Novel Strategies for Developing Next-Generation Vaccines to Combat Infectious Viral Diseases
Abstract
1. Introduction
2. Structure-Guided Antigen Design
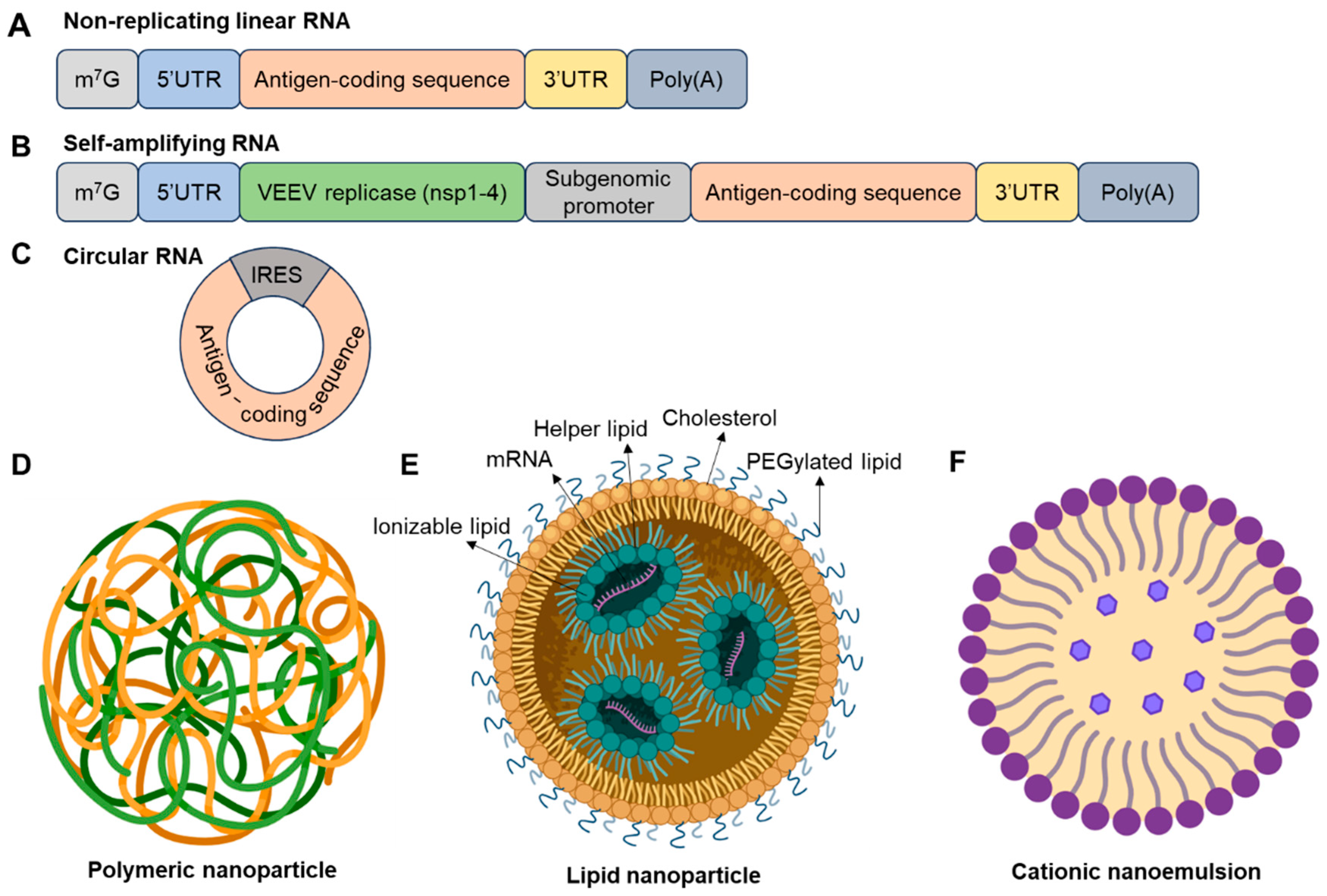
3. mRNA Technology and Its Future
| Delivery System | Composition and Structure | Mechanism of Action | Encapsulation Efficiency | Targeting Capability | Immunogenicity | Biodegradability and Safety | Clinical Status (Example) | Limitations | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Lipid-Based Nanoparticles (LNPs) | Ionizable lipids, cholesterol, phospholipids, PEG lipids | Endosomal escape via pH-sensitive ionizable lipids | High (>90%) | Passive; ligand conjugation possible | Moderate; PEG-related hypersensitivity | Biodegradable; well-tolerated | Approved: SARS-CoV-2 (Pfizer-BioNTech, Moderna) | Cold chain required; reactogenicity | [44,56,59,60] |
| Polymeric Nanoparticles | PLGA, PEI, PBAEs, other biodegradable polymers | Proton sponge effect; slow release | Moderate (40–80%) | Tunable via surface modification | Variable; PEI may be cytotoxic | Biodegradable; potential toxicity | Phase I: Zika virus mRNA vaccine (using PBAEs) | Lower transfection efficiency; cytotoxicity risk | |
| Cationic Nanoemulsions | Oil-in-water emulsion with cationic surfactants | Membrane fusion; mucosal uptake | Moderate | Suitable for mucosal targeting | Moderate to high; surfactant-dependent | Limited data; surfactant toxicity possible | Preclinical: Influenza A intranasal mRNA vaccine | Stability issues; limited clinical data |
4. Novel Adjuvant Development
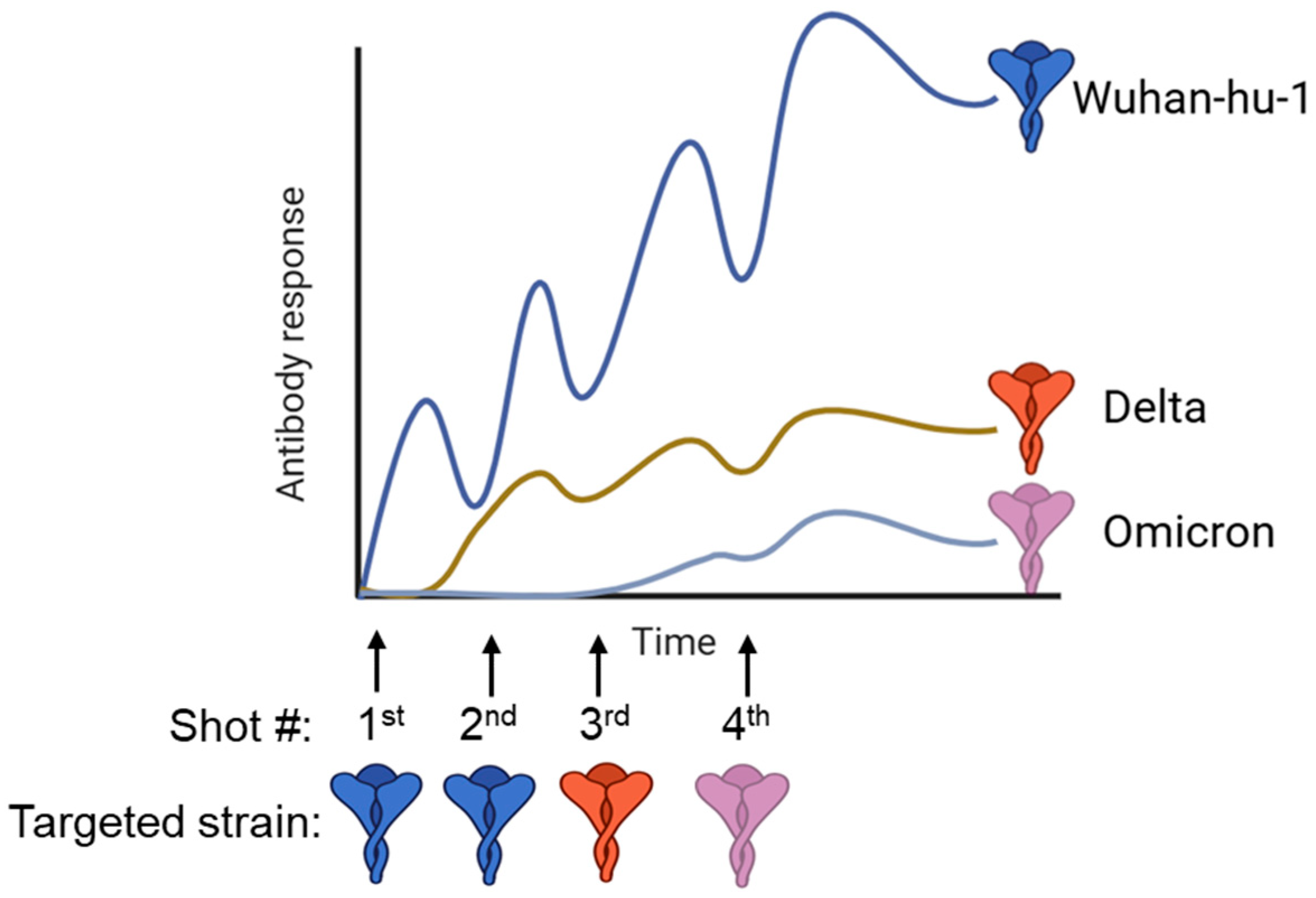
5. Universal Vaccines to Overcome Imprinting
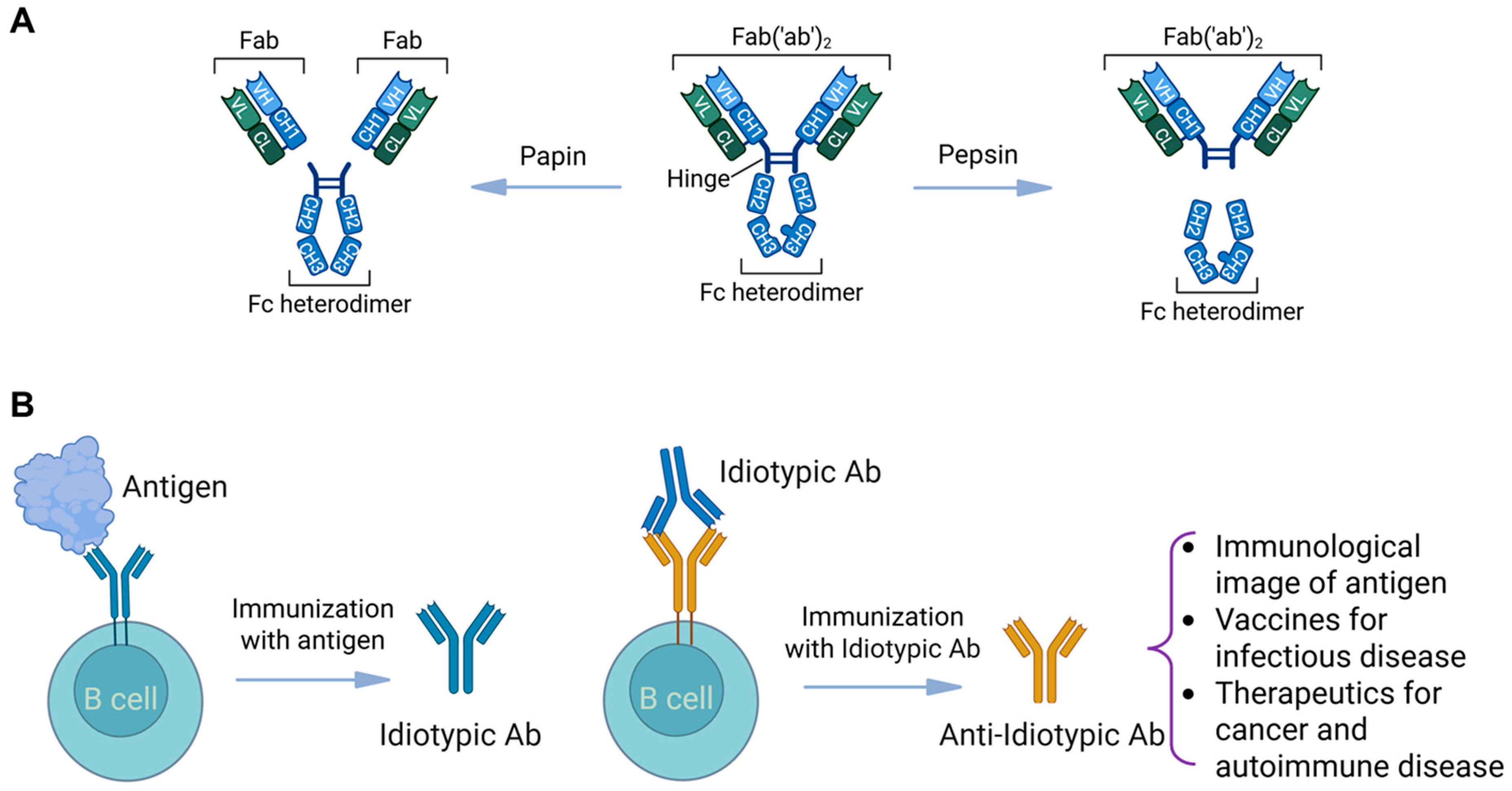
6. Anti-Idiotypic Vaccination Strategies
7. New Animal Models for Vaccine Testing
8. Global Collaboration and Education
9. Conclusions
Funding
Conflicts of Interest
References
- Pulendran, B.; Oh, J.Z.; Nakaya, H.I.; Ravindran, R.; Kazmin, D.A. Immunity to viruses: Learning from successful human vaccines. Immunol. Rev. 2013, 255, 243–255. [Google Scholar] [CrossRef]
- Amanna, I.J.; Slifka, M.K. Successful vaccines. In Vaccination Strategies Against Highly Variable Pathogens; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–30. [Google Scholar]
- Patel, M.; Zipursky, S.; Orenstein, W.; Garon, J.; Zaffran, M. Polio endgame: The global introduction of inactivated polio vaccine. Expert Rev. Vaccines 2015, 14, 749–762. [Google Scholar] [CrossRef]
- Saylor, K.; Gillam, F.; Lohneis, T.; Zhang, C. Designs of antigen structure and composition for improved protein-based vaccine efficacy. Front. Immunol. 2020, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Schellenbacher, C.; Roden, R.B.S.; Kirnbauer, R. Developments in L2-based human papillomavirus (HPV) vaccines. Virus Res. 2017, 231, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Prevec, L.; Campbell, J.B.; Christie, B.S.; Belbeck, L.; Graham, F.L. A recombinant human adenovirus vaccine against rabies. J. Infect. Dis. 1990, 161, 27–30. [Google Scholar] [CrossRef]
- Huang, C.Q.; Vishwanath, S.; Carnell, G.W.; Chan, A.C.Y.; Heeney, J.L. Immune imprinting and next-generation coronavirus vaccines. Nat. Microbiol. 2023, 8, 1971–1985. [Google Scholar] [CrossRef] [PubMed]
- Rock, D.L. Thoughts on African Swine Fever Vaccines. Viruses 2021, 13, 943. [Google Scholar] [CrossRef]
- Rock, D.L. Challenges for African swine fever vaccine development—“… perhaps the end of the beginning”. Vet. Microbiol. 2017, 206, 52–58. [Google Scholar] [CrossRef]
- Cameron, C.E. Syphilis vaccine development: Requirements, challenges, and opportunities. Sex. Transm. Dis. 2018, 45, S17–S19. [Google Scholar] [CrossRef]
- Williams, E.; Seib, K.L.; Fairley, C.K.; Pollock, G.L.; Hocking, J.S.; McCarthy, J.S.; Williamson, D.A. Neisseria gonorrhoeae vaccines: A contemporary overview. Clin. Microbiol. Rev. 2024, 37, e00094-23. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Boyoglu-Barnum, S.; Ellis, D.; Gillespie, R.A.; Hutchinson, G.B.; Park, Y.-J.; Moin, S.M.; Acton, O.J.; Ravichandran, R.; Murphy, M.; Pettie, D.; et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature 2021, 592, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Widge, A.T.; Hofstetter, A.R.; Houser, K.V.; Awan, S.F.; Chen, G.L.; Burgos Florez, M.C.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Holman, L.A.; et al. An influenza hemagglutinin stem nanoparticle vaccine induces cross-group 1 neutralizing antibodies in healthy adults. Sci. Transl. Med. 2023, 15, eade4790. [Google Scholar] [CrossRef]
- Withanage, K.; De Coster, I.; Cools, N.; Viviani, S.; Tourneur, J.; Chevandier, M.; Lambiel, M.; Willems, P.; Le Vert, A.; Nicolas, F.; et al. Phase 1 randomized, placebo-controlled, dose-escalating study to evaluate OVX836, a nucleoprotein-based influenza vaccine: Intramuscular results. J. Infect. Dis. 2022, 226, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, I.; Waerlop, G.; Tourneur, J.; De Boever, F.; Maes, C.; Bruhwyler, J.; Guyon-Gellin, D.; Moris, P.; Del Campo, J.; Willems, P.; et al. Randomized, double-blind, reference-controlled, phase 2a study evaluating the immunogenicity and safety of OVX836, a nucleoprotein-based influenza vaccine. Front. Immunol. 2022, 13, 852904. [Google Scholar] [CrossRef] [PubMed]
- Ingrole, R.S.J.; Shakya, A.K.; Joshi, G.; Lee, C.H.; Nesovic, L.D.; Compans, R.W.; Gill, H.S. Floss-based vaccination targets the gingival sulcus for mucosal and systemic immunization. Nat. Biomed. Eng. 2025. [Google Scholar] [CrossRef]
- Rathnasinghe, R.; Chang, L.A.; Pearl, R.; Jangra, S.; Aspelund, A.; Hoag, A.; Yildiz, S.; Mena, I.; Sun, W.; Loganathan, M.; et al. Sequential immunization with chimeric hemagglutinin ΔNS1 attenuated influenza vaccines induces broad humoral and cellular immunity. npj Vaccines 2024, 9, 169. [Google Scholar] [CrossRef]
- Singleton, E.V.; Gates, C.J.; David, S.C.; Hirst, T.R.; Davies, J.B.; Alsharifi, M. Enhanced Immunogenicity of a Whole-Inactivated Influenza A Virus Vaccine Using Optimised Irradiation Conditions. Front. Immunol. 2021, 12, 761632. [Google Scholar] [CrossRef]
- Ma, D.; Tian, S.; Qin, Q.; Yu, Y.; Jiao, J.; Xiong, X.; Guo, Y.; Zhang, X.; Ouyang, X. Construction of an inhalable recombinant M2e-FP-expressing Bacillus subtilis spores-based vaccine and evaluation of its protection efficacy against influenza in a mouse model. Vaccine 2023, 41, 4402–4413. [Google Scholar] [CrossRef]
- Lee, I.T.; Nachbagauer, R.; Ensz, D.; Schwartz, H.; Carmona, L.; Schaefers, K.; Avanesov, A.; Stadlbauer, D.; Henry, C.; Chen, R.; et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: Interim analysis. Nat. Commun. 2023, 14, 3631. [Google Scholar] [CrossRef]
- Ananworanich, J.; Lee, I.T.; Ensz, D.; Carmona, L.; Schaefers, K.; Avanesov, A.; Stadlbauer, D.; Choi, A.; Pucci, A.; McGrath, S.; et al. Safety and immunogenicity of mRNA-1010, an investigational seasonal influenza vaccine, in healthy adults: Final results from a phase 1/2 randomized trial. J. Infect. Dis. 2025, 231, e113–e122. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Li, X.-F.; Dai, X.-H.; Li, N.; Cheng, M.-L.; Huang, Z.; Shen, J.; Ge, Y.-H.; Shen, Z.-W.; Deng, Y.-Q.; et al. Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Microbe 2022, 3, e193–e202. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.D.; Rappaport, A.R.; Davis, M.J.; Hart, M.G.; Scallan, C.D.; Hong, S.-J.; Gitlin, L.; Kraemer, L.D.; Kounlavouth, S.; Yang, A.; et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: Phase 1 trial interim results. Nat. Med. 2022, 28, 1619–1629. [Google Scholar] [CrossRef]
- Smit, M.J.; Sander, A.F.; Ariaans, M.B.P.A.; Fougeroux, C.; Heinzel, C.; Fendel, R.; Esen, M.; Kremsner, P.G.; Ter Heine, R.; Wertheim, H.F.; et al. First-in-human use of a modular capsid virus-like vaccine platform: An open-label, non-randomised, phase 1 clinical trial of the SARS-CoV-2 vaccine ABNCoV2. Lancet Microbe 2023, 4, e140–e148. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef]
- Rumyantsev, A.; Wang, L.; Wang, S.; Kemp, T.; Wriggins, A.; Burks, A.; Fisher, D.; Brokke, K.; Fix, A.; Hensley, S.; et al. Safety and immunogenicity of UB-612 heterologous booster in adults primed with mRNA, adenovirus, or inactivated COVID-19 vaccines: A randomized, active-controlled, Phase 3 trial. EClinicalMedicine 2025, 86, 103349. [Google Scholar] [CrossRef]
- Shaw, C.A.; Essink, B.; Harper, C.; Mithani, R.; Kapoor, A.; Dhar, R.; Wilson, L.; Guo, R.; Panozzo, C.A.; Wilson, E.; et al. Safety and immunogenicity of an mRNA-based RSV vaccine including a 12-month booster in a phase 1 clinical trial in healthy older adults. J. Infect. Dis. 2024, 230, e647–e656. [Google Scholar] [CrossRef]
- Jenkin, D.; Wright, D.; Folegatti, P.M.; Platt, A.; Poulton, I.; Lawrie, A.; Tran, N.; Boyd, A.; Turner, C.; Gitonga, J.N.; et al. Safety and immunogenicity of a ChAdOx1 vaccine against Rift Valley fever in UK adults: An open-label, non-randomised, first-in-human phase 1 clinical trial. Lancet Infect. Dis. 2023, 23, 956–964. [Google Scholar] [CrossRef]
- Ruckwardt, T.J.; Morabito, K.M.; Phung, E.; Crank, M.C.; Costner, P.J.; Holman, L.A.; Chang, L.A.; Hickman, S.P.; Berkowitz, N.M.; Gordon, I.J.; et al. Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: A phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Respir. Med. 2021, 9, 1111–1120. [Google Scholar] [CrossRef]
- Rudman Spergel, A.K.; Ananworanich, J.; Guo, R.; Deng, W.; Carmona, L.; Schaefers, K.; Paila, Y.D.; Kandinov, B.; Eger, C.H.; Sinkiewicz, M.; et al. mRNA-based seasonal influenza and SARS-CoV-2 multicomponent vaccine in healthy adults: A phase 1/2 trial. Nat. Med. 2025, 31, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- August, A.; Shaw, C.A.; Lee, H.; Knightly, C.; Kalidindia, S.; Chu, L.; Essink, B.J.; Seger, W.; Zaks, T.; Smolenov, I.; et al. Safety and Immunogenicity of an mRNA-Based Human Metapneumovirus and Parainfluenza Virus Type 3 Combined Vaccine in Healthy Adults; Oxford University Press: Oxford, UK, 2022; p. ofac206. [Google Scholar]
- Essink, B.; Chu, L.; Seger, W.; Barranco, E.; Le Cam, N.; Bennett, H.; Faughnan, V.; Pajon, R.; Paila, Y.D.; Bollman, B.; et al. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: The results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis. 2023, 23, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, C.; Leroux–Roels, I.; Huang, K.B.; Bica, M.A.; Loeliger, E.; Schoenborn-Kellenberger, O.; Walz, L.; Leroux-Roels, G.; von Sonnenburg, F.; Oostvogels, L. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase 1 trial. Vaccine 2021, 39, 1310–1318. [Google Scholar] [CrossRef]
- Byrne, P.O.; McLellan, J.S. Principles and practical applications of structure-based vaccine design. Curr. Opin. Immunol. 2022, 77, 102209. [Google Scholar] [CrossRef]
- Graham, B.S.; Gilman, M.S.A.; McLellan, J.S. Structure-based vaccine antigen design. Annu. Rev. Med. 2019, 70, 91–104. [Google Scholar] [CrossRef]
- Ruckwardt, T.J. The road to approved vaccines for respiratory syncytial virus. npj Vaccines 2023, 8, 138. [Google Scholar] [CrossRef]
- Crank, M.C.; Ruckwardt, T.J.; Chen, M.; Morabito, K.M.; Phung, E.; Costner, P.J.; Holman, L.A.; Hickman, S.P.; Berkowitz, N.M.; Gordon, I.J.; et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019, 365, 505–509. [Google Scholar] [CrossRef]
- Kwong, P.D.; Wyatt, R.; Robinson, J.; Sweet, R.W.; Sodroski, J.; Hendrickson, W.A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 1998, 393, 648–659. [Google Scholar] [CrossRef]
- Zhang, P.; Gorman, J.; Tsybovsky, Y.; Lu, M.; Liu, Q.; Gopan, V.; Singh, M.; Lin, Y.; Miao, H.; Seo, Y.; et al. Design of soluble HIV-1 envelope trimers free of covalent gp120-gp41 bonds with prevalent native-like conformation. Cell Rep. 2024, 43, 114518. [Google Scholar] [CrossRef]
- Ellis, D.; Brunette, N.; Crawford, K.H.D.; Walls, A.C.; Pham, M.N.; Chen, C.; Herpoldt, K.-L.; Fiala, B.; Murphy, M.; Pettie, D.; et al. Stabilization of the SARS-CoV-2 spike receptor-binding domain using deep mutational scanning and structure-based design. Front. Immunol. 2021, 12, 710263. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.S.; Chen, M.; Joyce, M.G.; Sastry, M.; Stewart-Jones, G.B.E.; Yang, Y.; Zhang, B.; Chen, L.; Srivatsan, S.; Zheng, A.; et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013, 342, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Krarup, A.; Truan, D.; Furmanova-Hollenstein, P.; Bogaert, L.; Bouchier, P.; Bisschop, I.J.M.; Widjojoatmodjo, M.N.; Zahn, R.; Schuitemaker, H.; McLellan, J.S.; et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat. Commun. 2015, 6, 8143. [Google Scholar] [CrossRef]
- Brown, B.D.; Fauci, A.S.; Belkaid, Y.; Merad, M. RNA vaccines: A transformational advance. Immunity 2023, 56, 2665–2669. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Krammer, F. mRNA vaccines for infectious diseases—Advances, challenges and opportunities. Nat. Rev. Drug Discov. 2024, 23, 838–861. [Google Scholar] [CrossRef]
- Ramezani-Rad, P.; Marina-Zarate, E.; Maiorino, L.; Myers, A.; Michaels, K.K.; Pires, I.S.; Bloom, N.I.; Lopez, P.G.; Cottrell, C.A.; Burton, I.; et al. Dose-dependent regulation of immune memory responses against HIV by saponin monophosphoryl lipid A nanoparticle adjuvant. bioRxiv 2024. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Aditham, A.; Guo, J.; Huang, J.; Kostas, F.; Maher, K.; Friedrich, M.J.; Xavier, R.J.; Zhang, F. Chemical and topological design of multicapped mRNA and capped circular RNA to augment translation. Nat. Biotechnol. 2024, 43, 1128–1143. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Dousis, A.; Ravichandran, K.; Hobert, E.M.; Moore, M.J.; Rabideau, A.E. An engineered T7 RNA polymerase that produces mRNA free of immunostimulatory byproducts. Nat. Biotechnol. 2023, 41, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Y.; Li, S.; Jia, L.; Wang, H.; Li, M.; Deng, J.; Zhu, A.; Ma, L.; Li, W.; et al. The nano delivery systems and applications of mRNA. Eur. J. Med. Chem. 2022, 227, 113910. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, H.; Li, G.; Su, J.; Wei, Y.; Xu, C. Lipid nanovehicles overcome barriers to systemic RNA delivery: Lipid components, fabrication methods, and rational design. Acta Pharm. Sin. B 2024, 14, 579–601. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith Iii, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef]
- Ermilova, I.; Swenson, J. DOPC versus DOPE as a helper lipid for gene-therapies: Molecular dynamics simulations with DLin-MC3-DMA. Phys. Chem. Chem. Phys. 2020, 22, 28256–28268. [Google Scholar] [CrossRef]
- Berger, M.; Degey, M.; Leblond Chain, J.; Maquoi, E.; Evrard, B.; Lechanteur, A.; Piel, G. Effect of PEG anchor and serum on lipid nanoparticles: Development of a nanoparticles tracking method. Pharmaceutics 2023, 15, 597. [Google Scholar] [CrossRef]
- Kafil, V.; Omidi, Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in a431 cells. BioImpacts BI 2011, 1, 23. [Google Scholar]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef] [PubMed]
- Pateev, I.; Seregina, K.; Ivanov, R.; Reshetnikov, V. Biodistribution of RNA Vaccines and of Their Products: Evidence from Human and Animal Studies. Biomedicines 2024, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rudra, A.; Reed, K.; Langer, R.; Anderson, D.G. Advanced technologies for the development of infectious disease vaccines. Nat. Rev. Drug Discov. 2024, 23, 914–938. [Google Scholar] [CrossRef] [PubMed]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Chapin-Bardales, J.; Myers, T.; Gee, J.; Shay, D.K.; Marquez, P.; Baggs, J.; Zhang, B.; Licata, C.; Shimabukuro, T.T. Reactogenicity within 2 weeks after mRNA COVID-19 vaccines: Findings from the CDC v-safe surveillance system. Vaccine 2021, 39, 7066–7073. [Google Scholar] [CrossRef]
- SeyedAlinaghi, S.; Karimi, A.; Pashaei, Z.; Afzalian, A.; Mirzapour, P.; Ghorbanzadeh, K.; Ghasemzadeh, A.; Dashti, M.; Nazarian, N.; Vahedi, F. Safety and adverse events related to COVID-19 mRNA vaccines; a systematic review. Arch. Acad. Emerg. Med. 2022, 10, e41. [Google Scholar]
- Tahtinen, S.; Tong, A.-J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Senti, M.E.; de Jongh, C.A.; Dijkxhoorn, K.; Verhoef, J.J.F.; Szebeni, J.; Storm, G.; Hack, C.E.; Schiffelers, R.M.; Fens, M.H.; Boross, P. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J. Control. Release 2022, 341, 475–486. [Google Scholar] [CrossRef]
- Zschaler, J.; Schlorke, D.; Arnhold, J. Differences in innate immune response between man and mouse. Crit. Rev.™ Immunol. 2014, 34, 433–454. [Google Scholar] [CrossRef] [PubMed]
- McDonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. npj Vaccines 2021, 6, 74. [Google Scholar] [CrossRef]
- Han, X.; Gong, N.; Xue, L.; Billingsley, M.M.; El-Mayta, R.; Shepherd, S.J.; Alameh, M.-G.; Weissman, D.; Mitchell, M.J. Ligand-tethered lipid nanoparticles for targeted RNA delivery to treat liver fibrosis. Nat. Commun. 2023, 14, 75. [Google Scholar] [CrossRef]
- Klimstra, W.B.; Nangle, E.M.; Smith, M.S.; Yurochko, A.D.; Ryman, K.D. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell-and mammalian cell-derived viruses. J. Virol. 2003, 77, 12022–12032. [Google Scholar] [CrossRef]
- Kuhn, N.F.; Purdon, T.J.; van Leeuwen, D.G.; Lopez, A.V.; Curran, K.J.; Daniyan, A.F.; Brentjens, R.J. CD40 ligand-modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell 2019, 35, 473–488. [Google Scholar] [CrossRef]
- Sommermeyer, D.; Hill, T.; Shamah, S.M.; Salter, A.I.; Chen, Y.; Mohler, K.M.; Riddell, S.R. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia 2017, 31, 2191–2199. [Google Scholar] [CrossRef]
- Garanger, E.; Boturyn, D.; Dumy, P. Tumor targeting with RGD peptide ligands-design of new molecular conjugates for imaging and therapy of cancers. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2007, 7, 552–558. [Google Scholar] [CrossRef]
- Schottelius, M.; Herrmann, K.; Lapa, C. In vivo targeting of CXCR4—New horizons. Cancers 2021, 13, 5920. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Y.; Zhou, M.; Xu, S.; Varley, A.J.; Golubovic, A.; Lu, R.X.Z.; Wang, K.C.; Yeganeh, M.; Vosoughi, D.; et al. Combinatorial design of ionizable lipid nanoparticles for muscle-selective mRNA delivery with minimized off-target effects. Proc. Natl. Acad. Sci. USA 2023, 120, e2309472120. [Google Scholar]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.Y.; Witten, J.; Raji, I.O.; Eweje, F.; MacIsaac, C.; Meng, S.; Oladimeji, F.A.; Hu, Y.; Manan, R.S.; Langer, R. Combinatorial development of nebulized mRNA delivery formulations for the lungs. Nat. Nanotechnol. 2024, 19, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Lindblad, E.B. Aluminium adjuvants—In retrospect and prospect. Vaccine 2004, 22, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Haensler, J.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol. Sci. 2009, 30, 287–295. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Kazmin, D.; Napolitani, G.; Clutterbuck, E.A.; Pulendran, B.; Siegrist, C.-A.; Pollard, A.J. AS03-and MF59-adjuvanted influenza vaccines in children. Front. Immunol. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Freund, J.; McDermott, K. Sensitization to horse serum by means of adjuvants. Proc. Soc. Exp. Biol. Med. 1942, 49, 548–553. [Google Scholar]
- Maisonneuve, C.; Bertholet, S.; Philpott, D.J.; De Gregorio, E. Unleashing the potential of NOD-and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2014, 111, 12294–12299. [Google Scholar]
- Mata-Haro, V.; Cekic, C.; Martin, M.; Chilton, P.M.; Casella, C.R.; Mitchell, T.C. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 2007, 316, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, A.A.; Rosenthal, K.L. Toll-like receptor 9, CpG DNA and innate immunity. Curr. Mol. Med. 2002, 2, 545–556. [Google Scholar] [CrossRef]
- Yang, Y.; Che, Y.; Zhao, Y.; Wang, X. Prevention and treatment of cervical cancer by a single administration of human papillomavirus peptide vaccine with CpG oligodeoxynucleotides as an adjuvant in vivo. Int. Immunopharmacol. 2019, 69, 279–288. [Google Scholar] [CrossRef]
- Castrodeza-Sanz, J.; Sanz-Muñoz, I.; Eiros, J.M. Adjuvants for COVID-19 vaccines. Vaccines 2023, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, D.; Schmidt, J.J.; Capecchi, J.T.; Wightman, P.D. Vaccine adjuvant activity of 3M-052: An imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine 2011, 29, 5434–5442. [Google Scholar] [CrossRef]
- Behzad, H.; Huckriede, A.L.W.; Haynes, L.; Gentleman, B.; Coyle, K.; Wilschut, J.C.; Kollmann, T.R.; Reed, S.G.; McElhaney, J.E. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J. Infect. Dis. 2012, 205, 466–473. [Google Scholar] [CrossRef]
- Roman, F.; Burny, W.; Ceregido, M.A.; Laupèze, B.; Temmerman, S.T.; Warter, L.; Coccia, M. Adjuvant system AS01: From mode of action to effective vaccines. Expert Rev. Vaccines 2024, 23, 715–729. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, D.; Xiao, H.; Li, B.; Shang, K.; Zhao, D. Activating the cGAS-STING Pathway by Manganese-Based Nanoparticles Combined with Platinum-Based Nanoparticles for Enhanced Ovarian Cancer Immunotherapy. ACS Nano 2025, 19, 4346–4365. [Google Scholar] [CrossRef]
- Yan, H.; Chen, W. The promise and challenges of cyclic dinucleotides as molecular adjuvants for vaccine development. Vaccines 2021, 9, 917. [Google Scholar] [CrossRef]
- Wang, B.; Yu, W.; Jiang, H.; Meng, X.; Tang, D.; Liu, D. Clinical applications of STING agonists in cancer immunotherapy: Current progress and future prospects. Front. Immunol. 2024, 15, 1485546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Kurhade, C.; Zou, J.; Xia, H.; Liu, M.; Chang, H.C.; Ren, P.; Xie, X.; Shi, P.Y. Low neutralization of SARS-CoV-2 Omicron BA. 2.75. 2, BQ. 1.1 and XBB. 1 by parental mRNA vaccine or a BA. 5 bivalent booster. Nat. Med. 2023, 29, 344–347. [Google Scholar] [CrossRef]
- Chalkias, S.; Whatley, J.L.; Eder, F.; Essink, B.; Khetan, S.; Bradley, P.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; et al. Original SARS-CoV-2 monovalent and Omicron BA. 4/BA. 5 bivalent COVID-19 mRNA vaccines: Phase 2/3 trial interim results. Nat. Med. 2023, 29, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Docken, S.S.; Subbarao, K.; Kent, S.J.; Davenport, M.P.; Cromer, D. Predicting the efficacy of variant-modified COVID-19 vaccine boosters. Nat. Med. 2023, 29, 574–578. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef]
- Hauser, B.M.; Sangesland, M.; Denis, K.J.S.; Lam, E.C.; Case, J.B.; Windsor, I.W.; Feldman, J.; Caradonna, T.M.; Kannegieter, T.; Diamond, M.S.; et al. Rationally designed immunogens enable immune focusing following SARS-CoV-2 spike imprinting. Cell Rep. 2022, 38, 110561. [Google Scholar] [CrossRef]
- Hoffmann, M.; Behrens, G.M.N.; Arora, P.; Kempf, A.; Nehlmeier, I.; Cossmann, A.; Manthey, L.; Dopfer-Jablonka, A.; Pöhlmann, S. Effect of hybrid immunity and bivalent booster vaccination on omicron sublineage neutralisation. Lancet Infect. Dis. 2023, 23, 25–28. [Google Scholar] [CrossRef]
- Meade, P.; Strohmeier, S.; Bermúdez-González, M.C.; García-Sastre, A.; Palese, P.; Simon, V.; Krammer, F. Antigenic landscape analysis of individuals vaccinated with a universal influenza virus vaccine candidate reveals induction of cross-subtype immunity. J. Virol. 2023, 97, e01070-22. [Google Scholar] [CrossRef]
- Broecker, F.; Liu, S.T.H.; Suntronwong, N.; Sun, W.; Bailey, M.J.; Nachbagauer, R.; Krammer, F.; Palese, P. A mosaic hemagglutinin-based influenza virus vaccine candidate protects mice from challenge with divergent H3N2 strains. npj Vaccines 2019, 4, 31. [Google Scholar] [CrossRef]
- Sun, W.; Kirkpatrick, E.; Ermler, M.; Nachbagauer, R.; Broecker, F.; Krammer, F.; Palese, P. Development of influenza B universal vaccine candidates using the “mosaic” hemagglutinin approach. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Carnell, G.W.; Billmeier, M.; Vishwanath, S.; Suau Sans, M.; Wein, H.; George, C.L.; Neckermann, P.; Del Rosario, J.M.M.; Sampson, A.T.; Einhauser, S.; et al. Glycan masking of a non-neutralising epitope enhances neutralising antibodies targeting the RBD of SARS-CoV-2 and its variants. Front. Immunol. 2023, 14, 1118523. [Google Scholar] [CrossRef]
- Krammer, F. The quest for a universal flu vaccine: Headless HA 2.0. Cell Host Microbe 2015, 18, 395–397. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Joyce, M.G.; Gillespie, R.A.; Gallagher, J.R.; Andrews, S.F.; Yassine, H.M.; Wheatley, A.K.; Fisher, B.E.; Ambrozak, D.R.; Creanga, A.; et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat. Immunol. 2019, 20, 362–372. [Google Scholar] [CrossRef]
- Ross, T.M.; DiNapoli, J.; Giel-Moloney, M.; Bloom, C.E.; Bertran, K.; Balzli, C.; Strugnell, T.; e Silva, M.S.; Mebatsion, T.; Bublot, M.; et al. A computationally designed H5 antigen shows immunological breadth of coverage and protects against drifting avian strains. Vaccine 2019, 37, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Ross, T.M. Bivalent H1 and H3 COBRA recombinant hemagglutinin vaccines elicit seroprotective antibodies against H1N1 and H3N2 influenza viruses from 2009 to 2019. J. Virol. 2022, 96, e01652-21. [Google Scholar] [CrossRef]
- Del Campo, J.; Pizzorno, A.; Djebali, S.; Bouley, J.; Haller, M.; Pérez-Vargas, J.; Lina, B.; Boivin, G.; Hamelin, M.-E.; Nicolas, F.; et al. OVX836 a recombinant nucleoprotein vaccine inducing cellular responses and protective efficacy against multiple influenza A subtypes. npj Vaccines 2019, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, I.; Willems, P.; Waerlop, G.; Janssens, Y.; Tourneur, J.; De Boever, F.; Bruhwyler, J.; Alhatemi, A.; Jacobs, B.; Nicolas, F.; et al. Immunogenicity, safety, and preliminary efficacy evaluation of OVX836, a nucleoprotein-based universal influenza A vaccine candidate: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Infect. Dis. 2023, 23, 1360–1369. [Google Scholar] [CrossRef]
- Tudor Giurgea, L.; Han, A.; Czajkowski, L.; Ann Baus, H.; Mak, G.; Adao, K.; Bellayr, I.; Cervantes-Medina, A.; Gouzoulis, M.; Sherry, J.; et al. 593. Randomized, Double-Blinded, Placebo-Controlled, Phase 1 Study of the Safety of BPL-1357, A BPL-Inactivated, Whole-Virus, Universal Influenza Vaccine. Open Forum Infect. Dis. 2025, 12 (Suppl. 1), ofae631.188. [Google Scholar] [CrossRef]
- Zuccarino-Catania, G.V.; Sadanand, S.; Weisel, F.J.; Tomayko, M.M.; Meng, H.; Kleinstein, S.H.; Good-Jacobson, K.L.; Shlomchik, M.J. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat. Immunol. 2014, 15, 631–637. [Google Scholar] [CrossRef]
- Shokat, K.M.; Goodnow, C.C. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature 1995, 375, 334–338. [Google Scholar] [CrossRef]
- Pulendran, B.; Kannourakis, G.; Nouri, S.; Smith, K.G.C.; Nossal, G.J.V. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature 1995, 375, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Nguyen, T.H.; Philbrook, P.; Chu, M.; Sears, O.; Hatfield, S.; Abbott, R.K.; Kelsoe, G.; Sitkovsky, M.V. Targeted elimination of immunodominant B cells drives the germinal center reaction toward subdominant epitopes. Cell Rep. 2017, 21, 3672–3680. [Google Scholar] [CrossRef]
- Meyer-Hermann, M. Injection of antibodies against immunodominant epitopes tunes germinal centers to generate broadly neutralizing antibodies. Cell Rep. 2019, 29, 1066–1073. [Google Scholar] [CrossRef]
- Lee, K.-W.; Yam, J.W.; Mao, X. Dendritic Cell Vaccines: A Shift from Conventional Approach to New Generations. Cells 2023, 12, 2147. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef]
- Kurts, C.; Robinson, B.W.S.; Knolle, P.A. Cross-priming in health and disease. Nat. Rev. Immunol. 2010, 10, 403–414. [Google Scholar] [CrossRef]
- Caminschi, I.; Shortman, K. Boosting antibody responses by targeting antigens to dendritic cells. Trends Immunol. 2012, 33, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.A.; Huebsch, N.; Cao, L.; Dranoff, G.; Mooney, D.J. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 2009, 8, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Gale, E.C.; Powell, A.E.; Roth, G.A.; Meany, E.L.; Yan, J.; Ou, B.S.; Grosskopf, A.K.; Adamska, J.; Picece, V.C.T.M.; d’Aquino, A.I.; et al. Hydrogel-Based Slow Release of a Receptor-Binding Domain Subunit Vaccine Elicits Neutralizing Antibody Responses Against SARS-CoV-2. Adv. Mater. 2021, 33, 2104362. [Google Scholar] [CrossRef]
- Liu, H.; Moynihan, K.D.; Zheng, Y.; Szeto, G.L.; Li, A.V.; Huang, B.; Van Egeren, D.S.; Park, C.; Irvine, D.J. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 2014, 507, 519–522. [Google Scholar] [CrossRef]
- Stadler, B.M. Anti-IgE autoantibodies: A possible specific feedback on the cytokine network in allergy? Eur. Cytokine Netw. 1992, 3, 437–441. [Google Scholar] [PubMed]
- Jerne, N.K. Towards a network theory of the immune system. Ann. Immunol. 1974, 125, 373–389. [Google Scholar]
- Suciu-Foca, N.; Reed, E.; Rohowsky, C.; Kung, P.; King, D.W. Anti-idiotypic antibodies to anti-HLA receptors induced by pregnancy. Proc. Natl. Acad. Sci. USA 1983, 80, 830–834. [Google Scholar] [CrossRef]
- Ito, K.; Tanaka, T.; Tsutsumi, N.; Obata, F.; Kashiwagi, N. Possible mechanisms of immunotherapy for maintaining pregnancy in recurrent spontaneous aborters: Analysis of anti-idiotypic antibodies directed against autologous T-cell receptors. Hum. Reprod. 1999, 14, 650–655. [Google Scholar]
- Reed, E.; Ho, E.; Cohen, D.J.; Ramey, W.; Marboe, C.; D’Agati, V.; Rose, E.A.; Hardy, M.; Suciu-Foca, N. Anti-idiotypic antibodies specific for HLA in heart and kidney allograft recipients. Immunol. Res. 1993, 12, 1–11. [Google Scholar] [CrossRef]
- Kwak, L.W.; Thielemans, K.; Massaia, M. Idiotypic vaccination as therapy for multiple myeloma. Semin. Hematol. 1999, 36, 34–37. [Google Scholar] [PubMed]
- Foon, K.A.; Chakraborty, M.; John, W.J.; Sherratt, A.; Köhler, H.; Bhattacharya-Chatterjee, M. Immune response to the carcinoembryonic antigen in patients treated with an anti-idiotype antibody vaccine. J. Clin. Investig. 1995, 96, 334–342. [Google Scholar] [CrossRef]
- Keay, S.; Tacket, C.; Murphy, J.R.; Handwerger, B.S. Anti-CD4 Anti-Idiotype Antibodies in Volunteers Immunized with rgp 160 of HIV-1 or Infected with HIV-1. AIDS Res. Hum. Retroviruses 1992, 8, 1091–1098. [Google Scholar] [CrossRef]
- Deckert, P.M.; Ballmaier, M.; Lang, S.; Deicher, H.; Schedel, I. CD4-imitating human antibodies in HIV infection and anti-idiotypic vaccination. J. Immunol. 1996, 156, 826–833. [Google Scholar] [CrossRef]
- Kang, C.-Y.; Nara, P.; Chamat, S.; Caralli, V.; Chen, A.; Nguyen, M.-L.; Yoshiyama, H.; Morrow, W.J.; Ho, D.D.; Köhler, H. Anti-idiotype monoclonal antibody elicits broadly neutralizing anti-gp120 antibodies in monkeys. Proc. Natl. Acad. Sci. USA 1992, 89, 2546–2550. [Google Scholar] [CrossRef]
- Soodeen, S.; Justiz-Vaillant, A. Exploring Anti-Idiotypic HIV Vaccines through Oral Immunisation and Immunological Network Theory. arXiv 2025. [Google Scholar] [CrossRef]
- Stanova, A.K.; Ryabkova, V.A.; Tillib, S.V.; Utekhin, V.J.; Churilov, L.P.; Shoenfeld, Y. Anti-idiotypic agonistic antibodies: Candidates for the role of universal remedy. Antibodies 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yuhasz, S.C.; Amzel, L.M. Anti-idiotypic antibodies: Biological function and structural studies. FASEB J. 1995, 9, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.J.; Longo, D.L. A possible role for anti-idiotype antibodies in SARS-CoV-2 infection and vaccination. N. Engl. J. Med. 2022, 386, 394–396. [Google Scholar] [CrossRef]
- Junker, F.; Gordon, J.; Qureshi, O. Fc Gamma Receptors and Their Role in Antigen Uptake, Presentation, and T Cell Activation. Front. Immunol. 2020, 11, 1393. [Google Scholar] [CrossRef]
- Bakema, J.E.; van Egmond, M. The human immunoglobulin A Fc receptor FcαRI: A multifaceted regulator of mucosal immunity. Mucosal Immunol. 2011, 4, 612–624. [Google Scholar] [CrossRef]
- Kubagawa, H.; Oka, S.; Kubagawa, Y.; Torii, I.; Takayama, E.; Kang, D.-W.; Gartland, G.L.; Bertoli, L.F.; Mori, H.; Takatsu, H.; et al. Identity of the elusive IgM Fc receptor (FcμR) in humans. J. Exp. Med. 2009, 206, 2779–2793. [Google Scholar] [CrossRef] [PubMed]
- Spiegelberg, H.L. Fc Receptors for IgE and Interleukin-4 Induced IgE and IgG4 Secretion. J. Investig. Dermatol. 1990, 94 (Suppl. 6), s49–s52. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, D.; Blank, M.; Payne, A.S.; Anhalt, G.J.; Avivi, C.; Barshack, I.; David, M.; Shoenfeld, Y. Efficacy of intravenous immunoglobulin (IVIG) affinity-purified anti-desmoglein anti-idiotypic antibodies in the treatment of an experimental model of pemphigus vulgaris. Clin. Exp. Immunol. 2010, 162, 543–549. [Google Scholar] [CrossRef]
- Davis, T.A.; Maloney, D.G.; Czerwinski, D.K.; Liles, T.-M.; Levy, R. Anti-Idiotype Antibodies Can Induce Long-Term Complete Remissions in Non-Hodgkin’s Lymphoma Without Eradicating the Malignant Clone. Blood 1998, 92, 1184–1190. [Google Scholar] [CrossRef]
- Lode, H.N.; Schmidt, M.; Seidel, D.; Huebener, N.; Brackrock, D.; Bleeke, M.; Reker, D.; Brandt, S.; Mueller, H.-P.; Helm, C.; et al. Vaccination with anti-idiotype antibody ganglidiomab mediates a GD2-specific anti-neuroblastoma immune response. Cancer Immunol. Immunother. 2013, 62, 999–1010. [Google Scholar] [CrossRef]
- Ladjemi, M.Z.; Chardes, T.; Corgnac, S.; Garambois, V.; Morisseau, S.; Robert, B.; Bascoul-Mollevi, C.; Ait Arsa, I.; Jacot, W.; Pouget, J.-P.; et al. Vaccination with human anti-trastuzumab anti-idiotype scFv reverses HER2 immunological tolerance and induces tumor immunity in MMTV.f.huHER2(Fo5) mice. Breast Cancer Res. 2011, 13, R17. [Google Scholar] [CrossRef]
- Wilkinson, R.W.; Ross, E.L.; Lee-MacAry, A.E.; Laylor, R.; Burchell, J.; Taylor-Papadimitriou, J.; Snary, D. A transgenic mouse model for tumour immunotherapy: Induction of an anti-idiotype response to human MUC1. Br. J. Cancer 2000, 83, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Sheng, Y.; Zhang, G.; Sun, Y.; Wang, L.; Ji, P.; Zhu, J.; Wang, G.; Liu, B.; Zhou, E.-M.; et al. A novel strategy for an anti-idiotype vaccine: Nanobody mimicking neutralization epitope of porcine circovirus type 2. J. Virol. 2024, 98, e01650-23. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Kohler, H.; Pashov, A.; Kieber-Emmons, T. The Promise of Anti-idiotype Revisited. Front. Immunol. 2019, 10, 808. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Chia, Y.C.; Yee, H.R.; Fang Cheng, A.Y.; Anjum, C.E.; Kenisi, Y.; Chan, M.K.S.; Wong, M.B.F. Immunomodulatory Potential of anti-idiotypic Antibodies for the Treatment of Autoimmune Diseases. Future Sci. OA 2021, 7, FSO648. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, J.; Naninck, T.; Delache, B.; Creppy, J.; Huber, P.; Holzapfel, M.; Bouillier, C.; Contreras, V.; Martinon, F.; Kahlaoui, N.; et al. Non-human primate models of human respiratory infections. Mol. Immunol. 2021, 135, 147–164. [Google Scholar] [CrossRef]
- Santaniello, A.; Perruolo, G.; Cristiano, S.; Agognon, A.L.; Cabaro, S.; Amato, A.; Dipineto, L.; Borrelli, L.; Formisano, P.; Fioretti, A.; et al. SARS-CoV-2 Affects Both Humans and Animals: What Is the Potential Transmission Risk? A Literature Review. Microorganisms 2023, 11, 514. [Google Scholar] [CrossRef]
- Gurumurthy, C.B.; Quadros, R.M.; Richardson, G.P.; Poluektova, L.Y.; Mansour, S.L.; Ohtsuka, M. Genetically modified mouse models to help fight COVID-19. Nat. Protoc. 2020, 15, 3777–3787. [Google Scholar] [CrossRef]
- Yinda, C.K.; Port, J.R.; Bushmaker, T.; Offei Owusu, I.; Purushotham, J.N.; Avanzato, V.A.; Fischer, R.J.; Schulz, J.E.; Holbrook, M.G.; Hebner, M.J.; et al. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog. 2021, 17, e1009195. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.S.; Bailey, A.L.; Kafai, N.M.; Nair, S.; McCune, B.T.; Yu, J.; Fox, J.M.; Chen, R.E.; Earnest, J.T.; Keeler, S.P.; et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020, 21, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Dinnon Iii, K.H.; Leist, S.R.; Schäfer, A.; Edwards, C.E.; Martinez, D.R.; Montgomery, S.A.; West, A.; Yount, B.L., Jr.; Hou, Y.J.; Adams, L.E. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 2020, 586, 560–566. [Google Scholar] [CrossRef]
- Leist, S.R.; Dinnon, K.H.; Schäfer, A.; Longping, V.T.; Okuda, K.; Hou, Y.J.; West, A.; Edwards, C.E.; Sanders, W.; Fritch, E.J.; et al. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 2020, 183, 1070–1085. [Google Scholar] [CrossRef]
- Schulte, S.; Sukhova, G.K.; Libby, P. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am. J. Pathol. 2008, 172, 1500–1508. [Google Scholar] [CrossRef]
- Mosmann, T.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Fan, C.; Wu, Y.; Rui, X.; Yang, Y.; Ling, C.; Liu, S.; Liu, S.; Wang, Y. Animal models for COVID-19: Advances, gaps and perspectives. Signal Transduct. Target. Ther. 2022, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.; Yan, F.; Gao, Y.; Yang, S.; Xia, X. COVID-19 animal models and vaccines: Current landscape and future prospects. Vaccines 2021, 9, 1082. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Lowen, A.C. Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses 2010, 2, 1530–1563. [Google Scholar] [CrossRef]
- Michelitsch, A.; Wernike, K.; Ulrich, L.; Mettenleiter, T.C.; Beer, M. SARS-CoV-2 in animals: From potential hosts to animal models. Adv. Virus Res. 2021, 110, 59–102. [Google Scholar]
- van Riel, D.; Mittrücker, H.-W.; Engels, G.; Klingel, K.; Markert, U.R.; Gabriel, G. Influenza pathogenicity during pregnancy in women and animal models. Semin Immunopathol. 2016, 38, 719–726. [Google Scholar] [CrossRef]
- Ashby, B.; Best, A. Herd immunity. Curr. Biol. 2021, 31, R174–R177. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Hormeño-Holgado, A.; Jiménez, M.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Perez-Palencia, N.; Maestre-Serrano, R.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Dynamics of population immunity due to the herd effect in the COVID-19 pandemic. Vaccines 2020, 8, 236. [Google Scholar] [CrossRef]
- Baumann, B.M.; Rodriguez, R.M.; DeLaroche, A.M.; Rayburn, D.; Eucker, S.A.; Nadeau, N.L.; Drago, L.A.; Cullen, D.; Meskill, S.D.; Bialeck, S.; et al. Factors associated with parental acceptance of COVID-19 vaccination: A multicenter pediatric emergency department cross-sectional analysis. Ann. Emerg. Med. 2022, 80, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.P.; Lee, H.Y.; Alias, H.; Zimet, G.; Liu, T.; Lin, Y.; Hu, Z. Cost-based COVID-19 vaccination and willingness to pay: A post-pandemic review. Hum. Vaccines Immunother. 2024, 20, 2313860. [Google Scholar] [CrossRef]
- Excler, J.-L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2021, 27, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Te Yeh, M.; Bujaki, E.; Dolan, P.T.; Smith, M.; Wahid, R.; Konz, J.; Weiner, A.J.; Bandyopadhyay, A.S.; Van Damme, P.; De Coster, I.; et al. Engineering the live-attenuated polio vaccine to prevent reversion to virulence. Cell Host Microbe 2020, 27, 736–751. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Elkashif, A.; Alhashimi, M.; Sambhara, S.; Mittal, S.K. Adenoviral vector-based vaccine platforms for developing the next generation of influenza vaccines. Vaccines 2020, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Liu, Y.; Tam, R.C.-Y.; Chen, P.; Zhang, A.J.; Mok, B.W.-Y.; Long, T.; Kukic, A.; Zhou, R.; Xu, H.; et al. An intranasal influenza virus-vectored vaccine prevents SARS-CoV-2 replication in respiratory tissues of mice and hamsters. Nat. Commun. 2023, 14, 2081. [Google Scholar] [CrossRef]
- Zhu, F.; Huang, S.; Liu, X.; Chen, Q.; Zhuang, C.; Zhao, H.; Han, J.; Jaen, A.M.; Do, T.H.; Peter, J.G.; et al. Safety and efficacy of the intranasal spray SARS-CoV-2 vaccine dNS1-RBD: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2023, 11, 1075–1088. [Google Scholar] [CrossRef]
- Trimzi, M.A.; Ham, Y.-B. A needle-free jet injection system for controlled release and repeated biopharmaceutical delivery. Pharmaceutics 2021, 13, 1770. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.M. Can needle-free administration of vaccines become the norm in global immunization? Nat. Med. 2003, 9, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Immunization without needles. Nat. Rev. Immunol. 2005, 5, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Current status and future prospects of needle-free liquid jet injectors. Nat. Rev. Drug Discov. 2006, 5, 543–548. [Google Scholar] [CrossRef]
- Govender, M.; Indermun, S.; Choonara, Y.E. 3D bioprinted microneedles: Merging drug delivery and scaffold science for tissue-specific applications. Expert Opin. Drug Deliv. 2024, 21, 1559–1572. [Google Scholar] [CrossRef]
- Nadda, R.; Singh, P.K.; Das, D.B. Revolutionizing microneedle array fabrication using additive manufacturing technologies: Potential applications and clinical translation. J. Drug Deliv. Sci. Technol. 2024, 101, 106288. [Google Scholar] [CrossRef]
- Olowe, M.; Parupelli, S.K.; Desai, S. A Review of 3D-Printing of Microneedles. Pharmaceutics 2022, 14, 2693. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.M.; Lim, Y.J.L.; Tay, J.T.; Cheng, H.M.; Tey, H.L.; Liang, K. Design and fabrication of customizable microneedles enabled by 3D printing for biomedical applications. Bioact. Mater. 2024, 32, 222–241. [Google Scholar] [CrossRef]
- Falahi, S.; Kenarkoohi, A. Host factors and vaccine efficacy: Implications for COVID-19 vaccines. J. Med. Virol. 2022, 94, 1330–1335. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
| Vaccine Candidate | Target Virus | Platform | Route | Antigen Design | Clinical Trial Phase and ID | Unique Design Feature | Refs. |
|---|---|---|---|---|---|---|---|
| SteMos1 (NIAID) | Influenza | Nanoparticle (HA stem) | IM | Structure-guided HA stem + ALFQ adjuvant | Phase I: NCT07111078 | HA stem-only nanoparticle for universal flu vaccine | [13,14] |
| OVX835 (Osivax) | Influenza | Recombinant NP antigen | IM | Conserved nucleoprotein (NP) | Phase 2a: NCT04192500 | T cell-focused design targeting internal antigen | [15,16] |
| DentalFloss-M2e | Influenza | Gold nanoparticle | Floss-based | M2e peptide scaffold | N/A | Floss-like scaffold for mucosal delivery | [17] |
| cHA-ΔNS1-LAIV (CIVICs) | Influenza | Live attenuated vaccine | IN | Chimeric HA + NS1 deletion | N/A | NS1 deletion enhances safety and mucosal immunity | [18] |
| GammaFlu (Gamma Vaccines) | Influenza | Whole-virus inactivated | IM | Broad-spectrum antigen mix | N/A | Self-adjuvanting | [19] |
| RSM2eFP (CAS) | Influenza | Bacillus subtilis spore-based oral vaccine | Oral | M2e + fusion peptide | N/A | Thermostable spore-based oral delivery | [20] |
| mRNA-1010 (Moderna) | Influenza | mRNA-LNP | IM | HA antigens from 4 strains | Phase I/II: NCT04956575 | Quadrivalent seasonal mRNA flu vaccine | [21,22] |
| ARCoV (Walvax) | SARS-CoV-2 | mRNA-LNP | IM | RBD domain | Phase III: NCT04847102 | RBD-only design for thermostability | [23] |
| SAM-COVID (Gritstone) | SARS-CoV-2 | Self-amplifying mRNA | IM | Spike + T cell epitopes | Phase I: NCT04776317 | Self-replicating RNA for dose-sparing | [24] |
| ABNCoV2 (AdaptVac/Bavarian Nordic) | SARS-CoV-2 | VLP-mRNA hybrid | IM | RBD displayed on VLP | Phase I: NCT04839146 | Capsid VLP display enhances B cell activation | [25] |
| mRNA-1073 (Moderna) | SARS-CoV-2 + Influenza | mRNA-LNP | IM | Spike + HA antigens | Phase I: NCT05585632 | Dual-pathogen respiratory vaccine | [26] |
| UB-612 (Vaxxinity) | SARS-CoV-2 | Peptide-based subunit | IM | RBD + T cell epitopes | Phase III: NCT05293665 | Synthetic peptide for T cell targets | [27] |
| mRNA-1345 (Moderna) | RSV | mRNA-LNP | IM | prefusion F protein | Phase I: NCT04528719 | Structure-guided prefusion F design | [28] |
| ChAdOx1 RSV (Oxford) | RSV | Adenoviral vector | IM | Prefusion F protein | Phase I: NCT04754776 | ChAdOx1 vector with stabilized RSV antigen | [29] |
| DS-Cav1 (NIH) | RSV | Protein subunit | IM | prefusion F protein | Phase I: NCT03049488 | First rationally engineered RSV antigen | [30] |
| mRNA-1083 (Moderna) | RSV + SARS-CoV-2 | mRNA-LNP | IM | Spike + RSV F protein | Phase III: NCT05827926 | Dual-pathogen mRNA respiratory vaccine | [31] |
| mRNA-1653 (Moderna) | hMPV + PIV3 | mRNA-LNP | IM | Engineered fusion proteins | Phase I: NCT04144348 | Combined pediatric respiratory vaccine | [32] |
| mRNA-1893 (Moderna) | Zika virus | mRNA-LNP | IM | Zika envelope protein | Phase II: NCT04917861 | mRNA-encoding E protein | [33] |
| CV7202 (CureVac) | Rabies virus | Protamine-mRNA | IM | Rabies glycoprotein | Phase I: NCT03713086 | Protamine-complexed mRNA for enhanced stability | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, F.; Bluth, M.H. Novel Strategies for Developing Next-Generation Vaccines to Combat Infectious Viral Diseases. Vaccines 2025, 13, 979. https://doi.org/10.3390/vaccines13090979
Yuan F, Bluth MH. Novel Strategies for Developing Next-Generation Vaccines to Combat Infectious Viral Diseases. Vaccines. 2025; 13(9):979. https://doi.org/10.3390/vaccines13090979
Chicago/Turabian StyleYuan, Fangfeng, and Martin H. Bluth. 2025. "Novel Strategies for Developing Next-Generation Vaccines to Combat Infectious Viral Diseases" Vaccines 13, no. 9: 979. https://doi.org/10.3390/vaccines13090979
APA StyleYuan, F., & Bluth, M. H. (2025). Novel Strategies for Developing Next-Generation Vaccines to Combat Infectious Viral Diseases. Vaccines, 13(9), 979. https://doi.org/10.3390/vaccines13090979





