Challenges in the Vaccination of HIV-Infected Individuals
Abstract
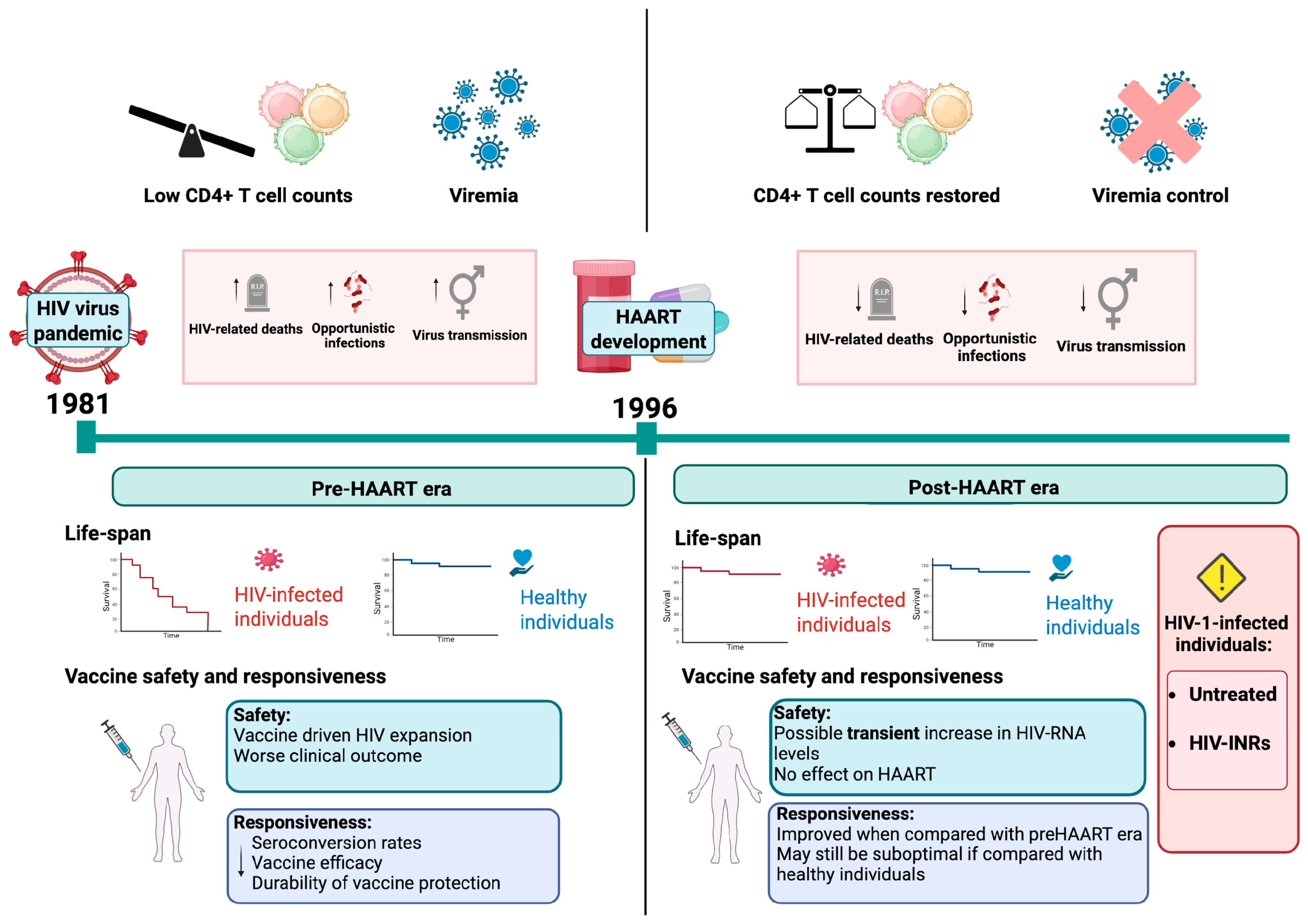
1. Introduction
2. The Impact of HAART on HIV Infection
3. Challenges of Vaccinating HIV-Infected Individuals
4. Vaccine Recommendations for PLWH
4.1. Vaccines Safe for Use in PLWH
4.2. Routine, Baseline of Care Vaccines
4.3. Vaccines Recommended Under Special Circumstances
4.4. Travel Vaccines
5. Therapeutic HIV Vaccination Towards a Functional Cure
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gottlieb, M.S.; Schroff, R.; Schanker, H.M.; Weisman, J.D.; Fan, P.T.; Wolf, R.A.; Saxon, A. Pneumocystis Carinii Pneumonia and Mucosal Candidiasis in Previously Healthy Homosexual Men—Evidence of a New Acquired Cellular Immunodeficiency. N. Engl. J. Med. 1981, 305, 1425–1431. [Google Scholar] [CrossRef]
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 17 November 2025).
- U.S. Department of Health & Human Services. HIV.gov. The Global HIV and AIDS Epidemic. 2025. Available online: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics (accessed on 17 November 2025).
- Okoye, A.A.; Picker, L.J. CD4+ T-cell Depletion in HIV Infection: Mechanisms of Immunological Failure. Immunol. Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef]
- Doitsh, G.; Greene, W.C. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe 2016, 19, 280–291. [Google Scholar] [CrossRef]
- Vijayan, K.K.V.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.E.; Carneiro, J.; Meier-Schellersheim, M.; Grossman, Z.; Victorino, R.M.M. CD4 T Cell Depletion Is Linked Directly to Immune Activation in the Pathogenesis of HIV-1 and HIV-2 but Only Indirectly to the Viral Load. J. Immunol. 2002, 169, 3400–3406. [Google Scholar] [CrossRef] [PubMed]
- Foxall, R.B.; Cortesão, C.S.; Albuquerque, A.S.; Soares, R.S.; Victorino, R.M.M.; Sousa, A.E. Gag-Specific CD4+ T-Cell Frequency Is Inversely Correlated with Proviral Load and Directly Correlated with Immune Activation in Infection with Human Immunodeficiency Virus Type 2 (HIV-2) but Not HIV-1. J. Virol. 2008, 82, 9795–9799. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.; Balde, A.T.; Roussilhon, C.; Aribot, G.; Sarthou, J.-L.; Gougeon, M.-L. Reduced Immune Activation and T Cell Apoptosis in Human Immunodeficiency Virus Type 2 Compared with Type 1: Correlation of T Cell Apoptosis with Β2 Microglobulin Concentration and Disease Evolution. J. Infect. Dis. 2000, 181, 64–75. [Google Scholar] [CrossRef]
- MacNeil, A.; Sarr, A.D.; Sankalé, J.-L.; Meloni, S.T.; Mboup, S.; Kanki, P. Direct Evidence of Lower Viral Replication Rates In Vivo in Human Immunodeficiency Virus Type 2 (HIV-2) Infection than in HIV-1 Infection. J. Virol. 2007, 81, 5325–5330. [Google Scholar] [CrossRef]
- Oboho, I.K.; Paulin, H.; Corcoran, C.; Hamilton, M.; Jordan, A.; Kirking, H.L.; Agyemang, E.; Podewils, L.J.; Pretorius, C.; Greene, G.; et al. Modelling the Impact of CD4 Testing on Mortality from TB and Cryptococcal Meningitis among Patients with Advanced HIV Disease in Nine Countries. J. Int. AIDS Soc. 2023, 26, e26070. [Google Scholar] [CrossRef]
- Perez-Molina, J.A.; Crespillo-Andújar, C.; Zamora, J.; Fernández-Félix, B.M.; Gaetano-Gil, A.; de Quirós, J.C.L.-B.; Serrano-Villar, S.; Moreno, S.; Álvarez-Díaz, N.; Berenguer, J. Contribution of Low CD4 Cell Counts and High Human Immunodeficiency Virus (HIV) Viral Load to the Efficacy of Preferred First-Line Antiretroviral Regimens for Treating HIV Infection: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2023, 76, 2027–2037. [Google Scholar] [CrossRef]
- Lehman, A.; Ellis, J.; Nalintya, E.; Bahr, N.C.; Loyse, A.; Rajasingham, R. Advanced HIV Disease: A Review of Diagnostic and Prophylactic Strategies. HIV Med. 2023, 24, 859–876. [Google Scholar] [CrossRef]
- Meya, D.B.; Tugume, L.; Nabitaka, V.; Namuwenge, P.; Phiri, S.; Oladele, R.; Jibrin, B.; Mobolaji-Bello, M.; Kanyama, C.; Maokola, W.; et al. Establishing Targets for Advanced HIV Disease: A Call to Action. South. Afr. J. HIV Med. 2021, 22, a1266. [Google Scholar] [CrossRef]
- Ford, N.; Meintjes, G.; Calmy, A.; Bygrave, H.; Migone, C.; Vitoria, M.; Penazzato, M.; Vojnov, L.; Doherty, M.; Guideline Development Group for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy. Managing Advanced HIV Disease in a Public Health Approach. Clin. Infect. Dis. 2018, 66, S106–S110. [Google Scholar] [CrossRef] [PubMed]
- Utay, N.S.; Hunt, P.W. Role of Immune Activation in Progression to AIDS. Curr. Opin. HIV AIDS 2016, 11, 131–137. [Google Scholar] [CrossRef]
- Lv, T.; Cao, W.; Li, T. HIV-Related Immune Activation and Inflammation: Current Understanding and Strategies. J. Immunol. Res. 2021, 2021, 7316456. [Google Scholar] [CrossRef]
- Walker, B.D.; Ahmed, R.; Plotkin, S. Moving Ahead an HIV Vaccine: Use Both Arms to Beat HIV. Nat. Med. 2011, 17, 1194–1195. [Google Scholar] [CrossRef]
- Scott, G.Y.; Worku, D. HIV Vaccination: Navigating the Path to a Transformative Breakthrough—A Review of Current Evidence. Heal. Sci. Rep. 2024, 7, e70089. [Google Scholar] [CrossRef] [PubMed]
- Sheila, M.B.; Alfredo, J.M.L.; Richard, M.N. Trial, Error, and Breakthrough: A Review of HIV Vaccine Development. J. AIDS Clin. Res. 2014, 5, 11. [Google Scholar] [CrossRef]
- Barouch, D.H. Challenges in the Development of an HIV-1 Vaccine. Nature 2008, 455, 613–619. [Google Scholar] [CrossRef]
- Ng’uni, T.; Chasara, C.; Ndhlovu, Z.M. Major Scientific Hurdles in HIV Vaccine Development: Historical Perspective and Future Directions. Front. Immunol. 2020, 11, 590780. [Google Scholar] [CrossRef] [PubMed]
- Meyerhans, A.; Cheynier, R.; Albert, J.; Seth, M.; Kwok, S.; Sninsky, J.; Morfeldt-Månson, L.; Asjö, B.; Wain-Hobson, S. Temporal Fluctuations in HIV Quasispecies in Vivo Are Not Reflected by Sequential HIV Isolations. Cell 1989, 58, 901–910. [Google Scholar] [CrossRef]
- Vartanian, J.P.; Meyerhans, A.; Asjö, B.; Wain-Hobson, S. Selection, Recombination, and G----A Hypermutation of Human Immunodeficiency Virus Type 1 Genomes. J. Virol. 1991, 65, 1779–1788. [Google Scholar] [CrossRef]
- Yazdanpanah, Y.; Sissoko, D.; Egger, M.; Mouton, Y.; Zwahlen, M.; Chêne, G. Clinical Efficacy of Antiretroviral Combination Therapy Based on Protease Inhibitors or Non-Nucleoside Analogue Reverse Transcriptase Inhibitors: Indirect Comparison of Controlled Trials. BMJ 2004, 328, 249. [Google Scholar] [CrossRef]
- Hogg, R.S.; Yip, B.; Kully, C.; Craib, K.J.; O’Shaughnessy, M.V.; Schechter, M.T.; Montaner, J.S. Improved Survival among HIV-Infected Patients after Initiation of Triple-Drug Antiretroviral Regimens. Can. Med. Assoc. J. 1999, 160, 659–665. [Google Scholar]
- McMyn, N.F.; Varriale, J.; Fray, E.J.; Zitzmann, C.; MacLeod, H.J.; Lai, J.; Singhal, A.; Moskovljevic, M.; Garcia, M.A.; Lopez, B.M.; et al. The Latent Reservoir of Inducible, Infectious HIV-1 Does Not Decrease despite Decades of Antiretroviral Therapy. J. Clin. Investig. 2023, 133, e171554. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Siliciano, R.F. In Vivo Dynamics of the Latent Reservoir for HIV-1: New Insights and Implications for Cure. Annu. Rev. Pathol. Mech. Dis. 2021, 17, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Mbonye, U.; Karn, J. The Cell Biology of HIV-1 Latency and Rebound. Retrovirology 2024, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Mahungu, T.W.; Rodger, A.J.; Johnson, M.A. HIV as a Chronic Disease. Clin. Med. 2009, 9, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.L.; Collier, A.C.; Kalish, L.A.; Assmann, S.F.; Para, M.F.; Flanigan, T.P.; Kumar, P.N.; Mintz, L.; Wallach, F.R.; Nemo, G.J.; et al. Highly Active Antiretroviral Therapy Decreases Mortality and Morbidity in Patients with Advanced HIV Disease. Ann. Intern. Med. 2001, 135, 17–26. [Google Scholar] [CrossRef]
- Gueler, A.; Moser, A.; Calmy, A.; Günthard, H.F.; Bernasconi, E.; Furrer, H.; Fux, C.A.; Battegay, M.; Cavassini, M.; Vernazza, P.; et al. Life Expectancy in HIV-Positive Persons in Switzerland. AIDS 2017, 31, 427–436. [Google Scholar] [CrossRef]
- The Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord; Lewden, C.; Bouteloup, V.; Wit, S.D.; Sabin, C.; Mocroft, A.; Wasmuth, J.C.; van Sighem, A.; Kirk, O.; Obel, N.; et al. All-Cause Mortality in Treated HIV-Infected Adults with CD4 ≥ 500/Mm3 Compared with the General Population: Evidence from a Large European Observational Cohort Collaboration†. Int. J. Epidemiol. 2012, 41, 433–445. [Google Scholar] [CrossRef]
- van Sighem, A.I.; Gras, L.A.J.; Reiss, P.; Brinkman, K.; de Wolf, F.; on Behalf of the Athena National Observational Cohort Study. Life Expectancy of Recently Diagnosed Asymptomatic HIV-Infected Patients Approaches That of Uninfected Individuals. AIDS 2010, 24, 1527–1535. [Google Scholar] [CrossRef]
- Sever, B.; Otsuka, M.; Fujita, M.; Ciftci, H. A Review of FDA-Approved Anti-HIV-1 Drugs, Anti-Gag Compounds, and Potential Strategies for HIV-1 Eradication. Int. J. Mol. Sci. 2024, 25, 3659. [Google Scholar] [CrossRef] [PubMed]
- Azzman, N.; Gill, M.S.A.; Hassan, S.S.; Christ, F.; Debyser, Z.; Mohamed, W.A.S.; Ahemad, N. Pharmacological Advances in Anti-retroviral Therapy for Human Immunodeficiency Virus-1 Infection: A Comprehensive Review. Rev. Méd. Virol. 2024, 34, e2529. [Google Scholar] [CrossRef]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. U.S. Department of Health & Human Services, 2025. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/drug-characteristics-tables (accessed on 17 November 2025).
- VeryWell Health. List of Approved HIV Antiretroviral Drugs. Available online: https://www.verywellhealth.com/list-of-approved-hiv-antiretroviral-drugs-49309 (accessed on 17 November 2025).
- Wang, W.; Zhao, S.; Wu, Y.; Duan, W.; Li, S.; Li, Z.; Guo, C.; Wang, W.; Zhang, T.; Wu, H.; et al. Safety and Efficacy of Long-Acting Injectable Agents for HIV-1: Systematic Review and Meta-Analysis. JMIR Public Health Surveill. 2023, 9, e46767. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, H.; Overton, E.T.; Richmond, G.; Rizzardini, G.; Andrade-Villanueva, J.F.; Mngqibisa, R.; Hermida, A.O.; Thalme, A.; Belonosova, E.; Ajana, F.; et al. Long-Acting Cabotegravir and Rilpivirine Dosed Every 2 Months in Adults with HIV-1 Infection (ATLAS-2M), 96-Week Results: A Randomised, Multicentre, Open-Label, Phase 3b, Non-Inferiority Study. Lancet HIV 2021, 8, e679–e689. [Google Scholar] [CrossRef]
- Gupta, S.K.; Berhe, M.; Crofoot, G.; Benson, P.; Ramgopal, M.; Sims, J.; McDonald, C.; Ruane, P.; Sanchez, W.E.; Scribner, A.; et al. Lenacapavir Administered Every 26 Weeks or Daily in Combination with Oral Daily Antiretroviral Therapy for Initial Treatment of HIV: A Randomised, Open-Label, Active-Controlled, Phase 2 Trial. Lancet HIV 2023, 10, e15–e23. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; D’Amico, R.; Sievers, J.; Brimhall, D.; Spears, B.; Taylor, D.; Dorey, D.; Benn, P.; Morgan, L.; Hareedy, R.; et al. Phase 1 Study of Cabotegravir Long- Acting Injectable Formulations Supports ≥ 4-Monthly Dose Interval. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Denver, CO, USA, 3–6 March 2024. [Google Scholar]
- Erb, P.; Battegay, M.; Zimmerli, W.; Rickenbach, M.; Egger, M. Effect of Antiretroviral Therapy on Viral Load, CD4 Cell Count, and Progression to Acquired Immunodeficiency Syndrome in a Community Human Immunodeficiency Virus–Infected Cohort. Arch. Intern. Med. 2000, 160, 1134–1140. [Google Scholar] [CrossRef]
- Thoueille, P.; Choong, E.; Cavassini, M.; Buclin, T.; Decosterd, L.A. Long-Acting Antiretrovirals: A New Era for the Management and Prevention of HIV Infection. J. Antimicrob. Chemother. 2021, 77, 290–302. [Google Scholar] [CrossRef]
- Detels, R. The Role of Epidemiology in Challenging the HIV/AIDS Pandemic. J. Epidemiol. 2007, 11, 95–102. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines on HIV, Viral Hepatitis and Sexually Transmitted Infections. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/guidelines (accessed on 17 November 2025).
- Gandhi, R.T.; Landovitz, R.J.; Sax, P.E.; Smith, D.M.; Springer, S.A.; Günthard, H.F.; Thompson, M.A.; Bedimo, R.J.; Benson, C.A.; Buchbinder, S.P.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV in Adults: 2024 Recommendations of the International Antiviral Society–USA Panel. JAMA 2025, 333, 609–628. [Google Scholar] [CrossRef]
- The INSIGHT START Study Group; Lundgren, J.D.; Babiker, A.G.; Gordin, F.; Emery, S.; Grund, B.; Sharma, S.; Avihingsanon, A.; Cooper, D.A.; Fätkenheuer, G.; et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N. Engl. J. Med. 2015, 373, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Brazier, E.; Maruri, F.; Duda, S.N.; Tymejczyk, O.; Wester, C.W.; Somi, G.; Ross, J.; Freeman, A.; Cornell, M.; Poda, A.; et al. Implementation of “Treat-all” at Adult HIV Care and Treatment Sites in the Global IeDEA Consortium: Results from the Site Assessment Survey. J. Int. AIDS Soc. 2019, 22, e25331. [Google Scholar] [CrossRef]
- Song, A.; Liu, X.; Huang, X.; Meyers, K.; Oh, D.-Y.; Hou, J.; Xia, W.; Su, B.; Wang, N.; Lu, X.; et al. From CD4-Based Initiation to Treating All HIV-Infected Adults Immediately: An Evidence-Based Meta-Analysis. Front. Immunol. 2018, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Rodger, A.J.; Sabin, C.A. How Have Guidelines on When to Start Antiretroviral Therapy Affected Survival of People Living with HIV Infection? Curr. Opin. HIV AIDS 2016, 11, 487–491. [Google Scholar] [CrossRef] [PubMed]
- TEMPRANO ANRS 12136 Study Group; Danel, C.; Moh, R.; Gabillard, D.; Badje, A.; Carrou, J.L.; Ouassa, T.; Ouattara, E.; Anzian, A.; Ntakpé, J.-B.; et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N. Engl. J. Med. 2015, 373, 808–822. [Google Scholar] [CrossRef]
- Mahy, M.I.; Sabin, K.M.; Feizzadeh, A.; Wanyeki, I. Progress towards 2020 Global HIV Impact and Treatment Targets. J. Int. AIDS Soc. 2021, 24, e25779. [Google Scholar] [CrossRef]
- Guedes, M.C.S.; Lopes-Araujo, H.F.; dos Santos, K.F.; Simões, E.; Carvalho-Silva, W.H.V.; Guimarães, R.L. How to Properly Define Immunological Nonresponse to Antiretroviral Therapy in People Living with HIV? An Integrative Review. Front. Immunol. 2025, 16, 1535565. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Loeliger, A.E.; Rijkers, G.T.; Aerts, P.; Been-Tiktak, A.; Hoepelman, A.I.M.; van Dijk, H.; Borleffs, J.C.C. Deficient Antipneumococcal Polysaccharide Responses in HIV-Seropositive Patients. FEMS Immunol. Méd. Microbiol. 1995, 12, 33–41. [Google Scholar] [CrossRef]
- Kroon, F.P.; van Dissel, J.T.; de Jong, J.C.; van Furth, R. Antibody Response to Influenza, Tetanus and Pneumococcal Vaccines in HIV-Seropositive Individuals in Relation to the Number of CD4+ Lymphocytes. AIDS 1994, 8, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Février, M.; Dorgham, K.; Rebollo, A. CD4+ T Cell Depletion in Human Immunodeficiency Virus (HIV) Infection: Role of Apoptosis. Viruses 2011, 3, 586–612. [Google Scholar] [CrossRef]
- Chamcha, V.; Reddy, P.B.J.; Kannanganat, S.; Wilkins, C.; Gangadhara, S.; Velu, V.; Green, R.; Law, G.L.; Chang, J.; Bowen, J.R.; et al. Strong TH1-Biased CD4 T Cell Responses Are Associated with Diminished SIV Vaccine Efficacy. Sci. Transl. Med. 2019, 11, eaav1800. [Google Scholar] [CrossRef]
- Kießling, M.; Cole, J.J.; Kübel, S.; Klein, P.; Korn, K.; Henry, A.R.; Laboune, F.; Fourati, S.; Harrer, E.; Harrer, T.; et al. Chronic Inflammation Degrades CD4 T Cell Immunity to Prior Vaccines in Treated HIV Infection. Nat. Commun. 2024, 15, 10200. [Google Scholar] [CrossRef]
- Kernéis, S.; Launay, O.; Turbelin, C.; Batteux, F.; Hanslik, T.; Boëlle, P.-Y. Long-Term Immune Responses to Vaccination in HIV-Infected Patients: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2014, 58, 1130–1139. [Google Scholar] [CrossRef]
- Schuetz, A.; Dirks, J.; Sester, U.; Haule, A.; Elias, N.; Geldmacher, C.; Sanga, E.; Maboko, L.; Reither, K.; Hoelscher, M.; et al. Pathogen Prevalence May Determine Maintenance of Antigen-Specific T-Cell Responses in HIV-Infected Individuals. AIDS 2012, 26, 695–700. [Google Scholar] [CrossRef]
- McGrath, B.; Broadhurst, M.; Roman, C. Infectious Disease Considerations in Immunocompromised Patients. JAAPA 2020, 33, 16–25. [Google Scholar] [CrossRef]
- Taramasso, L.; Labate, L.; Briano, F.; Brucci, G.; Mora, S.; Blanchi, S.; Giacomini, M.; Bassetti, M.; Biagio, A.D. CD4+ T Lymphocyte Recovery in the Modern Antiretroviral Therapy Era: Toward a New Threshold for Defining Immunological Non-Responders. Front. Virol. 2023, 2, 822153. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; He, Y.; Zhao, F.; Luo, Y.; Zhao, D.; Wu, H.; He, J.; Jiang, Y.; Liu, C.; et al. Two-Threshold Defined Immune Non-Responders Are Associated with Long-Term Morbidity in People with HIV: A Prospective Cohort Study. Emerg. Microbes Infect. 2025, 14, 2539198. [Google Scholar] [CrossRef]
- Zhang, W.; Ruan, L. Recent Advances in Poor HIV Immune Reconstitution: What Will the Future Look Like? Front. Microbiol. 2023, 14, 1236460. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Gaskell, K.M.; Richardson, M.; Klein, N.; Garner, P.; MacPherson, P. Discordant Immune Response with Antiretroviral Therapy in HIV-1: A Systematic Review of Clinical Outcomes. PLoS ONE 2016, 11, e0156099. [Google Scholar] [CrossRef]
- Karl, V.; Graeser, A.; Kremser, A.; Bauersfeld, L.; Emmerich, F.; Herkt, N.; Rieg, S.; Usadel, S.; Bengsch, B.; Boettler, T.; et al. Limited Vaccine-Induced CD8+ T Cell Immunity in HIV-Infected Immunological Nonresponders. JCI Insight 2025, 10, e195458. [Google Scholar] [CrossRef]
- Sisteré-Oró, M.; Andrade, N.; Wortmann, D.D.J.; Du, J.; Garcia-Giralt, N.; González-Cao, M.; Güerri-Fernández, R.; Meyerhans, A. Anti-SARS-CoV-2 Specific Immunity in HIV Immunological Non-Responders after mRNA-Based COVID-19 Vaccination. Front. Immunol. 2022, 13, 994173. [Google Scholar] [CrossRef]
- Alirezaylavasani, A.; Skeie, L.G.; Egner, I.M.; Chopra, A.; Dahl, T.B.; Prebensen, C.; Vaage, J.T.; Halvorsen, B.; Lund-Johansen, F.; Tonby, K.; et al. Vaccine Responses and Hybrid Immunity in People Living with HIV after SARS-CoV-2 Breakthrough Infections. npj Vaccines 2024, 9, 185. [Google Scholar] [CrossRef]
- Song, J.Y.; Cheong, H.J.; Noh, J.Y.; Choi, M.J.; Yoon, J.G.; Kim, W.J. Immunogenicity and Safety of 13-Valent Pneumococcal Conjugate Vaccine in HIV-Infected Adults in the Era of Highly Active Antiretroviral Therapy: Analysis Stratified by CD4 T-Cell Count. Hum. Vaccines Immunother. 2020, 16, 169–175. [Google Scholar] [CrossRef]
- Kojic, E.M.; Kang, M.; Cespedes, M.S.; Umbleja, T.; Godfrey, C.; Allen, R.T.; Firnhaber, C.; Grinsztejn, B.; Palefsky, J.M.; Webster-Cyriaque, J.Y.; et al. Immunogenicity and Safety of the Quadrivalent Human Papillomavirus Vaccine in HIV-1–Infected Women. Clin. Infect. Dis. 2014, 59, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Hua, W.; Wu, Y.; Zhang, T.; Wang, W.; Wu, H.; Guo, C.; Huang, X. Immune Response to Hepatitis B Virus Vaccine among People Living with HIV: A Meta-Analysis. Front. Immunol. 2021, 12, 745541. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.; Dyer, M.; Urban, M.A.; Vail, R.M.; Shah, S.S.; Fine, S.M.; McGowan, J.P.; Merrick, S.T.; Radix, A.E.; Monroe, A.K.; et al. Immunizations for Adults with HIV; Johns Hopkins University: Bethesda, MD, USA, 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK597807/ (accessed on 17 November 2025).
- University of Washington. Basic HIV Primary Care: Immunizations in Adults. 2025. Available online: https://www.hiv.uw.edu/go/basic-primary-care/immunizations (accessed on 17 November 2025).
- Pantazis, N.; Paparizos, V.; Papastamopoulos, V.; Metallidis, S.; Antoniadou, A.; Adamis, G.; Psichgiou, M.; Chini, M.; Sambatakou, H.; Chrysos, G.; et al. Low Pre-ART CD4 Count Is Associated with Increased Risk of Clinical Progression or Death Even after Reaching 500 CD4 Cells/μL on ART. PLoS ONE 2023, 18, e0283648. [Google Scholar] [CrossRef]
- Mocroft, A.; Furrer, H.J.; Miro, J.M.; Reiss, P.; Mussini, C.; Kirk, O.; Abgrall, S.; Ayayi, S.; Bartmeyer, B.; Braun, D.; et al. The Incidence of AIDS-Defining Illnesses at a Current CD4 Count ≥ 200 Cells/µL in the Post–Combination Antiretroviral Therapy Era. Clin. Infect. Dis. 2013, 57, 1038–1047. [Google Scholar] [CrossRef]
- Clinical Guidelines Program, New York State Department of Health AIDS Institute. Immunizations for Adults with HIV. 2025. Available online: https://www.hivguidelines.org/guideline/hiv-immunizations/ (accessed on 17 November 2025).
- Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. National Institutes of Health, 2025. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/immunizations (accessed on 17 November 2025).
- Centers for Disease Control and Prevention (CDC). Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States. 2025. Available online: https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/adult/adult-combined-schedule.pdf (accessed on 17 November 2025).
- European AIDS Clinical Society (EACS). Immunisation in Persons with HIV. Version 13.0. 2025. Available online: https://eacs.sanfordguide.com/en/eacs-hiv/eacs-section4/vaccination (accessed on 17 November 2025).
- Immunize.org. Vaccinations for Adults with HIV Infection. 2025. Available online: https://www.immunize.org/wp-content/uploads/catg.d/p4041.pdf (accessed on 17 November 2025).
- Nunes, M.C.; Madhi, S.A. Safety, Immunogenicity and Efficacy of Pneumococcal Conjugate Vaccine in HIV-Infected Individuals. Hum. Vaccines Immunother. 2012, 8, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Cohen, C.; von Gottberg, A. Introduction of Pneumococcal Conjugate Vaccine into the Public Immunization Program in South Africa: Translating Research into Policy. Vaccine 2012, 30, C21–C27. [Google Scholar] [CrossRef]
- Gabutti, G.; Guido, M.; Durando, P.; Donno, A.D.; Quattrocchi, M.; Bacilieri, S.; Ansaldi, F.; Cataldini, S.; Chiriacò, P.; Simone, M.D.; et al. Safety and Immunogenicity of Conventional Subunit and MF59-Adjuvanted Influenza Vaccines in Human Immunodeficiency Virus-1-Seropositive Patients. J. Int. Méd. Res. 2005, 33, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Shang, W.; Gao, P.; Wang, Y.; Liu, J.; Liu, M. Immunogenicity and Safety of COVID-19 Vaccines among People Living with HIV: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1569. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, X.; Feng, Y.; Wu, S.; Jiang, Y. Safety and Efficacy of Human Papillomavirus Vaccination for People Living with HIV: A Systematic Review and Meta-Analysis. Int. J. STD AIDS 2019, 30, 1105–1115. [Google Scholar] [CrossRef]
- Vito, A.D.; Colpani, A.; Trunfio, M.; Fiore, V.; Moi, G.; Fois, M.; Leoni, N.; Ruiu, S.; Babudieri, S.; Calcagno, A.; et al. Living with HIV and Getting Vaccinated: A Narrative Review. Vaccines 2023, 11, 896. [Google Scholar] [CrossRef]
- Geretti, A.M.; Brook, G.; Cameron, C.; Chadwick, D.; French, N.; Heyderman, R.; Ho, A.; Hunter, M.; Ladhani, S.; Lawton, M.; et al. British HIV Association Guidelines on the Use of Vaccines in HIV-Positive Adults 2015. HIV Med. 2016, 17, s2–s81. [Google Scholar] [CrossRef] [PubMed]
- Crum-Cianflone, N.F.; Sullivan, E. Vaccinations for the HIV-Infected Adult: A Review of the Current Recommendations, Part II. Infect. Dis. Ther. 2017, 6, 333–361. [Google Scholar] [CrossRef]
- Cioe, P.A.; Melbourne, K.; Larkin, J. An Immunization Update for HIV-Infected Adults in the United States: Review of the Literature. J. Assoc. Nurses AIDS Care 2015, 26, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Veit, O.; Niedrig, M.; Chapuis-Taillard, C.; Cavassini, M.; Mossdorf, E.; Schmid, P.; Bae, H.-G.; Litzba, N.; Staub, T.; Hatz, C.; et al. Immunogenicity and Safety of Yellow Fever Vaccination for 102 HIV-Infected Patients. Clin. Infect. Dis. 2009, 48, 659–666. [Google Scholar] [CrossRef]
- Sidibe, M.; Yactayo, S.; Kalle, A.; Sall, A.A.; Sow, S.; Ndoutabe, M.; Perea, W.; Avokey, F.; Lewis, R.F.; Veit, O. Immunogenicity and Safety of Yellow Fever Vaccine among 115 HIV-Infected Patients after a Preventive Immunisation Campaign in Mali. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 437–444. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Blanton, L.H.; Ferdinands, J.M.; Chung, J.R.; Broder, K.R.; Talbot, H.K. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023–2024Influenza Season. MMWR Recomm. Rep. 2023, 72, 1–25. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Contraindications and Precautions for Polio Vaccination. 2025. Available online: https://www.cdc.gov/vaccines/vpd/polio/hcp/contraindications-precautions.html (accessed on 18 November 2025).
- U.S. Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC). ACAM2000 Vaccine—Smallpox and Mpox (Vaccinia) Vaccine, Live. 2025. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/acam2000 (accessed on 18 November 2025).
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J.; et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR 2022, 71, 734–742. [Google Scholar] [CrossRef]
- Overton, E.T.; Stapleton, J.; Frank, I.; Hassler, S.; Goepfert, P.A.; Barker, D.; Wagner, E.; von Krempelhuber, A.; Virgin, G.; Weigl, J.; et al. Safety and Immunogenicity of Modified Vaccinia Ankara-Bavarian Nordic Smallpox Vaccine in Vaccinia-Naive and Experienced Human Immunodeficiency Virus-Infected Individuals: An Open-Label, Controlled Clinical Phase II Trial. Open Forum Infect. Dis. 2015, 2, ofv040. [Google Scholar] [CrossRef]
- Sisteré-Oró, M.; Du, J.; Wortmann, D.D.J.; Filippi, M.D.; Cañas-Ruano, E.; Arrieta-Aldea, I.; Marcos-Blanco, A.; Castells, X.; Grau, S.; García-Giralt, N.; et al. Pan-pox-specific T-cell Responses in HIV-1-infected Individuals after JYNNEOS Vaccination. J. Méd. Virol. 2024, 96, e29317. [Google Scholar] [CrossRef] [PubMed]
- Gispen, F.; Marks, K.M. Update on Vaccination Recommendations for Adults with HIV. Curr. HIVAIDS Rep. 2025, 22, 17. [Google Scholar] [CrossRef]
- Mofenson, L.M.; Brady, M.T.; Danner, S.P.; Dominguez, K.L.; Hazra, R.; Handelsman, E.; Havens, P.; Nesheim, S.; Read, J.S.; Serchuck, L.; et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-Exposed and HIV-Infected Children: Recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR 2009, 58, 1–166. [Google Scholar]
- Ołdakowska, A.; Pokorska-Śpiewak, M.; Marczyńska, M.M. Vaccination in Children Living with HIV—Review of Polish Recommendations. Pediatr. Med. Rodz. 2024, 20, 10–16. [Google Scholar] [CrossRef]
- Berghella, L.; Tancredi, S.; Cintori, C.; Kahfian, Z.; Rovesti, S.; Bargellini, A.; Mussini, C.; Casaletti, G. Update on Adherence to a Vaccination Protocol for Invasive Bacterial Diseases in HIV Patients. Eur. J. Public Health 2020, 30, ckaa166.1428. [Google Scholar] [CrossRef]
- Menson, E.N.; Mellado, M.J.; Bamford, A.; Castelli, G.; Duiculescu, D.; Marczyńska, M.; Navarro, M.L.; Scherpbier, H.J.; Heath, P.T.; Paediatric European Network for Treatment of AIDS (PENTA) Vaccines Group; et al. Guidance on Vaccination of HIV-infected Children in Europe. HIV Med. 2012, 13, 333–336. [Google Scholar] [CrossRef]
- Horberg, M.; Thompson, M.; Agwu, A.; Colasanti, J.; Haddad, M.; Jain, M.; McComsey, G.; Radix, A.; Rakhmanina, N.; Short, W.R.; et al. Primary Care Guidance for Providers Who Care for Persons with Human Immunodeficiency Virus: 2024 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 2024, ciae479. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Safety of BCG Vaccination in Immunocompromised Individuals. Global Advisory Committee on Vaccine Safety (GACVS) Meeting Reports, Including 2003, 2006, 2009, and 2017. Available online: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/bcg-vaccines (accessed on 18 November 2025).
- European AIDS Clinical Society. Guidelines for the Management of People Living with HIV in Europe (Version 13.0). 2025. Available online: https://www.eacsociety.org/guidelines/eacs-guidelines (accessed on 24 November 2025).
- TB Elimination. BCG Vaccine. Available online: https://www.cdc.gov/tb/publications/factsheets/prevention/bcg.pdf (accessed on 18 November 2025).
- World Health Organization. BCG Vaccines: WHO Position Paper—February 2018. Available online: https://www.who.int/publications/i/item/who-wer9308-73-96 (accessed on 24 November 2025).
- SAGE Evidence to Recommendations Framework. Available online: https://cdn.who.int/media/docs/default-source/immunization/position_paper_documents/tuberculosis-(bcg)/bcg-evidence-recommendation-table-hiv.pdf?sfvrsn=bce6051f_2 (accessed on 18 November 2025).
- Crum-Cianflone, N.F.; Wallace, M.R. Vaccination in HIV-Infected Adults. AIDS Patient Care STDs 2014, 28, 397–410. [Google Scholar] [CrossRef]
- Chadwick, D.R.; Geretti, A.M. Immunization of the HIV Infected Traveller. AIDS 2007, 21, 787–794. [Google Scholar] [CrossRef]
- Chang, L.; Lim, B.C.W.; Flaherty, G.T.; Torresi, J. Travel Vaccination Recommendations and Infection Risk in HIV-Positive Travellers. J. Travel Med. 2019, 26, taz034. [Google Scholar] [CrossRef] [PubMed]
- Franco-Paredes, C.; Hidron, A.; Tellez, I.; Lesesne, J.; Rio, C.D. HIV Infection and Travel: Pretravel Recommendations and Health-Related Risks. Top HIV Med. 2009, 17, 2–11. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention (CDC). Travelers with HIV. Available online: https://www.cdc.gov/yellow-book/hcp/travelers-with-additional-considerations/travelers-with-hiv.html (accessed on 17 November 2025).
- World Health Organization. Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy; World Health Organization: Geneva, Switzerland, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK475977/ (accessed on 17 November 2025).
- Bhadelia, N.; Klotman, M.; Caplivski, D. The HIV-Positive Traveler. Am. J. Med. 2007, 120, 574–580. [Google Scholar] [CrossRef]
- Schuhwerk, M.A.; Richens, J.; Zuckerman, J.N. HIV and Travel. Travel Med. Infect. Dis. 2006, 4, 174–183. [Google Scholar] [CrossRef]
- Casado, C.; Galvez, C.; Pernas, M.; Tarancon-Diez, L.; Rodriguez, C.; Sanchez-Merino, V.; Vera, M.; Olivares, I.; Pablo-Bernal, R.D.; Merino-Mansilla, A.; et al. Permanent Control of HIV-1 Pathogenesis in Exceptional Elite Controllers: A Model of Spontaneous Cure. Sci. Rep. 2020, 10, 1902. [Google Scholar] [CrossRef]
- Gasca-Capote, C.; Lian, X.; Gao, C.; Roseto, I.C.; Jiménez-León, M.R.; Gladkov, G.; Camacho-Sojo, M.I.; Pérez-Gómez, A.; Gallego, I.; Lopez-Cortes, L.E.; et al. The HIV-1 Reservoir Landscape in Persistent Elite Controllers and Transient Elite Controllers. J. Clin. Investig. 2024, 134, e174215. [Google Scholar] [CrossRef]
- Angeli, D.; Ferrell, J.E.; Sontag, E.D. Detection of Multistability, Bifurcations, and Hysteresis in a Large Class of Biological Positive-Feedback Systems. Proc. Natl. Acad. Sci. USA 2004, 101, 1822–1827. [Google Scholar] [CrossRef] [PubMed]
- Luzyanina, T.; Engelborghs, K.; Ehl, S.; Klenerman, P.; Bocharov, G. Low Level Viral Persistence after Infection with LCMV: A Quantitative Insight through Numerical Bifurcation Analysis. Math. Biosci. 2001, 173, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bocharov, G.; Grebennikov, D.; Argilaguet, J.; Meyerhans, A. Examining the Cooperativity Mode of Antibody and CD8+ T Cell Immune Responses for Vaccinology. Trends Immunol. 2021, 42, 852–855. [Google Scholar] [CrossRef]
- Greczmiel, U.; Kräutler, N.J.; Pedrioli, A.; Bartsch, I.; Agnellini, P.; Bedenikovic, G.; Harker, J.; Richter, K.; Oxenius, A. Sustained T Follicular Helper Cell Response Is Essential for Control of Chronic Viral Infection. Sci. Immunol. 2017, 2, eaam8686. [Google Scholar] [CrossRef]
- Mironov, I.; Khristichenko, M.Y.; Nechepurenko, Y.M.; Grebennikov, D.S.; Bocharov, G. Bifurcation Analysis of Multistability and Hysteresis in a Model of HIV Infection. Vavilov J. Genet. Breed. 2023, 27, 755–767. [Google Scholar] [CrossRef]
- Chen, Z.; Julg, B. Therapeutic Vaccines for the Treatment of HIV. Transl. Res. 2020, 223, 61–75. [Google Scholar] [CrossRef]
- Bailón, L.; Moltó, J.; Curran, A.; Cadiñanos, J.; de Quirós, J.C.L.B.; Santos, I.d.L.; Ambrosioni, J.; Imaz, A.; Benet, S.; Suanzes, P.; et al. Safety, Immunogenicity and Effect on Viral Rebound of HTI Vaccines Combined with a TLR7 Agonist in Early-Treated HIV-1 Infection: A Randomized, Placebo-Controlled Phase 2a Trial. Nat. Commun. 2025, 16, 2146. [Google Scholar] [CrossRef]
- Bailón, L.; Llano, A.; Cedeño, S.; Escribà, T.; Rosás-Umbert, M.; Parera, M.; Casadellà, M.; Lopez, M.; Pérez, F.; Oriol-Tordera, B.; et al. Safety, Immunogenicity and Effect on Viral Rebound of HTI Vaccines in Early Treated HIV-1 Infection: A Randomized, Placebo-Controlled Phase 1 Trial. Nat. Med. 2022, 28, 2611–2621. [Google Scholar] [CrossRef]
- Winckelmann, A.; Morcilla, V.; Shao, W.; Schleimann, M.H.; Hojen, J.F.; Schlub, T.E.; Benton, P.W.; Østergaard, L.; Søgaard, O.S.; Tolstrup, M.; et al. Genetic Characterization of the HIV-1 Reservoir after Vacc-4x and Romidepsin Therapy in HIV-1-Infected Individuals. AIDS 2018, 32, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Gay, C. ChAdOx1.HIVconsvX and MVA.HIVconsvX Vaccination Is Safe and Immunogenic in PWH on ART: The CM Study. In Proceedings of the CROI 2025, San Francisco, CA, USA, 9–12 March 2025. [Google Scholar]
- Grebennikov, D.; Bouchnita, A.; Volpert, V.; Bessonov, N.; Meyerhans, A.; Bocharov, G. Spatial Lymphocyte Dynamics in Lymph Nodes Predicts the Cytotoxic T Cell Frequency Needed for HIV Infection Control. Front. Immunol. 2019, 10, 1213. [Google Scholar] [CrossRef]
- Baiyegunhi, O.O.; Mann, J.; Khaba, T.; Nkosi, T.; Mbatha, A.; Ogunshola, F.; Chasara, C.; Ismail, N.; Ngubane, T.; Jajbhay, I.; et al. CD8 Lymphocytes Mitigate HIV-1 Persistence in Lymph Node Follicular Helper T Cells during Hyperacute-Treated Infection. Nat. Commun. 2022, 13, 4041. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, W.; Sun, M.; Li, T. Broadly Neutralizing Antibodies for HIV-1: Efficacies, Challenges and Opportunities. Emerg. Microbes Infect. 2020, 9, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, C.; Nogueira, L.; Stoffel, E.; Oliveira, T.Y.; Breton, G.; Millard, K.G.; Turroja, M.; Butler, A.; Ramos, V.; Seaman, M.S.; et al. Prolonged Viral Suppression with Anti-HIV-1 Antibody Therapy. Nature 2022, 606, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, P.; Gruell, H.; Nogueira, L.; Pai, J.A.; Butler, A.L.; Millard, K.; Lehmann, C.; Suárez, I.; Oliveira, T.Y.; Lorenzi, J.C.C.; et al. Combination Therapy with Anti-HIV-1 Antibodies Maintains Viral Suppression. Nature 2018, 561, 479–484. [Google Scholar] [CrossRef]
- Cohen, Y.Z.; Lorenzi, J.C.C.; Krassnig, L.; Barton, J.P.; Burke, L.; Pai, J.; Lu, C.-L.; Mendoza, P.; Oliveira, T.Y.; Sleckman, C.; et al. Relationship between Latent and Rebound Viruses in a Clinical Trial of Anti–HIV-1 Antibody 3BNC117. J. Exp. Med. 2018, 215, 2311–2324. [Google Scholar] [CrossRef]
- Niessl, J.; Baxter, A.E.; Mendoza, P.; Jankovic, M.; Cohen, Y.Z.; Butler, A.L.; Lu, C.-L.; Dubé, M.; Shimeliovich, I.; Gruell, H.; et al. Combination Anti-HIV-1 Antibody Therapy Is Associated with Increased Virus-Specific T Cell Immunity. Nat. Med. 2020, 26, 222–227. [Google Scholar] [CrossRef]
- Julg, B.; Walker-Sperling, V.E.K.; Wagh, K.; Aid, M.; Stephenson, K.E.; Zash, R.; Liu, J.; Nkolola, J.P.; Hoyt, A.; Castro, M.; et al. Safety and Antiviral Effect of a Triple Combination of HIV-1 Broadly Neutralizing Antibodies: A Phase 1/2a Trial. Nat. Med. 2024, 30, 3534–3543. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.S.; Chung, A.W.; Kent, S.J. Importance of Fc-Mediated Functions of Anti-HIV-1 Broadly Neutralizing Antibodies. Retrovirology 2018, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Danesh, A.; Ren, Y.; Jones, R.B. Roles of Fragment Crystallizable-Mediated Effector Functions in Broadly Neutralizing Antibody Activity against HIV. Curr. Opin. HIV AIDS 2020, 15, 316–323. [Google Scholar] [CrossRef]
- Alba, C.; Malhotra, S.; Horsfall, S.; Barnhart, M.E.; Bekker, A.; Chapman, K.; Cunningham, C.K.; Fast, P.E.; Fouda, G.G.; Freedberg, K.A.; et al. Cost-Effectiveness of Broadly Neutralizing Antibodies for HIV Prophylaxis for Infants Born in Settings with High HIV Burdens. PLoS ONE 2025, 20, e0318940. [Google Scholar] [CrossRef]
- Mahomed, S.; Pillay, K.; Hassan-Moosa, R.; Galvão, B.P.G.V.; Burgers, W.A.; Moore, P.L.; Rose-Abrahams, M.; Williamson, C.; Garrett, N. Clinical Trials of Broadly Neutralizing Monoclonal Antibodies in People Living with HIV—A Review. AIDS Res. Ther. 2025, 22, 44. [Google Scholar] [CrossRef]
- Peluso, M.; Sandel, D.; Deitchman, A.; Kim, S.J.; Dalhuisen, T.; Tummala, H.; Tiburcio, R.; Zemelko, L.; Borgo, G.; Singh, S.; et al. Combination Immunotherapy Induces Post-Intervention Control of HIV. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Gunst, J.D.; Højen, J.F.; Pahus, M.H.; Rosás-Umbert, M.; Stiksrud, B.; McMahon, J.H.; Denton, P.W.; Nielsen, H.; Johansen, I.S.; Benfield, T.; et al. Impact of a TLR9 Agonist and Broadly Neutralizing Antibodies on HIV-1 Persistence: The Randomized Phase 2a TITAN Trial. Nat. Med. 2023, 29, 2547–2558. [Google Scholar] [CrossRef]
- Hsu, D.C.; Mellors, J.W.; Vasan, S. Can Broadly Neutralizing HIV-1 Antibodies Help Achieve an ART-Free Remission? Front. Immunol. 2021, 12, 710044. [Google Scholar] [CrossRef]
- Moldt, B.; Chandrashekar, A.; Borducchi, E.N.; Nkolola, J.P.; Stephenson, H.; Nagel, M.; Hung, M.; Goldsmith, J.; Pace, C.S.; Carr, B.; et al. HIV Envelope Antibodies and TLR7 Agonist Partially Prevent Viral Rebound in Chronically SHIV-Infected Monkeys. PLoS Pathog. 2022, 18, e1010467. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P. Future of bNAbs in HIV Treatment. Curr. HIV/AIDS Rep. 2025, 22, 34. [Google Scholar] [CrossRef]
- Rosenberg, Y.J.; Montefiori, D.C.; LaBranche, C.C.; Lewis, M.G.; Sack, M.; Lees, J.P.; Jiang, X. Protection against SHIV Challenge by Subcutaneous Administration of the Plant-Derived PGT121 Broadly Neutralizing Antibody in Macaques. PLoS ONE 2016, 11, e0152760. [Google Scholar] [CrossRef] [PubMed]
- Benito, J.M.; Restrepo, C.; García-Foncillas, J.; Rallón, N. Immune Checkpoint Inhibitors as Potential Therapy for Reverting T-Cell Exhaustion and Reverting HIV Latency in People Living with HIV. Front. Immunol. 2023, 14, 1270881. [Google Scholar] [CrossRef] [PubMed]
- Khaitan, A.; Unutmaz, D. Revisiting Immune Exhaustion During HIV Infection. Curr. HIV/AIDS Rep. 2011, 8, 4–11. [Google Scholar] [CrossRef]
- Gubser, C.; Chiu, C.; Lewin, S.R.; Rasmussen, T.A. Immune Checkpoint Blockade in HIV. eBioMedicine 2022, 76, 103840. [Google Scholar] [CrossRef]
- Ward, A.R.; Mota, T.M.; Jones, R.B. Immunological Approaches to HIV Cure. Semin. Immunol. 2021, 51, 101412. [Google Scholar] [CrossRef]
- Zheltkova, V.; Argilaguet, J.; Peligero, C.; Bocharov, G.; Meyerhans, A. Prediction of PD-L1 Inhibition Effects for HIV-Infected Individuals. PLoS Comput. Biol. 2019, 15, e1007401. [Google Scholar] [CrossRef]
- Lee, J.; Whitney, J.B. Immune Checkpoint Inhibition as a Therapeutic Strategy for HIV Eradication: Current Insights and Future Directions. Curr. Opin. HIV AIDS 2024, 19, 179–186. [Google Scholar] [CrossRef]
- Tanaka, K.; Kim, Y.; Roche, M.; Lewin, S.R. The Role of Latency Reversal in HIV Cure Strategies. J. Méd. Primatol. 2022, 51, 278–283. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Hou, Y.; Liu, X.; Hong, J.; Shi, X.; Huang, X.; Zhang, T.; Liao, X.; Zhang, L. Novel TLR7/8 Agonists Promote Activation of HIV-1 Latent Reservoirs and Human T and NK Cells. Front. Microbiol. 2023, 14, 1033448. [Google Scholar] [CrossRef] [PubMed]
- Meås, H.Z.; Haug, M.; Beckwith, M.S.; Louet, C.; Ryan, L.; Hu, Z.; Landskron, J.; Nordbø, S.A.; Taskén, K.; Yin, H.; et al. Sensing of HIV-1 by TLR8 Activates Human T Cells and Reverses Latency. Nat. Commun. 2020, 11, 147. [Google Scholar] [CrossRef]
- Debrabander, Q.; Hensley, K.S.; Psomas, C.K.; Bramer, W.; Mahmoudi, T.; van Welzen, B.J.; Verbon, A.; Rokx, C. The Efficacy and Tolerability of Latency-Reversing Agents in Reactivating the HIV-1 Reservoir in Clinical Studies: A Systematic Review. J. Virus Erad. 2023, 9, 100342. [Google Scholar] [CrossRef]
- Miller, J.S.; Davis, Z.B.; Helgeson, E.; Reilly, C.; Thorkelson, A.; Anderson, J.; Lima, N.S.; Jorstad, S.; Hart, G.T.; Lee, J.H.; et al. Safety and Virologic Impact of the IL-15 Superagonist N-803 in People Living with HIV: A Phase 1 Trial. Nat. Med. 2022, 28, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Carrillo, M.A.; Kitchen, S.G. Engineering CAR T Cells to Target the HIV Reservoir. Front. Cell. Infect. Microbiol. 2020, 10, 410. [Google Scholar] [CrossRef]
- York, J.; Gowrishankar, K.; Micklethwaite, K.; Palmer, S.; Cunningham, A.L.; Nasr, N. Evolving Strategies to Eliminate the CD4 T Cells HIV Viral Reservoir via CAR T Cell Immunotherapy. Front. Immunol. 2022, 13, 873701. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, H.; Fang, S.-Y.; Chow, R.D.; Tang, K.; Majety, M.; Bai, M.; Dong, M.B.; Renauer, P.A.; Shang, X.; et al. CTLA-4 Tail Fusion Enhances CAR-T Antitumor Immunity. Nat. Immunol. 2023, 24, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Anthony-Gonda, K.; Ray, A.; Su, H.; Wang, Y.; Xiong, Y.; Lee, D.; Block, A.; Chilunda, V.; Weiselberg, J.; Zemelko, L.; et al. In Vivo Killing of Primary HIV-Infected Cells by Peripheral-Injected Early Memory–Enriched Anti-HIV duoCAR T Cells. JCI Insight 2022, 7, e161698. [Google Scholar] [CrossRef]
- Anthony-Gonda, K.; Bardhi, A.; Ray, A.; Flerin, N.; Li, M.; Chen, W.; Ochsenbauer, C.; Kappes, J.C.; Krueger, W.; Worden, A.; et al. Multispecific Anti-HIV duoCAR-T Cells Display Broad in Vitro Antiviral Activity and Potent in Vivo Elimination of HIV-Infected Cells in a Humanized Mouse Model. Sci. Transl. Med. 2019, 11, eaav5685. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, W.; Xia, B.; Jing, S.; Du, Y.; Zou, F.; Li, R.; Lu, L.; Chen, S.; Li, Y.; et al. Broadly Neutralizing Antibody-Derived CAR-T Cells Reduce Viral Reservoir in HIV-1-Infected Individuals. J. Clin. Investig. 2021, 131, e150211. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Liao, Q.; Zhu, Y.; Bi, M.; Zou, J.; Zheng, N.; Zhu, L.; Zhao, C.; Liu, Q.; Liu, L.; et al. Efficacy and Safety of Novel Multifunctional M10 CAR-T Cells in HIV-1-Infected Patients: A Phase I, Multicenter, Single-Arm, Open-Label Study. Cell Discov. 2024, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Kitchen, S.G.; Chen, I.S.Y.; Ng, H.L.; Zack, J.A.; Yang, O.O. HIV-1-Specific Chimeric Antigen Receptors Based on Broadly Neutralizing Antibodies. J. Virol. 2016, 90, 6999–7006. [Google Scholar] [CrossRef] [PubMed]
- A Comparison of the Cost of CAR T-Cell Therapy Around the World. Available online: https://car-t-access.org/comparison-cost-car-t-cell-therapy-around-world.html (accessed on 17 November 2025).
- Zarif, T.E.; Nassar, A.H.; Adib, E.; Fitzgerald, B.G.; Huang, J.; Mouhieddine, T.H.; Rubinstein, P.G.; Nonato, T.; McKay, R.R.; Li, M.; et al. Safety and Activity of Immune Checkpoint Inhibitors in People Living with HIV and Cancer: A Real-World Report From the Cancer Therapy Using Checkpoint Inhibitors in People Living with HIV-International (CATCH-IT) Consortium. J. Clin. Oncol. 2023, 41, 3712–3723. [Google Scholar] [CrossRef]
- Castelli, V.; Lombardi, A.; Palomba, E.; Bozzi, G.; Ungaro, R.; Alagna, L.; Mangioni, D.; Muscatello, A.; Bandera, A.; Gori, A. Immune Checkpoint Inhibitors in People Living with HIV/AIDS: Facts and Controversies. Cells 2021, 10, 2227. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sisteré-Oró, M.; Güerri-Fernandez, R.; Plana, M.; Bocharov, G.; Meyerhans, A. Challenges in the Vaccination of HIV-Infected Individuals. Vaccines 2026, 14, 40. https://doi.org/10.3390/vaccines14010040
Sisteré-Oró M, Güerri-Fernandez R, Plana M, Bocharov G, Meyerhans A. Challenges in the Vaccination of HIV-Infected Individuals. Vaccines. 2026; 14(1):40. https://doi.org/10.3390/vaccines14010040
Chicago/Turabian StyleSisteré-Oró, Marta, Roberto Güerri-Fernandez, Montserrat Plana, Gennady Bocharov, and Andreas Meyerhans. 2026. "Challenges in the Vaccination of HIV-Infected Individuals" Vaccines 14, no. 1: 40. https://doi.org/10.3390/vaccines14010040
APA StyleSisteré-Oró, M., Güerri-Fernandez, R., Plana, M., Bocharov, G., & Meyerhans, A. (2026). Challenges in the Vaccination of HIV-Infected Individuals. Vaccines, 14(1), 40. https://doi.org/10.3390/vaccines14010040






