Tattoo Delivery of a Semliki Forest Virus-Based Vaccine Encoding Human Papillomavirus E6 and E7
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Mice
2.3. Production, Purification and Titer Determination of SFV-EGFP, SFVLuc and SFVeE6,7 Particles
2.4. SFV-EGFP, SFVLuc and SFVeE6,7 Administration
2.5. Imaging of Luciferase Expression
2.6. Quantification of Luciferase Expression
2.7. Ex Vivo Detection of EGFP Expression
2.8. MHC Class I Tetramer Staining
2.9. Regular CTL and Micro-CTL Assay
2.10. IFN-γ Elispot Assay
2.11. Tumor Treatment Experiments
2.12. Statistical Analysis
3. Results
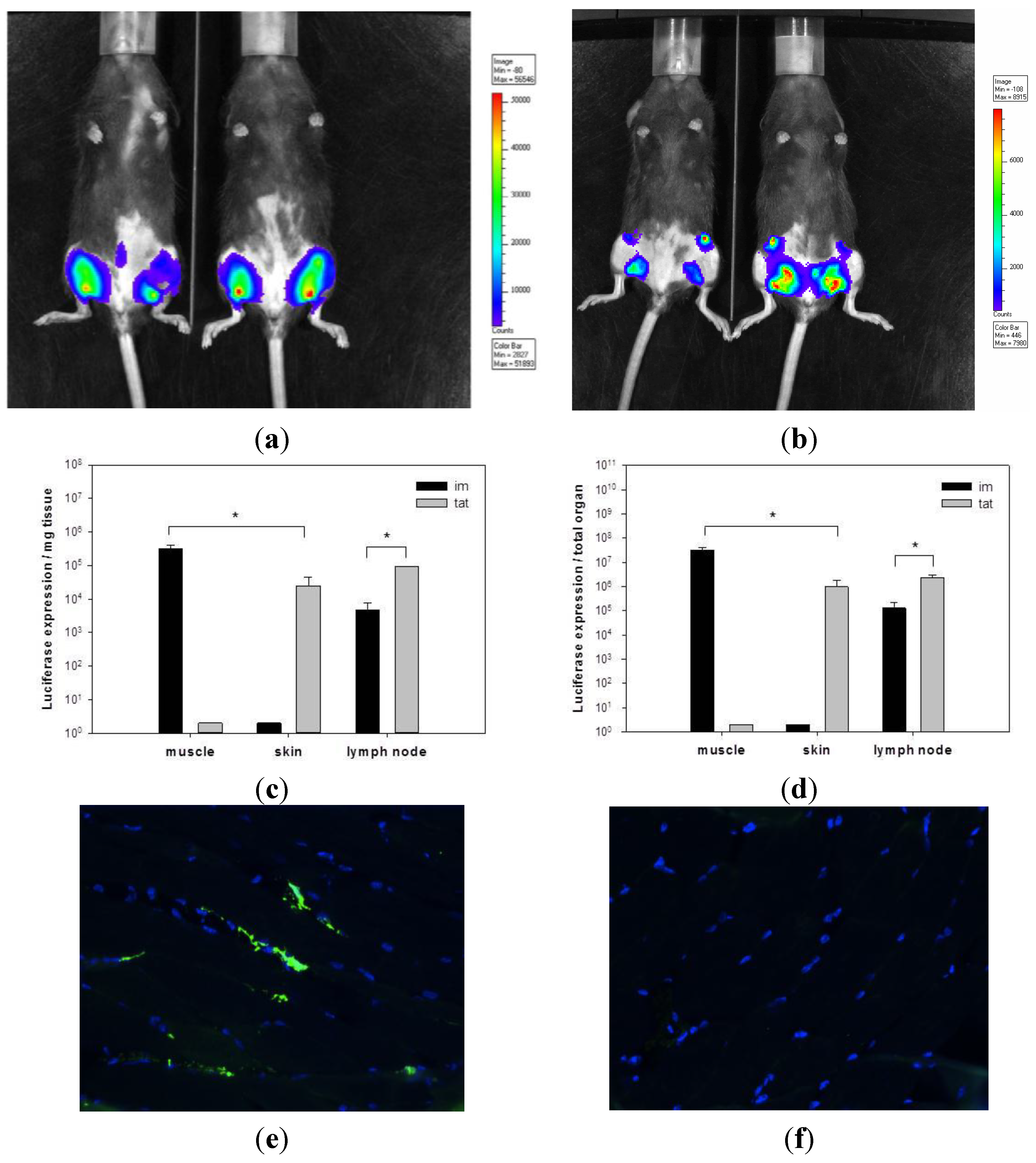
3.1. Transgene Expression upon Tattoo Injection of rSFV Replicon Particles
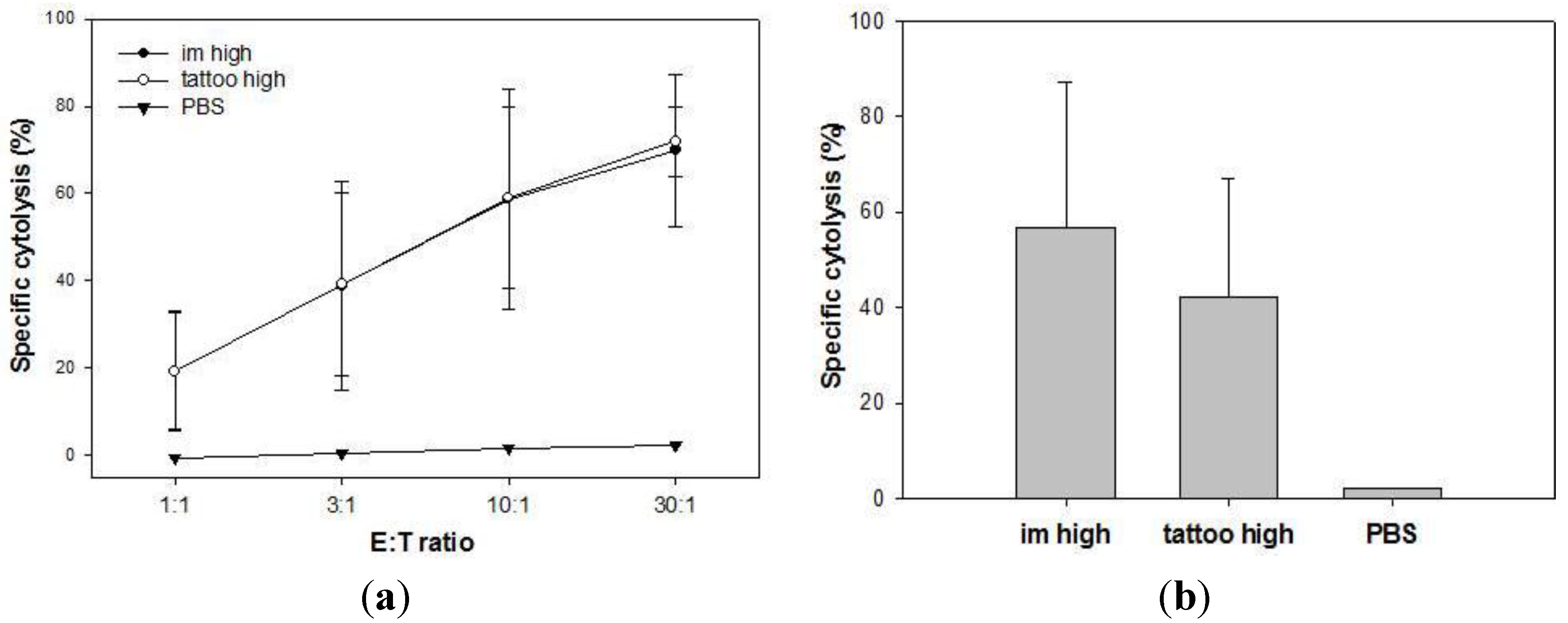
3.2. Induction of Cytotoxic T Lymphocytes upon Tattooing and Intramuscular Immunizations with SFVeE6,7

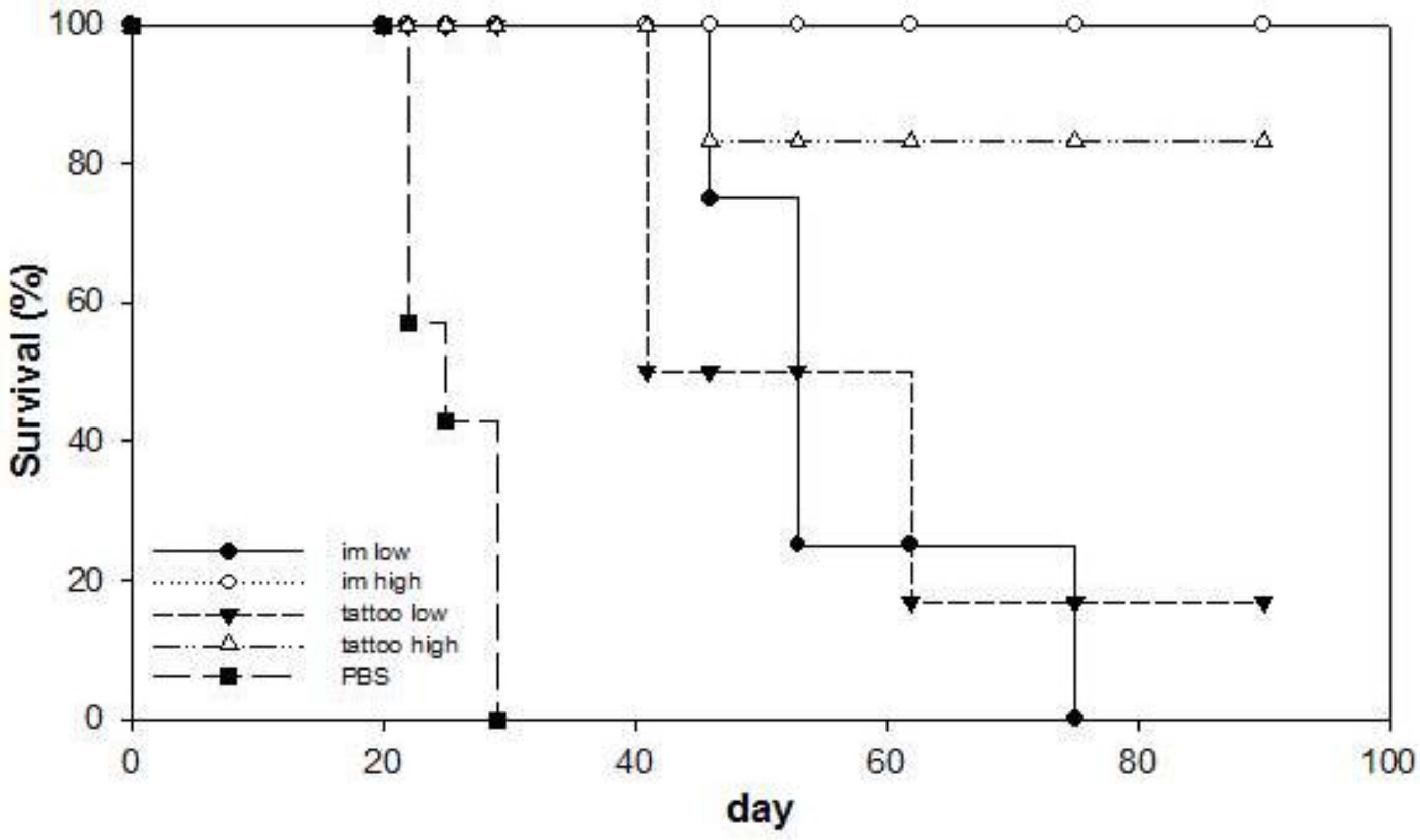
3.3. Anti-Tumor Therapeutic Efficacy of SFVeE6,7 Delivered via Tattooing
3.4. Memory T Cells Induced by SFVeE6,7 Tattoo Immunizations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Riezebos-Brilman, A.; Walczak, M.; Regts, J.; Rots, M.G.; Kamps, G.; Dontje, B.; Haisma, H.Y.; Wilschut, J.; Daemen, T. A comparative study on the immunotherapeutic efficacy of recombinant Semliki Forest virus and adenovirus vector systems in a murine model for cervical cancer. Gene Ther. 2007, 14, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; de Mare, A.; Riezebos-Brilman, A.; Regts, J.; Hoogeboom, B.N.; Visser, J.T.; Fiedler, M.; Jansen-Durr, P.; van der Zee, A.G.; Nijman, H.W.; et al. Heterologous prime-boost immunizations with a virosomal and an alphavirus replicon vaccine. Mol. Pharm. 2011, 8, 65–77. [Google Scholar] [CrossRef]
- Walczak, M.; Regts, J.; van Oosterhout, A.J.; Boon, L.; Wilschut, J.; Nijman, H.W.; Daemen, T. Role of regulatory T-cells in immunization strategies involving a recombinant alphavirus vector system. Antivir. Ther. 2011, 16, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Fehres, C.M.; Garcia-Vallejo, J.J.; Unger, W.W.; van Kooyk, Y. Skin-resident antigen-presenting cells: Instruction manual for vaccine development. Front. Immunol. 2013. [Google Scholar] [CrossRef]
- Clausen, B.E.; Kel, J.M. Langerhans cells: Critical regulators of skin immunity? Immunol. Cell Biol. 2010, 88, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Heath, W.R.; Carbone, F.R. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat. Immunol. 2009, 10, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Klechevsky, E.; Morita, R.; Aspord, C.; Cao, T.; Matsui, T.; di Pucchio, T.; Connolly, J.; Fay, J.W.; Pascual, V.; et al. Dendritic cell subsets in health and disease. Immunol. Rev. 2007, 219, 118–142. [Google Scholar] [CrossRef]
- Haniffa, M.; Gunawan, M.; Jardine, L. Human skin dendritic cells in health and disease. J. Dermatol. Sci. 2015, 77, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Heath, W.R.; Carbone, F.R. The skin-resident and migratory immune system in steady state and memory: Innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013, 14, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Elnekave, M.; Furmanov, K.; Nudel, I.; Arizon, M.; Clausen, B.E.; Hovav, A.H. Directly transfected langerin+ dermal dendritic cells potentiate CD8+ T cell responses following intradermal plasmid DNA immunization. J. Immunol. 2010, 185, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.H.; Laurent, P.E. Intradermal vaccine delivery: Will new delivery systems transform vaccine administration? Vaccine 2008, 26, 3197–3208. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.F.; Guy, B. Intradermal, epidermal and transcutaneous vaccination: From immunology to clinical practice. Expert Rev. Vaccines 2008, 7, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Picot, V. Intradermal immunization: An alternative route for vaccine administration. Articles as per sessions meeting report. Vaccine 2008, 26, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Vankerckhoven, V.; van Damme, P. Clinical studies assessing immunogenicity and safety of intradermally administered influenza vaccines. Expert Opin. Drug Deliv. 2010, 7, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Bachy, V.; Hervouet, C.; Becker, P.D.; Chorro, L.; Carlin, L.M.; Herath, S.; Papagatsias, T.; Barbaroux, J.B.; Oh, S.J.; Benlahrech, A.; et al. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc. Natl. Acad. Sci. USA 2013, 110, 3041–3046. [Google Scholar] [CrossRef]
- DeMuth, P.C.; Min, Y.; Huang, B.; Kramer, J.A.; Miller, A.D.; Barouch, D.H.; Hammond, P.T.; Irvine, D.J. Polymer multilayer tattooing for enhanced DNA vaccination. Nat. Mater. 2013, 12, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.N.; Sampson, J.M.; Jiang, X.; Zolla-Pazner, S.B.; Kong, X.P. Skin tattooing as a novel approach for DNA vaccine delivery. J. Vis. Exp. 2012. [Google Scholar] [CrossRef]
- Oosterhuis, K.; van den Berg, J.H.; Schumacher, T.N.; Haanen, J.B. DNA vaccines and intradermal vaccination by DNA tattooing. Curr. Top. Microbiol. Immunol. 2012, 351, 221–250. [Google Scholar] [PubMed]
- Van den Berg, J.H.; Oosterhuis, K.; Schumacher, T.N.; Haanen, J.B.; Bins, A.D. Intradermal vaccination by DNA tattooing. Methods Mol. Biol. 2014, 1143, 131–140. [Google Scholar] [PubMed]
- Wagemakers, A.; Mason, L.M.; Oei, A.; de Wever, B.; van der Poll, T.; Bins, A.D.; Hovius, J.W. Rapid outer-surface protein C DNA tattoo vaccination protects against Borrelia afzelii infection. Gene Ther. 2014, 21, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Babiarova, K.; Kutinova, L.; Zurkova, K.; Krystofova, J.; Brabcova, E.; Hainz, P.; Musil, J.; Nemeckova, S. Immunization with WT1-derived peptides by tattooing inhibits the growth of TRAMP-C2 prostate tumor in mice. J. Immunother. 2012, 35, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Bins, A.D.; Jorritsma, A.; Wolkers, M.C.; Hung, C.F.; Wu, T.C.; Schumacher, T.N.; Haanen, J.B. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat. Med. 2005, 11, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Pokorna, D.; Rubio, I.; Muller, M. DNA-vaccination via tattooing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet. Vaccines Ther. 2008. [Google Scholar] [CrossRef]
- Verstrepen, B.E.; Bins, A.D.; Rollier, C.S.; Mooij, P.; Koopman, G.; Sheppard, N.C.; Sattentau, Q.; Wagner, R.; Wolf, H.; Schumacher, T.N.; et al. Improved HIV-1 specific T-cell responses by short-interval DNA tattooing as compared to intramuscular immunization in non-human primates. Vaccine 2008, 26, 3346–3351. [Google Scholar] [CrossRef]
- Potthoff, A.; Schwannecke, S.; Nabi, G.; Hoffmann, D.; Grunwald, T.; Wildner, O.; Brockmeyer, N.H.; Uberla, K.; Tenbusch, M. Immunogenicity and efficacy of intradermal tattoo immunization with adenoviral vector vaccines. Vaccine 2009, 27, 2768–2774. [Google Scholar] [CrossRef] [PubMed]
- Daemen, T.; Riezebos-Brilman, A.; Regts, J.; Dontje, B.; van der Zee, A.; Wilschut, J. Superior therapeutic efficacy of alphavirus-mediated immunization against human papilloma virus type 16 antigens in a murine tumour model: Effects of the route of immunization. Antivir Ther. 2004, 9, 733–742. [Google Scholar] [PubMed]
- Daemen, T.; Regts, J.; Holtrop, M.; Wilschut, J. Immunization strategy against cervical cancer involving an alphavirus vector expressing high levels of a stable fusion protein of human papillomavirus 16 E6 and E7. Gene Ther. 2002, 9, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.H.; Nujien, B.; Beijnen, J.H.; Vincent, A.; van Tinteren, H.; Kluge, J.; Woerdeman, L.A.; Hennink, W.E.; Storm, G.; Schumacher, T.N.; et al. Optimization of intradermal vaccination by DNA tattooing in human skin. Hum. Gene Ther. 2009, 20, 181–189. [Google Scholar] [CrossRef]
- Kis, E.E.; Winter, G.; Myschik, J. Devices for intradermal vaccination. Vaccine 2012, 30, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Barnfield, C.; Naslund, T.I.; Fleeton, M.N.; Liljestrom, P. MyD88 expression is required for efficient cross-presentation of viral antigens from infected cells. J. Virol. 2005, 79, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Huckriede, A.; Bungener, L.; Holtrop, M.; de Vries, J.; Waarts, B.L.; Daemen, T.; Wilschut, J. Induction of cytotoxic T lymphocyte activity by immunization with recombinant Semliki Forest virus: Indications for cross-priming. Vaccine 2004, 22, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Bungener, L.; Serre, K.; Bijl, L.; Leserman, L.; Wilschut, J.; Daemen, T.; Machy, P. Virosome-mediated delivery of protein antigens to dendritic cells. Vaccine 2002, 20, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Schulz, O.; Diebold, S.S.; Chen, M.; Naslund, T.I.; Nolte, M.A.; Alexopoulou, L.; Azuma, Y.T.; Flavell, R.A.; Liljestrom, P.; Reis e Sousa, C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 2005, 433, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Belz, G.T.; Smith, C.M.; Eichner, D.; Shortman, K.; Karupiah, G.; Carbone, F.R.; Heath, W.R. Cutting edge: Conventional CD8alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J. Immunol. 2004, 172, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.S.; Smith, C.M.; Belz, G.T.; van Lint, A.L.; Wakim, L.M.; Heath, W.R.; Carbone, F.R. Epidermal viral immunity induced by CD8 alpha+ dendritic cells but not by Langerhans cells. Science 2003, 301, 1925–1928. [Google Scholar] [CrossRef] [PubMed]
- Shortman, K.; Heath, W.R. The CD8+ dendritic cell subset. Immunol. Rev. 2010, 234, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.S.; Waithman, J.; Bedoui, S.; Jones, C.M.; Villadangos, J.A.; Zhan, Y.; Lew, A.M.; Shortman, K.; Heath, W.R.; Carbone, F.R. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 2006, 25, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Stoitzner, P.; Tripp, C.H.; Eberhart, A.; Price, K.M.; Jung, J.Y.; Bursch, L.; Ronchese, F.; Romani, N. Langerhans cells cross-present antigen derived from skin. Proc. Natl. Acad. Sci. USA 2006, 103, 7783–7788. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Cubas, R.; Zhang, S.; Kwon, S.; Sevick-Muraca, E.M.; Li, M.; Chen, C.; Yao, Q. Virus-like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J. Immunother. 2009, 32, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.J.; Halliday, G.M.; King, N.J. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J. Investig. Dermatol. 2000, 114, 560–568. [Google Scholar] [CrossRef]
- Froelich, S.; Tai, A.; Kennedy, K.; Zubair, A.; Wang, P. Virus-receptor mediated transduction of dendritic cells by lentiviruses enveloped with glycoproteins derived from Semliki Forest virus. PLOS ONE 2011, 6, e21491. [Google Scholar] [CrossRef] [PubMed]
- De Witte, L.; Nabatov, A.; Geijtenbeek, T.B. Distinct roles for DC-SIGN+-dendritic cells and Langerhans cells in HIV-1 transmission. Trends Mol. Med. 2008, 14, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kissenpfennig, A.; Henri, S.; Dubois, B.; Laplace-Builhe, C.; Perrin, P.; Romani, N.; Tripp, C.H.; Douillard, P.; Leserman, L.; Kaiserlian, D.; et al. Dynamics and function of Langerhans cells in vivo: Dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 2005, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.H.; Quaak, S.G.; Beijnen, J.H.; Hennink, W.E.; Storm, G.; Schumacher, T.N.; Haanen, J.B.; Nuijen, B. Lipopolysaccharide contamination in intradermal DNA vaccination: Toxic impurity or adjuvant? Int. J. Pharm. 2010, 390, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Harden, L.M.; du Plessis, I.; Poole, S.; Laburn, H.P. Interleukin-6 and leptin mediate lipopolysaccharide-induced fever and sickness behavior. Physiol. Behav. 2006, 89, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, M.; Nakamura, T.; Kato, M.; Ishioka, T.; Kozawa, K.; Wakamatsu, K.; Kimura, H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol. Int. 2008, 32, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Gopee, N.V.; Cui, Y.; Olson, G.; Warbritton, A.R.; Miller, B.J.; Couch, L.H.; Wamer, W.G.; Howard, P.C. Response of mouse skin to tattooing: Use of SKH-1 mice as a surrogate model for human tattooing. Toxicol. Appl. Pharmacol. 2005, 209, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.N.; Halliday, G.M.; Johnston, L.J.; King, N.J. Interleukin-1beta but not tumor necrosis factor is involved in West Nile virus-induced Langerhans cell migration from the skin in C57BL/6 mice. J. Invest. Dermatol. 2001, 117, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Oosterhoff, D.; Heusinkveld, M.; Lougheed, S.M.; Kosten, I.; Lindstedt, M.; Bruijns, S.C.; van Es, T.; van Kooyk, Y.; van der Burg, S.H.; de Gruijl, T.D. Intradermal delivery of TLR agonists in a human explant skin model: Preferential activation of migratory dendritic cells by polyribosinic-polyribocytidylic acid and peptidoglycans. J. Immunol. 2013, 190, 3338–3345. [Google Scholar] [CrossRef]
- Schneider, L.P.; Schoonderwoerd, A.J.; Moutaftsi, M.; Howard, R.F.; Reed, S.G.; de Jong, E.C.; Teunissen, M.B. Intradermally administered TLR4 agonist GLA-SE enhances the capacity of human skin DCs to activate T cells and promotes emigration of Langerhans cells. Vaccine 2012, 30, 4216–4224. [Google Scholar] [CrossRef] [PubMed]
- Fehres, C.M.; Bruijns, S.C.; van Beelen, A.J.; Kalay, H.; Ambrosini, M.; Hooijberg, E.; Unger, W.W.; de Gruijl, T.D.; van Kooyk, Y. Topical rather than intradermal application of the TLR7 ligand imiquimod leads to human dermal dendritic cell maturation and CD8+ T-cell cross-priming. Eur. J. Immunol. 2014, 44, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.E.; Newell, L.; Taraban, V.Y.; Pickard, C.; Healy, E.; Friedmann, P.S.; al-Shamkhani, A.; Ardern-Jones, M.R. CD70-CD27 interaction augments CD8+ T-cell activation by human epidermal Langerhans cells. J. Investig. Dermatol. 2012, 132, 1636–1644. [Google Scholar]
- Flacher, V.; Tripp, C.H.; Mairhofer, D.G.; Steinman, R.M.; Stoitzner, P.; Idoyaga, J.; Romani, N. Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance. EMBO Mol. Med. 2014, 6, 1191–1204. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van de Wall, S.; Walczak, M.; Van Rooij, N.; Hoogeboom, B.-N.; Meijerhof, T.; Nijman, H.W.; Daemen, T. Tattoo Delivery of a Semliki Forest Virus-Based Vaccine Encoding Human Papillomavirus E6 and E7. Vaccines 2015, 3, 221-238. https://doi.org/10.3390/vaccines3020221
Van de Wall S, Walczak M, Van Rooij N, Hoogeboom B-N, Meijerhof T, Nijman HW, Daemen T. Tattoo Delivery of a Semliki Forest Virus-Based Vaccine Encoding Human Papillomavirus E6 and E7. Vaccines. 2015; 3(2):221-238. https://doi.org/10.3390/vaccines3020221
Chicago/Turabian StyleVan de Wall, Stephanie, Mateusz Walczak, Nienke Van Rooij, Baukje-Nynke Hoogeboom, Tjarko Meijerhof, Hans W. Nijman, and Toos Daemen. 2015. "Tattoo Delivery of a Semliki Forest Virus-Based Vaccine Encoding Human Papillomavirus E6 and E7" Vaccines 3, no. 2: 221-238. https://doi.org/10.3390/vaccines3020221
APA StyleVan de Wall, S., Walczak, M., Van Rooij, N., Hoogeboom, B.-N., Meijerhof, T., Nijman, H. W., & Daemen, T. (2015). Tattoo Delivery of a Semliki Forest Virus-Based Vaccine Encoding Human Papillomavirus E6 and E7. Vaccines, 3(2), 221-238. https://doi.org/10.3390/vaccines3020221




