterom, A.T.; de Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Witte, L. Inhibition of tumor growth and metastasis by targeting tumor-associated angiogenesis with antagonists to the receptors of vascular endothelial growth factor. Investig. New Drugs 1999, 17, 195–212. [Google Scholar] [CrossRef]
- Oshima, R.G.; Lesperance, J.; Munoz, V.; Hebbard, L.; Ranscht, B.; Sharan, N.; Muller, W.J.; Hauser, C.A.; Cardiff, R.D. Angiogenic acceleration of Neu induced mammary tumor progression and metastasis. Cancer Res. 2004, 64, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [PubMed]
- Baselga, J.; Swain, S.M. Novel anticancer targets: Revisiting ErbB2 and discovering ErbB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M.N.; Schernhammer, E.S.; Hankinson, S.E. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer 2004, 4, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M.N. Insulin-like growth factors and neoplasia. Nov. Found. Symp. 2004, 262, 84–98. [Google Scholar]
- Hudziak, R.M.; Lewis, G.D.; Winget, M.; Fendly, B.M.; Shepard, H.M.; Ullrich, A. P185her2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol. Cell. Biol. 1989, 9, 1165–1172. [Google Scholar] [PubMed]
- Shepard, H.M.; Lewis, G.D.; Sarup, J.C.; Fendly, B.M.; Maneval, D.; Mordenti, J.; Figari, I.; Kotts, C.E.; Palladino, M.A., Jr.; Ullrich, A.; et al. Monoclonal antibody therapy of human cancer: Taking the her2 protooncogene to the clinic. J. Clin. Immunol. 1991, 11, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Wedge, S.R. Zd6474—A novel inhibitor of VEGFR and EGFR tyrosine kinase activity. Br. J. Cancer 2005, 92, S6–S13. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ogasawara, A.K.; Yang, R.; Wei, W.; He, G.W.; Zioncheck, T.F.; Bunting, S.; de Vos, A.M.; Jin, H. Kdr (vegf receptor 2) is the major mediator for the hypotensive effect of VEGF. Hypertension 2002, 39, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Eskens, F.A.; Verweij, J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors: A review. Eur. J. Cancer 2006, 42, 3127–3139. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A. Recognizing and managing toxicities of molecular targeted therapies for colorectal cancer. Oncology (Williston Park) 2006, 20, 21–28. [Google Scholar] [PubMed]
- Ross, J.S.; McKenna, B.J. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Investig. 2001, 19, 554–568. [Google Scholar] [CrossRef]
- Rossi, E.; Grisanti, S.; Villanacci, V.; Della Casa, D.; Cengia, P.; Missale, G.; Minelli, L.; Buglione, M.; Cestari, R.; Bassotti, G. HER-2 overexpression/amplification in barrett’s oesophagus predicts early transition from dysplasia to adenocarcinoma: A clinico-pathologic study. J. Cell. Mol. Med. 2009, 13, 3826–3833. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.; Hollmen, M.; Junttila, T.T.; Kapanen, A.I.; Tommola, S.; Soini, Y.; Helin, H.; Salo, J.; Joensuu, H.; Sihvo, E.; et al. Amplification of her-2 in gastric carcinoma: Association with topoisomerase llalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann. Oncol. 2005, 16, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Gros, S.J.; Kurschat, N.; Dohrmann, T.; Reichelt, U.; Dancau, A.M.; Peldschus, K.; Adam, G.; Hoffman, R.M.; Izbicki, J.R.; Kaifi, J.T. Effective therapeutic targeting of the overexpressed HER-2 receptor in a highly metastatic orthotopic model of esophageal carcinoma. Mol. Cancer Ther. 2010, 9, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Okines, A.F.; Cunningham, D. Trastuzumab: A novel standard option for patients with HER-2-positive advanced gastric or gastro-oesophageal junction cancer. Ther. Adv. Gastroenterol. 2012, 5, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Swain, S.M.; Kim, S.B.; Cortes, J.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Knott, A.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (cleopatra study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Bosset, J.F.; Gignoux, M.; Triboulet, J.P.; Tiret, E.; Mantion, G.; Elias, D.; Lozach, P.; Ollier, J.C.; Pavy, J.J.; Mercier, M.; et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N. Engl. J. Med. 1997, 337, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wo, J.M.; Ray, M.B.; Jones, W.; Su, R.R.; Ellis, S.; Martin, R.C. Cyclooxygenase-2 and epithelial growth factor receptor up-regulation during progression of barrett's esophagus to adenocarcinoma. World J. Gastroenterol. 2006, 12, 928–934. [Google Scholar] [PubMed]
- Friess, H.; Fukuda, A.; Tang, W.H.; Eichenberger, A.; Furlan, N.; Zimmermann, A.; Korc, M.; Buchler, M.W. Concomitant analysis of the epidermal growth factor receptor family in esophageal cancer: Overexpression of epidermal growth factor receptor mRNA but not of c-erbB-2 and c-erbB-3. World J. Surg. 1999, 23, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Walch, A.; Specht, K.; Bink, K.; Zitzelsberger, H.; Braselmann, H.; Bauer, M.; Aubele, M.; Stein, H.; Siewert, J.R.; Hofler, H.; et al. HER-2/neu gene amplification, elevated mrna expression, and protein overexpression in the metaplasia-dysplasia-adenocarcinoma sequence of barrett’s esophagus. Lab. Investig. 2001, 81, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Geddert, H.; Zeriouh, M.; Wolter, M.; Heise, J.W.; Gabbert, H.E.; Sarbia, M. Gene amplification and protein overexpression of c-erb-B2 in barrett carcinoma and its precursor lesions. Am. J. Clin. Pathol. 2002, 118, 60–66. [Google Scholar] [PubMed]
- Mimura, K.; Kono, K.; Hanawa, M.; Mitsui, F.; Sugai, H.; Miyagawa, N.; Ooi, A.; Fujii, H. Frequencies of HER-2/neu expression and gene amplification in patients with oesophageal squamous cell carcinoma. Br. J. Cancer 2005, 92, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Marx, A.H.; Tharun, L.; Muth, J.; Dancau, A.M.; Simon, R.; Yekebas, E.; Kaifi, J.T.; Mirlacher, M.; Brummendorf, T.H.; Bokemeyer, C.; et al. HER-2 amplification is highly homogenous in gastric cancer. Hum. Pathol. 2009, 40, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Ayyappan, S.; Prabhakar, D.; Sharma, N. Epidermal growth factor receptor (EGFR)-targeted therapies in esophagogastric cancer. Anticancer Res. 2013, 33, 4139–4155. [Google Scholar] [PubMed]
- Gluz, O.; Liedtke, C.; Gottschalk, N.; Pusztai, L.; Nitz, U.; Harbeck, N. Triple-negative breast cancer—Current status and future directions. Ann. Oncol. 2009, 20, 1913–1927. [Google Scholar] [CrossRef] [PubMed]
- Livasy, C.A.; Karaca, G.; Nanda, R.; Tretiakova, M.S.; Olopade, O.I.; Moore, D.T.; Perou, C.M. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod. Pathol. 2006, 19, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.H.; Valero, V.; Hortobagyi, G.N. Emerging targeted therapies for breast cancerd. J. Clin. Oncol. 2010, 28, 3366–3379. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Jang, M.H.; Kim, E.J.; Kim, H.J.; Lee, H.J.; Kim, Y.J.; Kim, J.H.; Kang, E.; Kim, S.W.; Kim, I.A.; et al. High EGFR gene copy number predicts poor outcome in triple-negative breast cancer. Mod. Pathol. 2014, 27, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Sayed-Ahmed, M.M.; Hafez, M.M.; Al-Shabanah, O.A.; Al-Rejaie, S.S.; Aleisa, A.M.; Al-Yahya, A.A.; Alsheikh, A.; Al Diab, A.I.; Al-Akeely, M.H. Increased expression of biological markers as potential therapeutic targets in saudi women with triple-negative breast cancer. Tumori 2013, 99, 545–554. [Google Scholar] [PubMed]
- Carey, L.A.; Rugo, H.S.; Marcom, P.K.; Mayer, E.L.; Esteva, F.J.; Ma, C.X.; Liu, M.C.; Storniolo, A.M.; Rimawi, M.F.; Forero-Torres, A.; et al. Tbcrc 001: Randomized phase ii study of cetuximab in combination with carboplatin in stage iv triple-negative breast cancer. J. Clin. Oncol. 2012, 30, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Gomez, P.; Greil, R.; Braga, S.; Climent, M.A.; Wardley, A.M.; Kaufman, B.; Stemmer, S.M.; Pego, A.; Chan, A.; et al. Randomized phase ii study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 2013, 31, 2586–2592. [Google Scholar] [CrossRef] [PubMed]
- Davison, Z.; de Blacquiere, G.E.; Westley, B.R.; May, F.E. Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: Implications for therapy. Neoplasia 2011, 13, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Litzenburger, B.C.; Creighton, C.J.; Tsimelzon, A.; Chan, B.T.; Hilsenbeck, S.G.; Wang, T.; Carboni, J.M.; Gottardis, M.M.; Huang, F.; Chang, J.C.; et al. High igf-ir activity in triple-negative breast cancer cell lines and tumorgrafts correlates with sensitivity to anti-IGF-IR therapy. Clin. Cancer Res. 2011, 17, 2314–2327. [Google Scholar] [CrossRef] [PubMed]
- Morgillo, F.; Woo, J.K.; Kim, E.S.; Hong, W.K.; Lee, H.Y. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006, 66, 10100–10111. [Google Scholar] [CrossRef] [PubMed]
- Kuribayashi, A.; Kataoka, K.; Kurabayashi, T.; Miura, M. Evidence that basal activity, but not transactivation, of the epidermal growth factor receptor tyrosine kinase is required for insulin-like growth factor I-induced activation of extracellular signal-regulated kinase in oral carcinoma cells. Endocrinology 2004, 145, 4976–4984. [Google Scholar] [CrossRef] [PubMed]
- Vicari, D.; Foy, K.C.; Liotta, E.M.; Kaumaya, P.T. Engineered conformation-dependent vegf peptide mimics are effective in inhibiting vegf signaling pathways. J. Biol. Chem. 2011, 286, 13612–13625. [Google Scholar] [CrossRef] [PubMed]
- Foy, K.C.; Miller, M.J.; Moldovan, N.; Carson, W.E.; Kaumaya, P.T.P. Combined vaccination with HER-2 peptide followed by therapy with VEGF peptide mimics exerts effective anti-tumor and anti-angiogenic effects in vitro and in vivo. OncoImmunology 2012, 1, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Guix, M.; Faber, A.C.; Wang, S.E.; Olivares, M.G.; Song, Y.; Qu, S.; Rinehart, C.; Seidel, B.; Yee, D.; Arteaga, C.L.; et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J. Clin. Investig. 2008, 118, 2609–2619. [Google Scholar] [PubMed]
- Li, P.; Veldwijk, M.R.; Zhang, Q.; Li, Z.B.; Xu, W.C.; Fu, S. Co-inhibition of epidermal growth factor receptor and insulin-like growth factor receptor 1 enhances radiosensitivity in human breast cancer cells. BMC Cancer 2013. [Google Scholar] [CrossRef] [PubMed]
- Camirand, A.; Zakikhani, M.; Young, F.; Pollak, M. Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells. Breast Cancer Res. 2005, 7, R570–R579. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Zhu, X.F.; Zhong, G.S.; Deng, B.G.; Gao, Z.T.; Wang, H. Lapatinib, a dual inhibitor of EGFR and HER2, has synergistic effects with 5-fluorouracil on esophageal carcinoma. Oncol. Rep. 2012, 27, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Leung, R.; Kwong, A.; Chiu, J.; Liang, R.; Swanton, C.; Yau, T. Integrating molecular mechanisms and clinical evidence in the management of trastuzumab resistant or refractory HER-2(+) metastatic breast cancer. Oncologist 2011, 16, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Luo, H.S.; Li, Y.; Li, J.C.; Cai, Z.Q.; Su, X.Y.; Dai, D.Q.; Du, W.; Chen, T.X.; Chen, M. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem. Biophys. 2012, 62, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Dancey, J.E.; Chen, H.X. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nat. Rev. Drug Discov. 2006, 5, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Moulder, S.L.; Arteaga, C.L. A phase I/II trial of trastuzumab and gefitinib in patients with metastatic breast cancer that overexpresses HER2/neu (ErbB-2). Clin. Breast Cancer 2003, 4, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Lenz, H.J.; Kindler, H.L.; Hochster, H.S.; Wadler, S.; Hoff, P.M.; Kemeny, N.E.; Hollywood, E.M.; Gonen, M.; Quinones, M.; et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: The bond-2 study. J. Clin. Oncol. 2007, 25, 4557–4561. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.D.; Garrett, J.T.; Rawale, S.V.; Jones, A.L.; Phillips, G.; Forni, G.; Morris, J.C.; Oshima, R.G.; Kaumaya, P.T. Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. J. Immunol. 2007, 179, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Garrett, J.T.; Rawale, S.; Allen, S.D.; Phillips, G.; Forni, G.; Morris, J.C.; Kaumaya, P.T. Novel engineered trastuzumab conformational epitopes demonstrate in vitro and in vivo antitumor properties against HER-2/neu. J. Immunol. 2007, 178, 7120–7131. [Google Scholar] [CrossRef] [PubMed]
- Foy, K.C.; Wygle, R.M.; Miller, M.J.; Overholser, J.P.; Bekaii-Saab, T.; Kaumaya, P.T. Peptide vaccines and peptidomimetics of EGFR (HER-1) ligand binding domain inhibit cancer cell growth in vitro and in vivo. J. Immunol. 2013, 191, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Foy, K.C.; Miller, M.J.; Moldovan, N.; Bozanovic, T.; Carson Iii, W.E.; Kaumaya, P.T. Immunotherapy with HER-2 and VEGF peptide mimics plus metronomic paclitaxel causes superior antineoplastic effects in transplantable and transgenic mouse models of human breast cancer. Oncoimmunology 2012, 1, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.M.; Otero, D.A.; Banks, W.A.; O’Brien, J.S. Retro-inverso prosaptide peptides retain bioactivity, are stable in vivo, and are blood-brain barrier permeable. J. Pharmacol. Exp. Ther. 2000, 295, 190–194. [Google Scholar] [PubMed]
- Dakappagari, N.K.; Pyles, J.; Parihar, R.; Carson, W.E.; Young, D.C.; Kaumaya, P.T. A chimeric multi-human epidermal growth factor receptor-2 B cell epitope peptide vaccine mediates superior antitumor responses. J. Immunol. 2003, 170, 4242–4253. [Google Scholar] [CrossRef] [PubMed]
- Foy, K.C.; Liu, Z.; Phillips, G.; Miller, M.; Kaumaya, P.T. Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo. J. Biol. Chem. 2011, 286, 13626–13637. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Foy, K.C.; Overholser, J.P.; Nahta, R.; Kaumaya, P.T.P. HER-3 peptide vaccines/mimics: Combined therapy with IGF-1R, HER-2, and HER-1 peptides induces synergistic antitumor effects against breast and pancreatic cancer cells. Oncoimmunology 2014, 3, e956012. [Google Scholar] [CrossRef] [PubMed]
- Foy, K.C.; Miller, M.J.; Overholser, J.; Donnelly, S.M.; Nahta, R.; Kaumaya, P.T.P. IGF-1R peptide vaccines/mimics inhibit the growth of BxPC3 and JIMT-1 cancer cells and exhibit synergistic antitumor effects with HER-1 and HER-2 peptides. Oncoimmunology 2014, 3, e956005. [Google Scholar] [CrossRef] [PubMed]
- Kaumaya, P.T.P.; Foy, K.C.; Garrett, J.; Rawale, S.V.; Vicari, D.; Thurmond, J.M.; Lamb, T.; Mani, A.; Kane, Y.; Balint, C.R.; et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. J. Clin. Oncol. 2009, 27, 5270–5277. [Google Scholar] [CrossRef] [PubMed]
- Epa, V.C.; Ward, C.W. Model for the complex between the insulin-like growth factor I and its receptor: Towards designing antagonists for the IGF-1 receptor. Protein Eng. Des. Sel. 2006, 19, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Power, D.G.; Reynolds, J.V. Localized adenocarcinoma of the esophagogastric junction—Is there a standard of care? Cancer Treat. Rev. 2010, 36, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Fox, C.; Peng, S.; Pusung, M.; Pectasides, E.; Matthee, E.; Hong, Y.S.; Do, I.G.; Jang, J.; Thorner, A.R.; et al. Preexisting oncogenic events impact trastuzumab sensitivity in ErbB2-amplified gastroesophageal adenocarcinoma. J. Clin. Investig. 2014, 124, 5145–5158. [Google Scholar] [CrossRef] [PubMed]
- Privitera, G.; Luca, T.; Musso, N.; Vancheri, C.; Crimi, N.; Barresi, V.; Condorelli, D.; Castorina, S. In vitro antiproliferative effect of trastuzumab (herceptin®) combined with cetuximab (erbitux®) in a model of human non-small cell lung cancer expressing EGFR and HER2. Clin. Exp. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Harari, D.; Yarden, Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene 2000, 19, 6102–6114. [Google Scholar] [CrossRef] [PubMed]
- Balana, M.E.; Labriola, L.; Salatino, M.; Movsichoff, F.; Peters, G.; Charreau, E.H.; Elizalde, P.V. Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene 2001, 20, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Camirand, A.; Lu, Y.; Pollak, M. Co-targeting HER2/ErbB2 and insulin-like growth factor-1 receptors causes synergistic inhibition of growth in HER2-overexpressing breast cancer cells. Med. Sci. Monit. 2002, 8, BR521–526. [Google Scholar] [PubMed]
- Nahta, R.; Yuan, L.X.; Zhang, B.; Kobayashi, R.; Esteva, F.J. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005, 65, 11118–11128. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.E.; Epa, V.C.; Garrett, T.P.; Ward, C.W. Structure and function of the type 1 insulin-like growth factor receptor. Cell. Mol. Life Sci. 2000, 57, 1050–1093. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Overholser, J.; Ambegaokar, K.H.; Eze, S.M.; Sanabria-Figueroa, E.; Nahta, R.; Bekaii-Saab, T.; Kaumaya, P.T.P. Anti-Tumor Effects of Peptide Therapeutic and Peptide Vaccine Antibody Co-targeting HER-1 and HER-2 in Esophageal Cancer (EC) and HER-1 and IGF-1R in Triple-Negative Breast Cancer (TNBC). Vaccines 2015, 3, 519-543. https://doi.org/10.3390/vaccines3030519
Overholser J, Ambegaokar KH, Eze SM, Sanabria-Figueroa E, Nahta R, Bekaii-Saab T, Kaumaya PTP. Anti-Tumor Effects of Peptide Therapeutic and Peptide Vaccine Antibody Co-targeting HER-1 and HER-2 in Esophageal Cancer (EC) and HER-1 and IGF-1R in Triple-Negative Breast Cancer (TNBC). Vaccines. 2015; 3(3):519-543. https://doi.org/10.3390/vaccines3030519
Chicago/Turabian StyleOverholser, Jay, Kristen Henkins Ambegaokar, Siobhan M. Eze, Eduardo Sanabria-Figueroa, Rita Nahta, Tanios Bekaii-Saab, and Pravin T.P. Kaumaya. 2015. "Anti-Tumor Effects of Peptide Therapeutic and Peptide Vaccine Antibody Co-targeting HER-1 and HER-2 in Esophageal Cancer (EC) and HER-1 and IGF-1R in Triple-Negative Breast Cancer (TNBC)" Vaccines 3, no. 3: 519-543. https://doi.org/10.3390/vaccines3030519
APA StyleOverholser, J., Ambegaokar, K. H., Eze, S. M., Sanabria-Figueroa, E., Nahta, R., Bekaii-Saab, T., & Kaumaya, P. T. P. (2015). Anti-Tumor Effects of Peptide Therapeutic and Peptide Vaccine Antibody Co-targeting HER-1 and HER-2 in Esophageal Cancer (EC) and HER-1 and IGF-1R in Triple-Negative Breast Cancer (TNBC). Vaccines, 3(3), 519-543. https://doi.org/10.3390/vaccines3030519