Recent Advancements in Non-Invasive Vaccination Strategies
Abstract
1. Introduction
2. Oral Vaccines
2.1. Early Development of Oral Vaccines
2.2. The New Wave of Oral Vaccine Research
2.2.1. Nanoparticle and Microparticle Delivery Systems
2.2.2. Recombinant Microbial Vectors in Oral Vaccines
2.2.3. Mucoadhesive and Mucus-Penetrating Formulations in Oral Vaccine Delivery
2.2.4. Bacterium-like Particles (BLPs) and Lipid-Coated Systems in Oral Vaccines
2.2.5. Plant-Based Oral Vaccines
3. Inhalation Vaccines
3.1. Intranasal Vaccines
3.1.1. Mechanism of Action of Intranasal Administration
3.1.2. Limitations and Advancements in the Field
3.2. Oral Inhalation
3.2.1. Mechanism
3.2.2. Limitations of Inhalation Route
4. Microneedles Vaccine Delivery
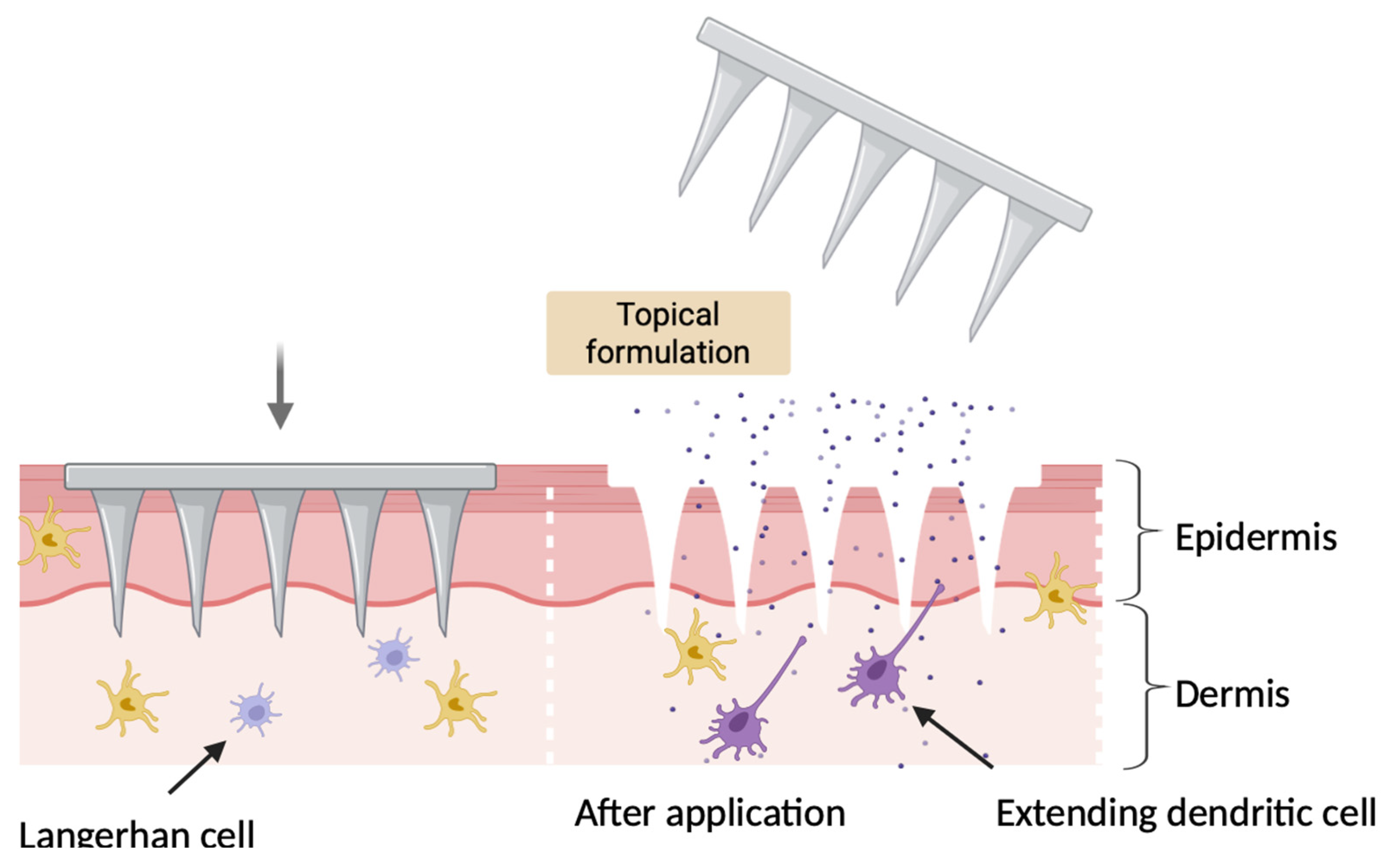
4.1. Mechanism of Action of Microneedles
4.2. Evolution of MN Technology in Vaccines
4.3. Solid Microneedles
4.4. Hollow Microneedles
4.5. Coated Microneedles
4.6. Dissolving Microneedles
4.7. Hydrogel/Swelling Microneedles
5. Buccal Route of Administration
5.1. Mechanistic Insights into Buccal Vaccine Delivery
5.2. Current Research and Development
6. Sublingual Vaccination
6.1. Mechanism of Sublingual Immunization
6.2. Sublingual Tablets
6.3. Patch-Based Systems
7. Vaginal Vaccines
7.1. Mechanism of Action
7.2. History and Current Field of Vaginal Vaccines
7.3. Limitations and Advancements
7.4. Mucoadhesive and Thermoresponsive Gels
7.5. Mucus-Penetrating Vaccines
7.6. Intravaginal Rings
On-Going Clinical Trials
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, Z.; Diaz-Arévalo, D.; Guan, H.; Zeng, M. Noninvasive Vaccination against Infectious Diseases. Hum. Vaccines Immunother. 2018, 14, 1717–1733. [Google Scholar] [CrossRef] [PubMed]
- Giudice, E.; Campbell, J. Needle-Free Vaccine Delivery☆. Adv. Drug Deliv. Rev. 2006, 58, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Sun, Y.; Cui, H.; Zhu, S.J.; Qiu, H.-J. Mucosal Vaccines: Strategies and Challenges. Immunol. Lett. 2020, 217, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weken, H.; Jahantigh, H.R.; Cox, E.; Devriendt, B. Targeted Delivery of Oral Vaccine Antigens to Aminopeptidase N Protects Pigs against Pathogenic E. coli Challenge Infection. Front. Immunol. 2023, 14, 1192715. [Google Scholar] [CrossRef]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current State and Challenges in Developing Oral Vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Kim, L.; Martinez, C.J.; Hodgson, K.A.; Trager, G.R.; Brandl, J.R.; Sandefer, E.P.; Doll, W.J.; Liebowitz, D.; Tucker, S.N. Systemic and Mucosal Immune Responses Following Oral Adenoviral Delivery of Influenza Vaccine to the Human Intestine by Radio Controlled Capsule. Sci. Rep. 2016, 6, 37295. [Google Scholar] [CrossRef]
- Chowdhury, F.; Bhuiyan, T.R.; Akter, A.; Bhuiyan, M.S.; Khan, A.I.; Tauheed, I.; Ahmed, T.; Ferdous, J.; Dash, P.; Basher, S.R.; et al. Augmented Immune Responses to a Booster Dose of Oral Cholera Vaccine in Bangladeshi Children Less than 5 Years of Age: Revaccination after an Interval of over Three Years of Primary Vaccination with a Single Dose of Vaccine. Vaccine 2020, 38, 1753–1761. [Google Scholar] [CrossRef]
- Malembaka, E.B.; Bugeme, P.M.; Hutchins, C.; Xu, H.; Hulse, J.D.; Demby, M.N.; Gallandat, K.; Saidi, J.M.; Rumedeka, B.B.; Itongwa, M.; et al. Effectiveness of One Dose of Killed Oral Cholera Vaccine in an Endemic Community in the Democratic Republic of the Congo: A Matched Case-Control Study. Lancet Infect. Dis. 2024, 24, 514–522. [Google Scholar] [CrossRef]
- Marasini, N.; Skwarczynski, M.; Toth, I. Oral Delivery of Nanoparticle-Based Vaccines. Expert Rev. Vaccines 2014, 13, 1361–1376. [Google Scholar] [CrossRef]
- Diaz-Arévalo, D.; Zeng, M. Nanoparticle-Based Vaccines: Opportunities and limitations. In Nanopharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–150. ISBN 978-0-12-817778-5. [Google Scholar]
- Borges, O.; Lebre, F.; Bento, D.; Borchard, G.; Junginger, H.E. Mucosal Vaccines: Recent Progress in Understanding the Natural Barriers. Pharm. Res. 2010, 27, 211–223. [Google Scholar] [CrossRef]
- Li, M.; Kaminskas, L.M.; Marasini, N. Recent Advances in Nano/Microparticle-Based Oral Vaccines. J. Pharm. Investig. 2021, 51, 425–438. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Khurana, N.; Vyas, M.; Sharma, V.; Batiha, G.E.-S.; Kaur, H.; Singh, J.; Kumar, D.; Sharma, N.; Kaushik, A.; et al. Aspects of Nanotechnology for COVID-19 Vaccine Development and Its Delivery Applications. Pharmaceutics 2023, 15, 451. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, T.; D’Souza, B.; Uddin, M.N.; D’Souza, M.J. Oral Delivery of Microparticles Containing Plasmid DNA Encoding Hepatitis-B Surface Antigen. J. Drug Target. 2012, 20, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Song, Y.; Liu, C.; Yu, L.; Shang, Y.; Tang, H.; Sun, S.; Wang, F. Application of Bacillus subtilis as a Live Vaccine Vector: A Review. J. Vet. Med. Sci. 2020, 82, 1693–1699. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Langella, P. Use of lactic acid bacteria as mucosal vaccines. Rev. Francoph. Lab. 2009, 2009, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, P. Yeast-Based Vaccines: New Perspective in Vaccine Development and Application. FEMS Yeast Res. 2019, 19, foz007. [Google Scholar] [CrossRef]
- Amuguni, H.; Lee, S.; Kerstein, K.; Brown, D.; Belitsky, B.; Herrmann, J.; Keusch, G.; Sonenshein, A.; Tzipori, S. Sublingual Immunization with an Engineered Bacillus subtilis Strain Expressing Tetanus Toxin Fragment C Induces Systemic and Mucosal Immune Responses in Piglets. Microbes Infect. 2012, 14, 447–456. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, R.; Sun, S.; Cui, Z.; Liang, B.; Li, E.; Wang, T.; Feng, Y.; Yang, S.; Yan, F.; et al. Bacillus subtilis Vector Based Oral Rabies Vaccines Induced Potent Immune Response and Protective Efficacy in Mice. Front. Microbiol. 2023, 14, 1126533. [Google Scholar] [CrossRef]
- Lee, S.; Belitsky, B.R.; Brinker, J.P.; Kerstein, K.O.; Brown, D.W.; Clements, J.D.; Keusch, G.T.; Tzipori, S.; Sonenshein, A.L.; Herrmann, J.E. Development of a Bacillus subtilis -Based Rotavirus Vaccine. Clin. Vaccine Immunol. 2010, 17, 1647–1655. [Google Scholar] [CrossRef]
- Yu, J.; Fu, J.; Liu, H.; Kang, C.; Wang, Z.; Jin, Y.; Wu, S.; Li, T.; Yang, R.; Jin, M.; et al. Application of Recombinant Lactic Acid Bacteria (LAB) Live Vector Oral Vaccine in the Prevention of F4+ Enterotoxigenic Escherichia coli. Vaccines 2024, 12, 304. [Google Scholar] [CrossRef]
- Li, L.; Hao, J.; Jiang, Y.; Hao, P.; Gao, Y.; Chen, J.; Zhang, G.; Jin, N.; Wang, M.; Li, C. A Micro-Sized Vaccine Based on Recombinant Lactiplantibacillus plantarum Fights against SARS-CoV-2 Infection via Intranasal Immunization. Acta Pharm. Sin. B 2023, 13, 3168–3176. [Google Scholar] [CrossRef]
- Del Rio, B.; Dattwyler, R.J.; Aroso, M.; Neves, V.; Meirelles, L.; Seegers, J.F.M.L.; Gomes-Solecki, M. Oral Immunization with Recombinant Lactobacillus plantarum Induces a Protective Immune Response in Mice with Lyme Disease. Clin. Vaccine Immunol. 2008, 15, 1429–1435. [Google Scholar] [CrossRef]
- King, T.H.; Kemmler, C.B.; Guo, Z.; Mann, D.; Lu, Y.; Coeshott, C.; Gehring, A.J.; Bertoletti, A.; Ho, Z.Z.; Delaney, W.; et al. A Whole Recombinant Yeast-Based Therapeutic Vaccine Elicits HBV X, S and Core Specific T Cells in Mice and Activates Human T Cells Recognizing Epitopes Linked to Viral Clearance. PLoS ONE 2014, 9, e101904. [Google Scholar] [CrossRef]
- Lei, H.; Xie, B.; Gao, T.; Cen, Q.; Ren, Y. Yeast Display Platform Technology to Prepare Oral Vaccine against Lethal H7N9 Virus Challenge in Mice. Microb. Cell Fact. 2020, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.H. Current and New Approaches for Mucosal Vaccine Delivery. In Mucosal Vaccines; Elsevier: Amsterdam, The Netherlands, 2020; pp. 325–356. ISBN 978-0-12-811924-2. [Google Scholar]
- Garg, N.K.; Mangal, S.; Khambete, H.; Sharma, P.K.; Tyagi, R.K. Mucosal Delivery of Vaccines: Role of Mucoadhesive/Biodegradable Polymers. Recent Pat. Drug Deliv. Formul. 2010, 4, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.-Y.; Hanes, J. Mucus-Penetrating Nanoparticles for Drug and Gene Delivery to Mucosal Tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Mokabari, K.; Iriti, M.; Varoni, E.M. Mucoadhesive Vaccine Delivery Systems for the Oral Mucosa. J. Dent. Res. 2023, 102, 709–718. [Google Scholar] [CrossRef]
- Amin, M.K.; Boateng, J.S. Enhancing Stability and Mucoadhesive Properties of Chitosan Nanoparticles by Surface Modification with Sodium Alginate and Polyethylene Glycol for Potential Oral Mucosa Vaccine Delivery. Mar. Drugs 2022, 20, 156. [Google Scholar] [CrossRef]
- Anggraeni, R.; Ana, I.D.; Wihadmadyatami, H. Development of Mucosal Vaccine Delivery: An Overview on the Mucosal Vaccines and Their Adjuvants. Clin. Exp. Vaccine Res. 2022, 11, 235. [Google Scholar] [CrossRef]
- Paul, S.; Bhuyan, S.; Balasoupramanien, D.D.; Palaniappan, A. Muco-Adhesive and Muco-Penetrative Formulations for the Oral Delivery of Insulin. ACS Omega 2024, 9, 24121–24141. [Google Scholar] [CrossRef]
- Zhao, K.; Xie, Y.; Lin, X.; Xu, W. The Mucoadhesive Nanoparticle-Based Delivery System in the Development of Mucosal Vaccines. Int. J. Nanomed. 2022, 17, 4579–4598. [Google Scholar] [CrossRef] [PubMed]
- Russell-Jones, G.J. Oral Vaccine Delivery. J. Control. Release 2000, 65, 49–54. [Google Scholar] [CrossRef]
- De, X.; Gao, M.; Jia, Z.; Ren, H.; Liu, R.; Zhou, X.; Guo, J.; Wang, J.; Yu, Q.; Qu, N.; et al. A Novel Oral Vaccine Delivery System for Enhancing Stability and Immune Protection: Bacterium-like Particle with Functional Coating. Front. Microbiol. 2024, 15, 1481514. [Google Scholar] [CrossRef] [PubMed]
- Akalkotkar, A.; Chablani, L.; Tawde, S.A.; D’Souza, C.; D’Souza, M.J. Development of a Microparticulate Prostate Cancer Vaccine and Evaluating the Effect of Route of Administration on Its Efficacy via the Skin. J. Microencapsul. 2015, 32, 281–289. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.J.; Lee, Y.-K.; Cho, S. Oral Vaccine Delivery for Intestinal Immunity—Biological Basis, Barriers, Delivery System, and M Cell Targeting. Polymers 2018, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Van Braeckel-Budimir, N.; Haijema, B.J.; Leenhouts, K. Bacterium-like Particles for Efficient Immune Stimulation of Existing Vaccines and New Subunit Vaccines in Mucosal Applications. Front. Immunol. 2013, 4, 282. [Google Scholar] [CrossRef]
- Chan, H.; Daniell, H. Plant-made Oral Vaccines against Human Infectious Diseases—Are We There Yet? Plant Biotechnol. J. 2015, 13, 1056–1070. [Google Scholar] [CrossRef]
- Kwon, K.-C.; Verma, D.; Singh, N.D.; Herzog, R.; Daniell, H. Oral Delivery of Human Biopharmaceuticals, Autoantigens and Vaccine Antigens Bioencapsulated in Plant Cells. Adv. Drug Deliv. Rev. 2013, 65, 782–799. [Google Scholar] [CrossRef]
- Vo, D.-K.; Trinh, K.T.L. Molecular Farming for Immunization: Current Advances and Future Prospects in Plant-Produced Vaccines. Vaccines 2025, 13, 191. [Google Scholar] [CrossRef]
- Feng, H.; Li, X.; Song, W.; Duan, M.; Chen, H.; Wang, T.; Dong, J. Oral Administration of a Seed-Based Bivalent Rotavirus Vaccine Containing VP6 and NSP4 Induces Specific Immune Responses in Mice. Front. Plant Sci. 2017, 8, 910. [Google Scholar] [CrossRef]
- Tokuhara, D.; Álvarez, B.; Mejima, M.; Hiroiwa, T.; Takahashi, Y.; Kurokawa, S.; Kuroda, M.; Oyama, M.; Kozuka-Hata, H.; Nochi, T.; et al. Rice-Based Oral Antibody Fragment Prophylaxis and Therapy against Rotavirus Infection. J. Clin. Investig. 2013, 123, 3829–3838. [Google Scholar] [CrossRef]
- Pudhuvai, B.; Koul, B.; Mishra, A.K. Insights into the World of Edible Vaccines: From Lab to Reality. Curr. Res. Biotechnol. 2025, 9, 100290. [Google Scholar] [CrossRef]
- Kong, Q.; Richter, L.; Yang, Y.F.; Arntzen, C.J.; Mason, H.S.; Thanavala, Y. Oral Immunization with Hepatitis B Surface Antigen Expressed in Transgenic Plants. Proc. Natl. Acad. Sci. USA 2001, 98, 11539–11544. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Carter, N.J.; Curran, M.P. Live Attenuated Influenza Vaccine (FluenzTM): A Guide to Its Use in the Prevention of Seasonal Influenza in Children in the EU. Pediatr. Drugs 2012, 14, 271–279. [Google Scholar] [CrossRef]
- FDA Approves Nasal Spray Influenza Vaccine for Self- or Caregiver-Administration. Available online: https://Www.Fda.Gov/News-Events/Press-Announcements/Fda-Approves-Nasal-Spray-Influenza-Vaccine-Self-or-Caregiver-Administration (accessed on 10 September 2025).
- Kehagia, E.; Papakyriakopoulou, P.; Valsami, G. Advances in Intranasal Vaccine Delivery: A Promising Non-Invasive Route of Immunization. Vaccine 2023, 41, 3589–3603. [Google Scholar] [CrossRef]
- Jabbal-Gill, I. Nasal Vaccine Innovation. J. Drug Target. 2010, 18, 771–786. [Google Scholar] [CrossRef]
- Heida, R.; Hinrichs, W.L.; Frijlink, H.W. Inhaled Vaccine Delivery in the Combat against Respiratory Viruses: A 2021 Overview of Recent Developments and Implications for COVID-19. Expert Rev. Vaccines 2022, 21, 957–974. [Google Scholar] [CrossRef]
- Fokkens, W.J. Antigen-presenting Cells in Nasal Allergy. Allergy 1999, 54, 1130–1141. [Google Scholar] [CrossRef]
- Shim, S.; Soh, S.H.; Im, Y.B.; Ahn, C.; Park, H.-T.; Park, H.-E.; Park, W.B.; Kim, S.; Yoo, H.S. Induction of Systemic Immunity through Nasal-Associated Lymphoid Tissue (NALT) of Mice Intranasally Immunized with Brucella Abortus Malate Dehydrogenase-Loaded Chitosan Nanoparticles. PLoS ONE 2020, 15, e0228463. [Google Scholar] [CrossRef]
- Ramvikas, M.; Arumugam, M.; Chakrabarti, S.R.; Jaganathan, K.S. Nasal Vaccine Delivery. In Micro and Nanotechnology in Vaccine Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 279–301. ISBN 978-0-323-39981-4. [Google Scholar]
- Lee, H.; Ruane, D.; Law, K.; Ho, Y.; Garg, A.; Rahman, A.; Esterházy, D.; Cheong, C.; Goljo, E.; Sikora, A.G.; et al. Phenotype and Function of Nasal Dendritic Cells. Mucosal Immunol. 2015, 8, 1083–1098. [Google Scholar] [CrossRef]
- Seefeld, M.L.; Templeton, E.L.; Lehtinen, J.M.; Sinclair, N.; Yadav, D.; Hartwell, B.L. Harnessing the Potential of the NALT and BALT as Targets for Immunomodulation Using Engineering Strategies to Enhance Mucosal Uptake. Front. Immunol. 2024, 15, 1419527. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gulani, M.; Vijayanand, S.; Arte, T.; Adediran, E.; Pasupuleti, D.; Patel, P.; Ferguson, A.; Uddin, M.; Zughaier, S.M.; et al. An Intranasal Quadruple Variant Vaccine Approach Using SARS-CoV-2 and Influenza A: Delta, Omicron, H1N1 and H3N2. Int. J. Pharm. 2025, 683, 126043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Y.; Huang, L.; Yuan, L.; Huang, S.; Zeng, Z.; Cao, Y.; Wei, X.; Wang, X.; Shi, M.; et al. Nanotechnology-Driven Advances in Intranasal Vaccine Delivery Systems against Infectious Diseases. Front. Immunol. 2025, 16, 1573037. [Google Scholar] [CrossRef] [PubMed]
- Nian, X.; Zhang, J.; Huang, S.; Duan, K.; Li, X.; Yang, X. Development of Nasal Vaccines and the Associated Challenges. Pharmaceutics 2022, 14, 1983. [Google Scholar] [CrossRef]
- Kar, S.; Devnath, P.; Emran, T.B.; Tallei, T.E.; Mitra, S.; Dhama, K. Oral and Intranasal Vaccines against SARS-CoV-2: Current Progress, Prospects, Advantages, and Challenges. Immun. Inflam Dis. 2022, 10, e604. [Google Scholar] [CrossRef]
- Mashkoor, Y.; Nadeem, A.; Fatima, T.; Aamir, M.; Vohra, L.I.; Habib, A.; Khan, A.; Raufi, N.; Habte, A. Neurological Complications of Influenza Vaccination: Navigating the Spectrum with a Focus on Acute Disseminated Encephalomyelitis (ADEM). Ann. Med. Surg. 2024, 86, 1029–1041. [Google Scholar] [CrossRef]
- Wu, L.; Xu, W.; Jiang, H.; Yang, M.; Cun, D. Respiratory Delivered Vaccines: Current Status and Perspectives in Rational Formulation Design. Acta Pharm. Sin. B 2024, 14, 5132–5160. [Google Scholar] [CrossRef]
- He, X.; Chen, X.; Wang, H.; Du, G.; Sun, X. Recent Advances in Respiratory Immunization: A Focus on COVID-19 Vaccines. J. Control Release 2023, 355, 655–674. [Google Scholar] [CrossRef]
- Masjedi, M.; Montahaei, T.; Sharafi, Z.; Jalali, A. Pulmonary Vaccine Delivery: An Emerging Strategy for Vaccination and Immunotherapy. J. Drug Deliv. Sci. Technol. 2022, 69, 103184. [Google Scholar] [CrossRef]
- Randall, T.D. Bronchus-Associated Lymphoid Tissue (BALT). In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 187–241. ISBN 978-0-12-381300-8. [Google Scholar]
- Next-Gen Inhaled COVID Vaccine Boosts Lung Immunity. Bioengineer, 21 July 2025.
- Tang, J.; Sun, J. Lung Tissue-Resident Memory T Cells: The Gatekeeper to Respiratory Viral (Re)-Infection. Curr. Opin. Immunol. 2023, 80, 102278. [Google Scholar] [CrossRef]
- Blank, F.; Stumbles, P.; Von Garnier, C. Opportunities and Challenges of the Pulmonary Route for Vaccination. Expert Opin. Drug Deliv. 2011, 8, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Tonnis, W.F.; Kersten, G.F.; Frijlink, H.W.; Hinrichs, W.L.J.; De Boer, A.H.; Amorij, J.-P. Pulmonary Vaccine Delivery: A Realistic Approach? J. Aerosol Med. Pulm. Drug Deliv. 2012, 25, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Price, D.N.; Kunda, N.K.; Muttil, P. Challenges Associated with the Pulmonary Delivery of Therapeutic Dry Powders for Preclinical Testing. KONA Powder Part. J. 2019, 36, 129–144. [Google Scholar] [CrossRef]
- Hossain, M.K.; Ahmed, T.; Bhusal, P.; Subedi, R.K.; Salahshoori, I.; Soltani, M.; Hassanzadeganroudsari, M. Microneedle Systems for Vaccine Delivery: The Story so Far. Expert Rev. Vaccines 2020, 19, 1153–1166. [Google Scholar] [CrossRef]
- Mansoor, I.; Eassa, H.A.; Mohammed, K.H.A.; Abd El-Fattah, M.A.; Abdo, M.H.; Rashad, E.; Eassa, H.A.; Saleh, A.; Amin, O.M.; Nounou, M.I.; et al. Microneedle-Based Vaccine Delivery: Review of an Emerging Technology. AAPS PharmSciTech 2022, 23, 103. [Google Scholar] [CrossRef]
- Stoitzner, P.; Tripp, C.H.; Eberhart, A.; Price, K.M.; Jung, J.Y.; Bursch, L.; Ronchese, F.; Romani, N. Langerhans Cells Cross-Present Antigen Derived from Skin. Proc. Natl. Acad. Sci. USA 2006, 103, 7783–7788. [Google Scholar] [CrossRef]
- Mikszta, J.A.; Alarcon, J.B.; Brittingham, J.M.; Sutter, D.E.; Pettis, R.J.; Harvey, N.G. Improved Genetic Immunization via Micromechanical Disruption of Skin-Barrier Function and Targeted Epidermal Delivery. Nat. Med. 2002, 8, 415–419. [Google Scholar] [CrossRef]
- Quan, F.-S.; Kim, Y.-C.; Vunnava, A.; Yoo, D.-G.; Song, J.-M.; Prausnitz, M.R.; Compans, R.W.; Kang, S.-M. Intradermal Vaccination with Influenza Virus-Like Particles by Using Microneedles Induces Protection Superior to That with Intramuscular Immunization. J. Virol. 2010, 84, 7760–7769. [Google Scholar] [CrossRef]
- Edens, C.; Collins, M.L.; Goodson, J.L.; Rota, P.A.; Prausnitz, M.R. A Microneedle Patch Containing Measles Vaccine Is Immunogenic in Non-Human Primates. Vaccine 2015, 33, 4712–4718. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Hu, H.; Wong, Y.-Y.; Yao, X.; He, M.-L. Microneedles: An Emerging Vaccine Delivery Tool and a Prospective Solution to the Challenges of SARS-CoV-2 Mass Vaccination. Pharmaceutics 2023, 15, 1349. [Google Scholar] [CrossRef]
- Intradermal Injections Made Easy. Available online: https://www.worldpharmaceuticals.net/companies/becton-dickinson/ (accessed on 1 June 2025).
- Nguyen, H.X. Beyond the Needle: Innovative Microneedle-Based Transdermal Vaccination. Medicines 2025, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Pamornpathomkul, B.; Wongkajornsilp, A.; Laiwattanapaisal, W.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. A Combined Approach of Hollow Microneedles and Nanocarriers for Skin Immunization with Plasmid DNA Encoding Ovalbumin. Int. J. Nanomed. 2017, 12, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Ogai, N.; Nonaka, I.; Toda, Y.; Ono, T.; Minegishi, S.; Inou, A.; Hachiya, M.; Fukamizu, H. Enhanced Immunity in Intradermal Vaccination by Novel Hollow Microneedles. Ski. Res. Technol. 2018, 24, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Li, J.; Jiang, W.; Zhang, M.; Ma, Y.; Gu, Q.; Wang, X.; Cai, L.; Shi, L.; Sun, M. Dose-Sparing Intradermal DTaP-sIPV Immunization with a Hollow Microneedle Leads to Superior Immune Responses. Front. Microbiol. 2021, 12, 757375. [Google Scholar] [CrossRef]
- Niu, L.; Chu, L.Y.; Burton, S.A.; Hansen, K.J.; Panyam, J. Intradermal Delivery of Vaccine Nanoparticles Using Hollow Microneedle Array Generates Enhanced and Balanced Immune Response. J. Control. Release 2019, 294, 268–278. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Quan, F.-S.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Formulation and Coating of Microneedles with Inactivated Influenza Virus to Improve Vaccine Stability and Immunogenicity. J. Control. Release 2010, 142, 187–195. [Google Scholar] [CrossRef]
- Choi, I.-J.; Cha, H.-R.; Hwang, S.J.; Baek, S.-K.; Lee, J.M.; Choi, S.-O. Live Vaccinia Virus-Coated Microneedle Array Patches for Smallpox Vaccination and Stockpiling. Pharmaceutics 2021, 13, 209. [Google Scholar] [CrossRef]
- Park, S.H.; Shah, I.R.; Jhumur, N.C.; Mo, Y.; Tendolkar, S.; Lallow, E.O.; Shan, J.W.; Zahn, J.D.; Maslow, J.N.; Pelegri, A.A.; et al. Microneedle Arrays Coated with Middle East Respiratory Syndrome Coronavirus DNA Vaccine via Electrospray Deposition. Soft Matter 2025, 21, 3207–3214. [Google Scholar] [CrossRef]
- Kale, A.; Joshi, D.; Menon, I.; Bagwe, P.; Patil, S.; Vijayanand, S.; Braz Gomes, K.; Uddin, M.; D’Souza, M. Zika Vaccine Microparticles (MPs)-Loaded Dissolving Microneedles (MNs) Elicit a Significant Immune Response in a Pre-Clinical Murine Model. Vaccines 2023, 11, 583. [Google Scholar] [CrossRef]
- Adediran, E.; Arte, T.; Pasupuleti, D.; Vijayanand, S.; Singh, R.; Patel, P.; Gulani, M.; Ferguson, A.; Uddin, M.; Zughaier, S.M.; et al. Delivery of PLGA-Loaded Influenza Vaccine Microparticles Using Dissolving Microneedles Induces a Robust Immune Response. Pharmaceutics 2025, 17, 510. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, J.; Kim, J.C.; Park, H.; Yang, E.; Park, J.S.; Song, M.; Park, J.-H. Microneedles with Dual Release Pattern for Improved Immunological Efficacy of Hepatitis B Vaccine. Int. J. Pharm. 2020, 591, 119928. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, A.J.; Rodgers, A.M.; McCrudden, M.T.C.; McCarthy, H.O.; Donnelly, R.F. Novel Hydrogel-Forming Microneedle Array for Intradermal Vaccination in Mice Using Ovalbumin as a Model Protein Antigen. Mol. Pharm. 2019, 16, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Trincado, V.; Gala, R.P.; Morales, J.O. Buccal and Sublingual Vaccines: A Review on Oral Mucosal Immunization and Delivery Systems. Vaccines 2021, 9, 1177. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Azegami, T. The Mucosal Immune System: From Dentistry to Vaccine Development. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2015, 91, 423–439. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef]
- Gala, R.P.; Popescu, C.; Knipp, G.T.; McCain, R.R.; Ubale, R.V.; Addo, R.; Bhowmik, T.; Kulczar, C.D.; D’Souza, M.J. Physicochemical and Preclinical Evaluation of a Novel Buccal Measles Vaccine. AAPS PharmSciTech 2017, 18, 283–292. [Google Scholar] [CrossRef]
- Kraan, H.; Vrieling, H.; Czerkinsky, C.; Jiskoot, W.; Kersten, G.; Amorij, J.-P. Buccal and Sublingual Vaccine Delivery. J. Control. Release 2014, 190, 580–592. [Google Scholar] [CrossRef]
- Hensley, C.; Zhou, P.; Schnur, S.; Mahsoub, H.M.; Liang, Y.; Wang, M.-X.; Page, C.; Yuan, L.; Bronshtein, V. Thermostable, Dissolvable Buccal Film Rotavirus Vaccine Is Highly Effective in Neonatal Gnotobiotic Pig Challenge Model. Vaccines 2021, 9, 437. [Google Scholar] [CrossRef]
- Yoon, K.-W.; Mao, J.; Eom, G.-D.; Heo, S.I.; Chu, K.B.; Lee, M.S.; Quan, F.-S. Orally Dissolving Film-Based Influenza Vaccines Confer Superior Protection Compared to the Oral Administration of Inactivated Influenza Virus. Vaccines 2025, 13, 600. [Google Scholar] [CrossRef]
- Cui, Z.; Mumper, R.J. Bilayer Films for Mucosal (Genetic) Immunization via the Buccal Route in Rabbits. Pharm. Res. 2002, 19, 947–953. [Google Scholar] [CrossRef]
- Mašek, J.; Lubasová, D.; Lukáč, R.; Turánek-Knotigová, P.; Kulich, P.; Plocková, J.; Mašková, E.; Procházka, L.; Koudelka, Š.; Sasithorn, N.; et al. Multi-Layered Nanofibrous Mucoadhesive Films for Buccal and Sublingual Administration of Drug-Delivery and Vaccination Nanoparticles—Important Step towards Effective Mucosal Vaccines. J. Control Release 2017, 249, 183–195. [Google Scholar] [CrossRef]
- Esih, H.; Mezgec, K.; Billmeier, M.; Malenšek, Š.; Benčina, M.; Grilc, B.; Vidmar, S.; Gašperlin, M.; Bele, M.; Zidarn, M.; et al. Mucoadhesive Film for Oral Delivery of Vaccines for Protection of the Respiratory Tract. J. Control Release 2024, 371, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Patel, P.; Ferguson, A.; Bagwe, P.; Kale, A.; Adediran, E.; Singh, R.; Arte, T.; Pasupuleti, D.; Uddin, M.; et al. Buccal Administration of a Zika Virus Vaccine Utilizing 3D-Printed Oral Dissolving Films in a Mouse Model. Vaccines 2024, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Anggraeni, R.; Ana, I.D.; Agustina, D.; Martien, R. Induction of Protein Specific Antibody by Carbonated Hydroxy Apatite as a Candidate for Mucosal Vaccine Adjuvant. Dent. Mater. J. 2022, 41, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Yang, G.; Schluns, K.S.; Anthony, S.M.; Sastry, K.J. Sublingual Vaccination Induces Mucosal and Systemic Adaptive Immunity for Protection against Lung Tumor Challenge. PLoS ONE 2014, 9, e90001. [Google Scholar] [CrossRef]
- Goswami, T.; Jasti, B.; Li, X. Sublingual Drug Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 449–484. [Google Scholar] [CrossRef]
- Saha, P.; Verma, S.; Das, P.S. Sublingual drug delivery: An indication of potential alternative route. Int. J. Curr. Pharm. Res. 2017, 9, 5–7. [Google Scholar] [CrossRef]
- Mazzinelli, E.; Favuzzi, I.; Arcovito, A.; Castagnola, R.; Fratocchi, G.; Mordente, A.; Nocca, G. Oral Mucosa Models to Evaluate Drug Permeability. Pharmaceutics 2023, 15, 1559. [Google Scholar] [CrossRef]
- Hua, S. Advances in Nanoparticulate Drug Delivery Approaches for Sublingual and Buccal Administration. Front. Pharmacol. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Mangla, B.; Javed, S.; Sultan, M.H.; Ahsan, W.; Aggarwal, G.; Kohli, K. Nanocarriers-Assisted Needle-Free Vaccine Delivery Through Oral and Intranasal Transmucosal Routes: A Novel Therapeutic Conduit. Front. Pharmacol. 2022, 12, 757761. [Google Scholar] [CrossRef]
- Stie, M.B.; Gätke, J.R.; Wan, F.; Chronakis, I.S.; Jacobsen, J.; Nielsen, H.M. Swelling of Mucoadhesive Electrospun Chitosan/Polyethylene Oxide Nanofibers Facilitates Adhesion to the Sublingual Mucosa. Carbohydr. Polym. 2020, 242, 116428. [Google Scholar] [CrossRef]
- Kusumoto, Y.; Ueda, M.; Hashimoto, M.; Takeuchi, H.; Okada, N.; Yamamoto, J.; Nishii, A.; Fujino, A.; Kurahashi, A.; Satoh, M.; et al. Sublingual Immune Cell Clusters and Dendritic Cell Distribution in the Oral Cavity. JCI Insight 2024, 9, e167373. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Shiraishi, D.; Tanaka, Y.; Nagasawa, Y.; Ohwada, S.; Shimauchi, H.; Aso, H.; Endo, Y.; Sugawara, S. Transportation of Sublingual Antigens across Sublingual Ductal Epithelial Cells to the Ductal Antigen-presenting Cells in Mice. Clin. Exp. Allergy 2015, 45, 677–686. [Google Scholar] [CrossRef]
- Hervouet, C.; Luci, C.; Bekri, S.; Juhel, T.; Bihl, F.; Braud, V.M.; Czerkinsky, C.; Anjuère, F. Antigen-Bearing Dendritic Cells from the Sublingual Mucosa Recirculate to Distant Systemic Lymphoid Organs to Prime Mucosal CD8 T Cells. Mucosal Immunol. 2014, 7, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Shim, B.-S.; Choi, Y.; Cheon, I.S.; Song, M.K. Sublingual Delivery of Vaccines for the Induction of Mucosal Immunity. Immune Netw. 2013, 13, 81–85. [Google Scholar] [CrossRef]
- Benito-Villalvilla, C.; Cirauqui, C.; Diez-Rivero, C.M.; Casanovas, M.; Subiza, J.L.; Palomares, O. MV140, a Sublingual Polyvalent Bacterial Preparation to Treat Recurrent Urinary Tract Infections, Licenses Human Dendritic Cells for Generating Th1, Th17, and IL-10 Responses via Syk and MyD88. Mucosal Immunol. 2017, 10, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Monge, C.; Ayad, C.; Paris, A.-L.; Rovera, R.; Colomb, E.; Verrier, B. Mucosal Adjuvants Delivered by a Mucoadhesive Patch for Sublingual Administration of Subunit Vaccines. Int. J. Mol. Sci. 2022, 23, 13440. [Google Scholar] [CrossRef]
- Kim, Y.; Park, I.H.; Shin, J.; Choi, J.; Jeon, C.; Jeon, S.; Shin, J.-S.; Jung, H. Sublingual Dissolving Microneedle (SLDMN)-Based Vaccine for Inducing Mucosal Immunity against SARS-CoV-2. Adv. Healthc. Mater. 2023, 12, e2300889. [Google Scholar] [CrossRef]
- Sexually Transmitted Infections (STIs). Available online: https://www.who.int/health-topics/sexually-transmitted-infections#tab=tab_1 (accessed on 9 June 2025).
- VanBenschoten, H.M.; Woodrow, K.A. Vaginal Delivery of Vaccines. Adv. Drug Deliv. Rev. 2021, 178, 113956. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Way, S.S.; Chen, K. Immunology of the Uterine and Vaginal Mucosae. Trends Immunol. 2018, 39, 302–314. [Google Scholar] [CrossRef]
- Johansson, E.-L.; Wassén, L.; Holmgren, J.; Jertborn, M.; Rudin, A. Nasal and Vaginal Vaccinations Have Differential Effects on Antibody Responses in Vaginal and Cervical Secretions in Humans. Infect. Immun. 2001, 69, 7481–7486. [Google Scholar] [CrossRef]
- Baker, J.R.; Farazuddin, M.; Wong, P.T.; O’Konek, J.J. The Unfulfilled Potential of Mucosal Immunization. J. Allergy Clin. Immunol. 2022, 150, 1–11. [Google Scholar] [CrossRef]
- Seavey, M.M.; Mosmann, T.R. Estradiol-Induced Vaginal Mucus Inhibits Antigen Penetration and CD8+ T Cell Priming in Response to Intravaginal Immunization. Vaccine 2009, 27, 2342–2349. [Google Scholar] [CrossRef]
- Vaginal Administration. Available online: https://www.cd-bioparticles.net/vaginal-administration?srsltid=afmbooouyjio3ah_8bcj1tnbgstaip2fykfmnax9r8bxzetwk1f2gyru (accessed on 5 June 2025).
- Han, I.-K.; Kim, Y.B.; Kang, H.-S.; Sul, D.; Jung, W.-W.; Cho, H.J.; Oh, Y.-K. Thermosensitive and Mucoadhesive Delivery Systems of Mucosal Vaccines. Methods 2006, 38, 106–111. [Google Scholar] [CrossRef]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The Pig: A Model for Human Infectious Diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Oh, Y.; Kang, M.; Kim, C. Enhanced Mucosal and Systemic Immune Responses Following Intravaginal Immunization with Human Papillomavirus 16 L1 Virus-like Particle Vaccine in Thermosensitive Mucoadhesive Delivery Systems. J. Med. Virol. 2003, 70, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Song, X.; Zhao, Y.; Hu, X.; Yang, H.; Jin, R.; Nie, Y. Mucus-Penetrating Nonviral Gene Vaccine Processed in the Epithelium for Inducing Advanced Vaginal Mucosal Immune Responses. Acta Pharm. Sin. B 2023, 13, 1287–1302. [Google Scholar] [CrossRef] [PubMed]
- McKay, P.F.; Mann, J.F.S.; Pattani, A.; Kett, V.; Aldon, Y.; King, D.; Malcolm, R.K.; Shattock, R.J. Intravaginal Immunisation Using a Novel Antigen-Releasing Ring Device Elicits Robust Vaccine Antigen-Specific Systemic and Mucosal Humoral Immune Responses. J. Control Release 2017, 249, 74–83. [Google Scholar] [CrossRef]
- FluGen’s Intranasal Vaccine Shows Promise in Boosting Flu Protection for Older Adults. Available online: https://www.contagionlive.com/view/flugen-s-intranasal-vaccine-shows-promise-in-boosting-flu-protection-for-older-adults (accessed on 1 June 2025).
- Nasal COVID Vax Shows Promise in Phase 1 Clinical Trial. Available online: https://scienceblog.cincinnatichildrens.org/nasal-covid-vax-shows-promise-in-phase-1-clinical-trial/ (accessed on 3 June 2025).
- Chu, K.; Quan, J.; Liu, X.; Chen, Q.; Zang, X.; Jiang, H.; Liu, D.; Chu, X.; Zhuang, C.; Han, J.; et al. A Randomized Phase I Trial of Intranasal SARS-CoV-2 Vaccine dNS1-RBD in Children Aged 3–17 Years. npj Vaccines 2025, 10, 50. [Google Scholar] [CrossRef]
- Singh, C.; Verma, S.; Reddy, P.; Diamond, M.S.; Curiel, D.T.; Patel, C.; Jain, M.K.; Redkar, S.V.; Bhate, A.S.; Gundappa, V.; et al. Phase III Pivotal Comparative Clinical Trial of Intranasal (iNCOVACC) and Intramuscular COVID 19 Vaccine (Covaxin®). npj Vaccines 2023, 8, 125. [Google Scholar] [CrossRef]
- Intravacc to Begin Clinical Trials for Nasal COVID Vaccine. Available online: https://manufacturingchemist.com/intravacc-to-begin-clinical-trials-for-nasal-covid-vaccine-197961 (accessed on 14 June 2025).
- Human Challenge Study to Evaluate the Efficacy of MV-012-968 Vaccine. Available online: https://clinicaltrials.gov/study/nct04690335 (accessed on 14 June 2025).
- Keech, C.; Miller, V.E.; Rizzardi, B.; Hoyle, C.; Pryor, M.J.; Ferrand, J.; Solovay, K.; Thalen, M.; Noviello, S.; Goldstein, P.; et al. Immunogenicity and Safety of BPZE1, an Intranasal Live Attenuated Pertussis Vaccine, versus Tetanus–Diphtheria–Acellular Pertussis Vaccine: A Randomised, Double-Blind, Phase 2b Trial. Lancet 2023, 401, 843–855. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; D’Agostino, M.R.; Satia, I.; Fritz, D.K.; Miyasaki, K.; Ang, J.C.; Zganiacz, A.; Howie, K.J.; Swinton, M.; et al. Induction of Lung Mucosal Immunity by a Next-Generation Inhaled Aerosol COVID-19 Vaccine: An Open-Label, Multi-Arm Phase 1 Clinical Trial. Nat. Commun. 2025, 16, 6000. [Google Scholar] [CrossRef]
- McMaster’s Inhaled COVID-19 Vaccine Boasts Safety and Robust Mucosal Immunity After phase-1 Human Trials. Available online: https://healthsci.mcmaster.ca/mcmasters-inhaled-covid-19-vaccine-boasts-safety-and-robust-mucosal-immunity-after-phase-1-human-trials/ (accessed on 2 June 2025).
- Inhaled COVID Vaccine Begins Recruitment for Phase 2. Available online: https://respiratory-therapy.com/products-treatment/pharmaceuticals/intl-pharmaceuticals/inhaled-covid-vaccine-begins-recruitment-for-phase- (accessed on 5 July 2025).
- A Phase 2 Clinical Trial to Evaluate the Recombinant Vaccine for COVID-19 (Adenovirus Vector). Available online: https://clinicaltrials.gov/study/NCT04341389 (accessed on 10 June 2025).
- Hepatitis B Vaccine Delivered Transdermally by MAP. Available online: https://clinicaltrials.gov/study/nct06800131?cond=hepatitis%20b&viewtype=table&rank=5 (accessed on 7 June 2025).
- Establishing Immunogenicity and Safety of Needle-free Intradermal Delivery of mRNA COVID-19 Vaccine (MILESTONE). Available online: https://www.clinicaltrials.gov/study/nct05315362 (accessed on 20 June 2025).
- Measles and Rubella Vaccine Microneedle Patch Phase 1–2 Age De-Escalation Trial. Available online: https://clinicaltrials.gov/study/nct04394689 (accessed on 27 June 2025).
- CDC-9 Inactivated Rotavirus Vaccine (IRV) Microneedle Patch (MNP) in Healthy Adults. Available online: https://clinicaltrials.gov/study/nct06962904 (accessed on 10 June 2025).
- Inactivated Influenza Vaccine Delivered by Microneedle Patch or by Hypodermic Needle. Available online: https://clinicaltrials.gov/study/nct02438423?tab=results (accessed on 15 June 2025).
- Garg, N.; Tellier, G.; Vale, N.; Kluge, J.; Portman, J.L.; Markowska, A.; Tussey, L. Phase 1, Randomized, Rater and Participant Blinded Placebo-Controlled Study of the Safety, Reactogenicity, Tolerability and Immunogenicity of H1N1 Influenza Vaccine Delivered by VX-103 (a MIMIX Microneedle Patch [MAP] System) in Healthy Adults. PLoS ONE 2024, 19, e0303450. [Google Scholar] [CrossRef] [PubMed]
- A Pilot Study to Evaluate the Safety and Immunogenicity of Low Dose Flu Vaccines (NANOVAX). Available online: https://www.clinicaltrials.gov/study/nct00558649 (accessed on 1 July 2025).
- Immunogenicity of Inactivated and Live Polio Vaccines. Available online: https://clinicaltrials.gov/study/nct01813604 (accessed on 1 July 2025).
- ImmunityBio’s hAd5 T-Cell COVID-19 Vaccine Candidate Shows Complete Protection of Airways in Non-Human Primates. Available online: https://immunitybio.com/immunitybios-had5-t-cell-covid-19-vaccine-candidate-shows-complete-protection-of-airways-in-non-human-primates/ (accessed on 10 July 2025).
- Initiation of Phase I Clinical Trial for Seasonal Influenza HA Vaccine Sublingual Tablet. Available online: https://www.nitto.com/us/en/press/2016/1102.jsp (accessed on 5 July 2025).
- Safety of Sublingual dmLT for ETEC. Available online: https://clinicaltrials.gov/study/nct02052934 (accessed on 5 July 2025).
- Yuki, Y.; Kurokawa, S.; Sugiura, K.; Kashima, K.; Maruyama, S.; Yamanoue, T.; Honma, A.; Mejima, M.; Takeyama, N.; Kuroda, M.; et al. MucoRice-CTB Line 19A, a New Marker-Free Transgenic Rice-Based Cholera Vaccine Produced in an LED-Based Hydroponic System. Front. Plant Sci. 2024, 15, 1342662. [Google Scholar] [CrossRef] [PubMed]
- Herbst-Kralovetz, M.; Mason, H.S.; Chen, Q. Norwalk Virus-like Particles as Vaccines. Expert Rev. Vaccines 2010, 9, 299–307. [Google Scholar] [CrossRef] [PubMed]
- El Jaddaoui, I.; Al Idrissi, N.; Hamdi, S.; Wakrim, L.; Nejjari, C.; Amzazi, S.; Elouahabi, A.; Bakri, Y.; Ghazal, H. Plant-Based Vaccines Against COVID-19 for Massive Vaccination in Africa. Front. Drug Deliv. 2022, 2, 909958. [Google Scholar] [CrossRef]
- Mason, H.S.; Haq, T.A.; Clements, J.D.; Arntzen, C.J. Edible Vaccine Protects Mice against Escherichia coli Heat-Labile Enterotoxin (LT): Potatoes Expressing a Synthetic LT-B Gene. Vaccine 1998, 16, 1336–1343. [Google Scholar] [CrossRef]
- Safety and Immunogenicity Study of a Virosomal Vaccine Against Recurrent Vulvovaginal Candida Infection. Available online: https://clinicaltrials.gov/study/nct01067131?cond=%22candidiasis%22&intr=%22vaccines%22&viewtype=table&rank=3 (accessed on 7 July 2025).
- Hopkins, W.J.; Elkahwaji, J.; Beierle, L.M.; Leverson, G.E.; Uehling, D.T. Vaginal Mucosal Vaccine for Recurrent Urinary Tract Infections in Women: Results of a Phase 2 Clinical Trial. J. Urol. 2007, 177, 1349–1353. [Google Scholar] [CrossRef]
- Lewis, D.J.; Fraser, C.A.; Mahmoud, A.N.; Wiggins, R.C.; Woodrow, M.; Cope, A.; Cai, C.; Giemza, R.; Jeffs, S.A.; Manoussaka, M.; et al. Phase I Randomised Clinical Trial of an HIV-1CN54, Clade C, Trimeric Envelope Vaccine Candidate Delivered Vaginally. PLoS ONE 2011, 6, e25165. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; St. John, A.L. Promises and Challenges of Mucosal COVID-19 Vaccines. Vaccine 2023, 41, 4042–4049. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Das, S.; Malik, A. Vaccine and Malnutrition: A Narrative Review. J. Fam. Med. Prim. Care 2023, 12, 1808–1813. [Google Scholar] [CrossRef]
- Hong, S.-H. Influence of Microbiota on Vaccine Effectiveness: “Is the Microbiota the Key to Vaccine-Induced Responses?”. J. Microbiol. 2023, 61, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zou, Y.; Zhao, D.; Yu, J. Optimising Vaccine Immunogenicity in Ageing Populations: Key Strategies. Lancet Infect. Dis. 2025, 25, e23–e33. [Google Scholar] [CrossRef]
- Kanojia, G.; Have, R.T.; Soema, P.C.; Frijlink, H.; Amorij, J.-P.; Kersten, G. Developments in the Formulation and Delivery of Spray Dried Vaccines. Hum. Vaccines Immunother. 2017, 13, 2364–2378. [Google Scholar] [CrossRef]
- Ghaemmaghamian, Z.; Zarghami, R.; Walker, G.; O’Reilly, E.; Ziaee, A. Stabilizing Vaccines via Drying: Quality by Design Considerations. Adv. Drug Deliv. Rev. 2022, 187, 114313. [Google Scholar] [CrossRef]






| Disease | Phase of Development | Name | Platform of Vaccine | Antigen and Adjuvant | |
|---|---|---|---|---|---|
| Intranasal Vaccines | |||||
| Influenza | Phase 1 completed [128] | FluGen + Fluzone High-Dose inactivated influenza vaccine | M2SR H3N2 | Coadministration of the H3N2 M2SR vaccine with Fluzone HD | |
| COVID-19 | Phase 2 Single-dose vaccine [129] | CVXGA Intranasal COVID-19 Vaccine in Adults | Parainfluenza virus 5 (PIV5) vector expressing SARS-CoV-2 spike | Omicron XBB.1.5 variant—Spike protein, chimeric virus formulated by inserting spike gene into the PIV5 genome | |
| COVID-19 + Influenza vaccine | Phase 3 Phase 1 trial—children aged 3–17 years [130] | Pneucolin®, ChiCTR2300068044 | SARS-CoV-2 vaccine dNS1-RBD based on a live attenuated influenza virus vector | Cold-adapted influenza strain (CA4) without the non-structural protein 1 (NS1) as the genetic backbone, into which receptor-binding domain (RBD) gene from ancestral SARS-CoV-2 is inserted by gene reassortment | |
| COVID-19 | Phase 3—completed [131] | BBV154 (iNCOVACC®) of 0.5 mL | Chimpanzee adenovirus-vectored, single-dose intranasal COVID-19 booster vaccine | Prefusion-stabilized SARS-CoV-2 spike | |
| COVID-19 | Phase 2 trial [132] | NANOVAC (Intravacc) | Nanoparticulate peptide vaccine | HBcAg (hepatitis B core antigen) as an adjuvant and mucosal targeting carrier. soluble nanospheres containing synthetic mini-proteins from spike and other conserved coronavirus epitopes | |
| COVID-19 | Phase 1 [63] | COVI-VACTM | Live-attenuated SARS-COV-2 synthetic viral vaccine | Attenuated through deletion of the furin cleavage site and introduction of 283 silent deoptimizing mutations that maintain viral amino acid sequence | |
| RSV | Phase 2 completed [133] | MV-012-968 | recombinant, live attenuated RSV vaccine. | ||
| RSV | Phase 1 in RSV-Seronegative Children | RSV/ΔNS2/Δ1313/I1314L | live attenuated, recombinant version of RSV strain A2 | (1) A 523 nucleotide deletion of the NS2 gene and (2) a codon deletion in the L gene (Δ1313; deletion of S1313) plus the adjacent missense mutation I1314L that prevents the compensatory deattenuating mutation I1314T. The virus was generated from cDNA on World Health Organization Vero cells by reverse genetics | |
| Pertussis (whooping cough) | Phase 2b completed [134] | BPZE1 vaccine | Genetically modified, live attenuated strain of Bordetella pertussis | Modification by deletion of Dermonecrotic Toxin that eliminates local tissue damage, genetic inactivation of Pertussis Toxin (PT) to remove toxic effect of virus and gene expression reduced of Tracheal Cytotoxin that will prevent ciliary damage in airway epithelium | |
| Oral Inhalation Vaccines | |||||
| Disease | Phase/Status | Product Name | Platform | Antigen and Adjuvant Preparation | |
| COVID-19 | Phase 2 (Canada) [135,136] | ChAd-triCoV/Mac (McMaster) | Viral vector (adenovirus, inhaled aerosol) | Triple-antigen: S1 spike, nucleocapsid, truncated RNA polymerase/non-adjuvanted | |
| COVID-19 | Phase 2 (Canada) [137] | AeroVax (McMaster) | Viral vector (inhaled aerosol) | Adenovirus vector with 3 SARS-CoV-2 gene segments | |
| COVID-19 | Phase 1–2 (China) [138] | Convidecia Air/Ad5-nCoV | Adenoviral vector (inhaled aerosol) | Adenovirus-vectored spike protein/non-adjuvanted | |
| Microneedle vaccines | |||||
| Title of study | Type of Microneedle | Sponsor | |||
| Phase 1: Hepatitis B Vaccine Delivered Trans-dermally by MAP [139] | Microneedle array patch (MAP) | International Vaccine Institute | |||
| Phase 2a, Establishing Immunogenicity and Safety of Needle-free Intradermal Delivery by Solid Micro Needle Skin Patch of mRNA SARS-CoV-2 Vaccine as a Revaccination Strategy in Healthy Volunteers [140] | Solid microneedle skin patch | Leiden University Medical Center | |||
| A Phase I/II, Double-blind, Randomized, Active-controlled, Age De-escalation Trial to Assess Safety and Immunogenicity of a Measles Rubella Vaccine (MRV) Microneedle Patch (MRV-MNP) in Adults, MRV-primed Toddlers, and MRV-naïve Infants [141]. | Dissolving microneedle patch | Micron Biomedical, Inc. | |||
| A Phase 1 Study to Evaluate the Safety and Immunogenicity of CDC-9 Inactivated Rotavirus Vaccine for Intradermal Administration by Microneedle Patch in Healthy Adults [142]. | Dissolving microneedle patch | Centers for Disease Control and Prevention | |||
| A Phase I Study of The Safety, Reactogenicity, Acceptability and Immunogenicity of Inactivated Influenza Vaccine Delivered either by Microneedle Patch or by Hypodermic Needle [143]. | - | Mark Prausnitz | |||
| Phase 1 Evaluation of H1 Influenza Vaccine Delivered by MIMIX MAP [144]. | MIMIX Microneedle Array Patch (MAP) System | Vaxess Technologies | |||
| A Pilot, Controlled, Comparative and Single Blinded Study to Evaluate the Safety and Immunogenicity of Low Dose Flu Vaccines Administered Intradermally Using Microneedle Injectors as Compared With Standard Dose Intramuscular Flu Vaccines as Reference [145]. | - | NanoPass Technologies Ltd. | |||
| Phase III Clinical Trial to Assess the Immunogenicity of a Sequential Dose of Fractional Inactivated Polio Vaccine (f-IPV) and Oral Polio Vaccine (OPV) [146]. | MicroJet 600 microneedle | Centers for Disease Control and Prevention | |||
| Sublingual Vaccination | |||||
| Disease | Phase | Name | Platform Type | Antigen/Adjuvant | |
| COVID-19 | Phase 1 [147] | hAd5 T cell vaccine | Adenoviral Vector | Spike + Nucleocapsid proteins | |
| Influenza (QIV) | Phase 1 [148] | Tablet (NIBRG-14) | Inactivated virus tablet | H5N1, inulin glass | |
| ETEC (traveler’s diarrhea) | Phase 1 [149] | CfaEB-dmLT vaccine | Subnit + vaccine | CfaEB/CfaE-LTB, dmLT (mutant LT) | |
| Oral vaccination | |||||
| Disease | Phase of Development | Name/Developer | Vaccine Platform | Plant Used | Antigen and Adjuvant |
| Cholera | Phase I/II [150] | MucoRice-CTB, Seed-based vaccines | Subunit (CTB, VLP) | Rice, tobacco | CTB (cholera toxin B); none/various |
| Norwalk virus | Early Clinical [151] | Various | VLP | Potato, tobacco | Norwalk virus capsid protein; none |
| Rotavirus | Phase I [42] | Maize-based bivalent vaccine, Ro-VLP | Subunit (VP6, NSP4, VLP) | Maize, potato, alfalfa | VP6, NSP4, Ro-VLP; LTB (E. coli heat-labile toxin B) |
| COVID-19 | Phase III, Registered [152] | Covifenz (Medicago/GSK), Baiya Phytopharm | Virus-like Particle (VLP) | Nicotiana benthamiana | Spike (S) protein; AS03/CpG1018 adjuvant |
| Enterotoxigenic E. coli | Phase I (human), Preclinical (animal) [153] | - | Subunit | Potato, maize | LT-B (heat-labile toxin B subunit); none |
| Intravaginal Vaccines | |||||
| Disease | Phase | Name of product | Platform | Antigen and Adjuvant | |
| Recurrent Vulvovaginal Candidiasis | Phase I (completed) [154] | PEV7 | Virosome-formulated subunit vaccine delivered either via intravaginal capsule (PEV7C) or IM injection (PEV7B) | Sap2 protein (Candida albicans)/non-adjuvanted | |
| Recurrent UTIs | Phase II [155] | Urovac™ | Vaginal suppository | 10 strains of uropathogenic bacteria that were heat-killed/non-adjuvant | |
| HIV-1 | Phase I [156] | CN54 gp140 | Recombinant HIV-1 enveloped protein formulated in a Carbopol gel, delivered via applicator | HIV-1 (CN54 gp140)/non-adjuvanted | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulani, M.; Arte, T.; Ferguson, A.; Pasupuleti, D.; Adediran, E.; Harsoda, Y.; Nicolas McCommon, A.; Gala, R.; D’Souza, M.J. Recent Advancements in Non-Invasive Vaccination Strategies. Vaccines 2025, 13, 978. https://doi.org/10.3390/vaccines13090978
Gulani M, Arte T, Ferguson A, Pasupuleti D, Adediran E, Harsoda Y, Nicolas McCommon A, Gala R, D’Souza MJ. Recent Advancements in Non-Invasive Vaccination Strategies. Vaccines. 2025; 13(9):978. https://doi.org/10.3390/vaccines13090978
Chicago/Turabian StyleGulani, Mahek, Tanisha Arte, Amarae Ferguson, Dedeepya Pasupuleti, Emmanuel Adediran, Yash Harsoda, Andrew Nicolas McCommon, Rikhav Gala, and Martin J. D’Souza. 2025. "Recent Advancements in Non-Invasive Vaccination Strategies" Vaccines 13, no. 9: 978. https://doi.org/10.3390/vaccines13090978
APA StyleGulani, M., Arte, T., Ferguson, A., Pasupuleti, D., Adediran, E., Harsoda, Y., Nicolas McCommon, A., Gala, R., & D’Souza, M. J. (2025). Recent Advancements in Non-Invasive Vaccination Strategies. Vaccines, 13(9), 978. https://doi.org/10.3390/vaccines13090978







