CD63-Mediated SARS-CoV-2 RBD Fusion Neoantigen DNA Vaccine Enhances Antitumor Immune Response in a Mouse Panc02 Model via EV-Targeted Delivery
Abstract
1. Introduction
2. Materials and Methods
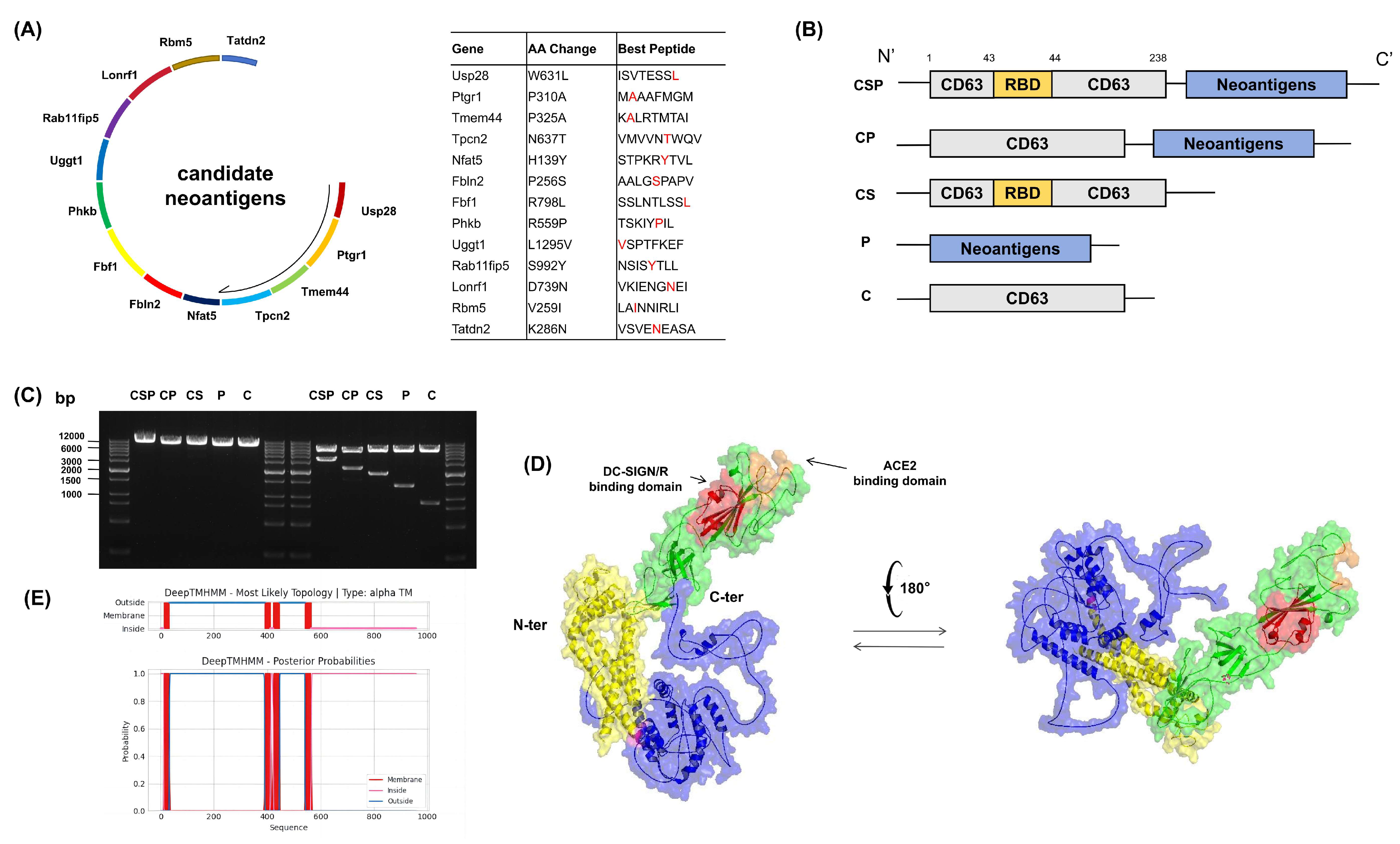
2.1. Somatic Mutation Analysis and Screening
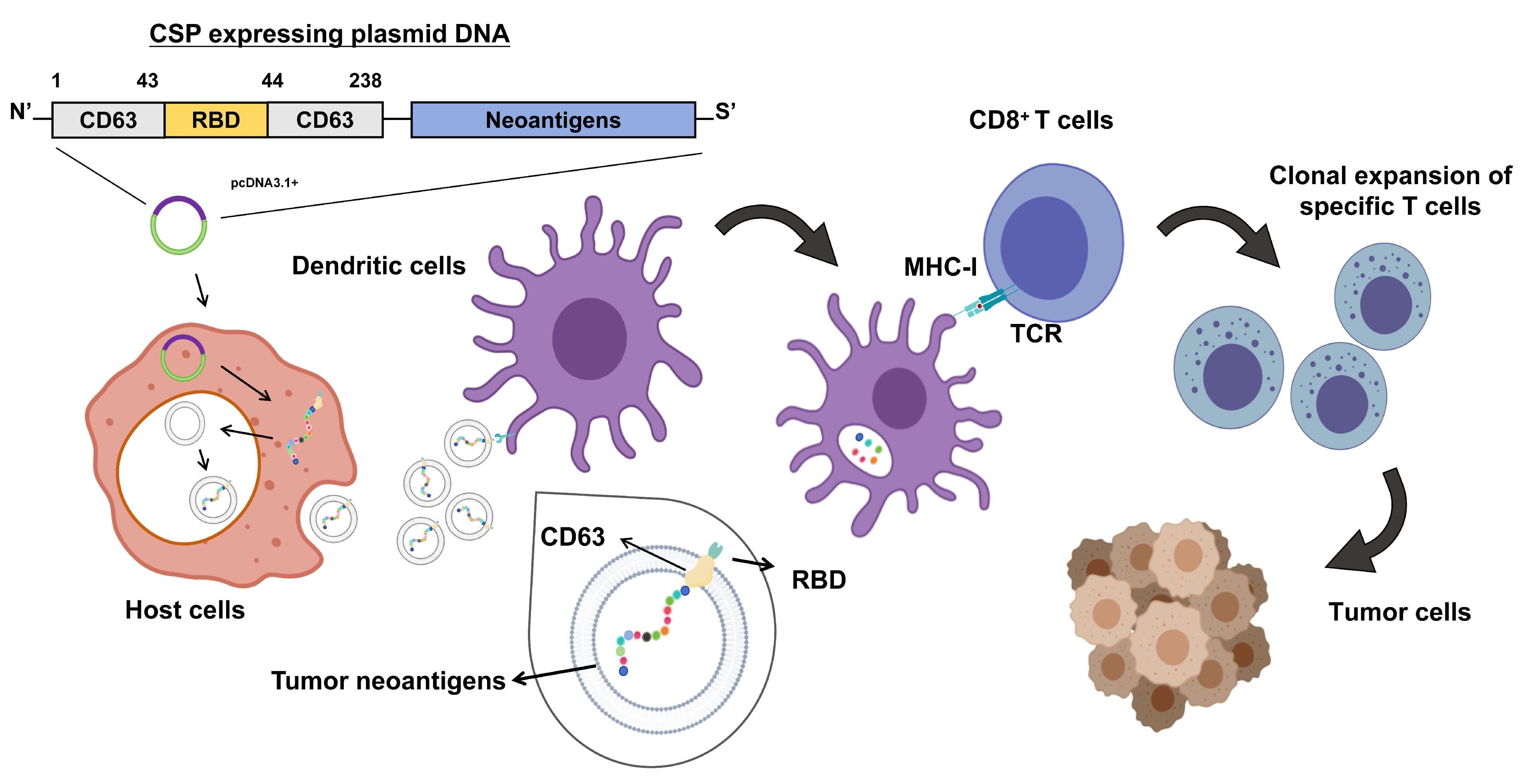
2.2. DNA Vaccine Construction
2.3. Protein Structure Prediction
2.4. Cell Culture and Preparation
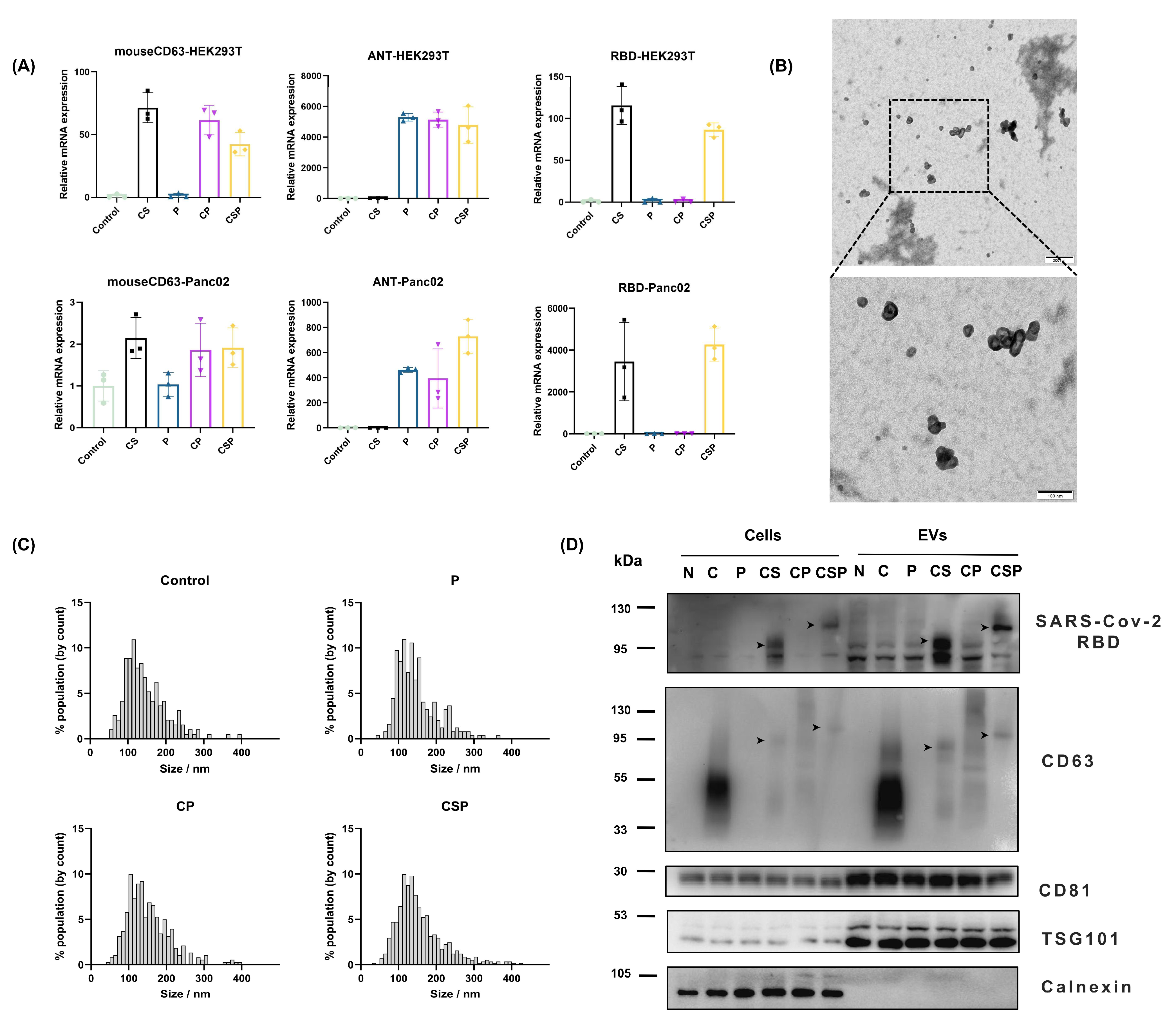
2.5. EV Isolation and Characterization
2.6. Western Blotting
2.7. RNA Isolation and Quantitative Real-Time PCR
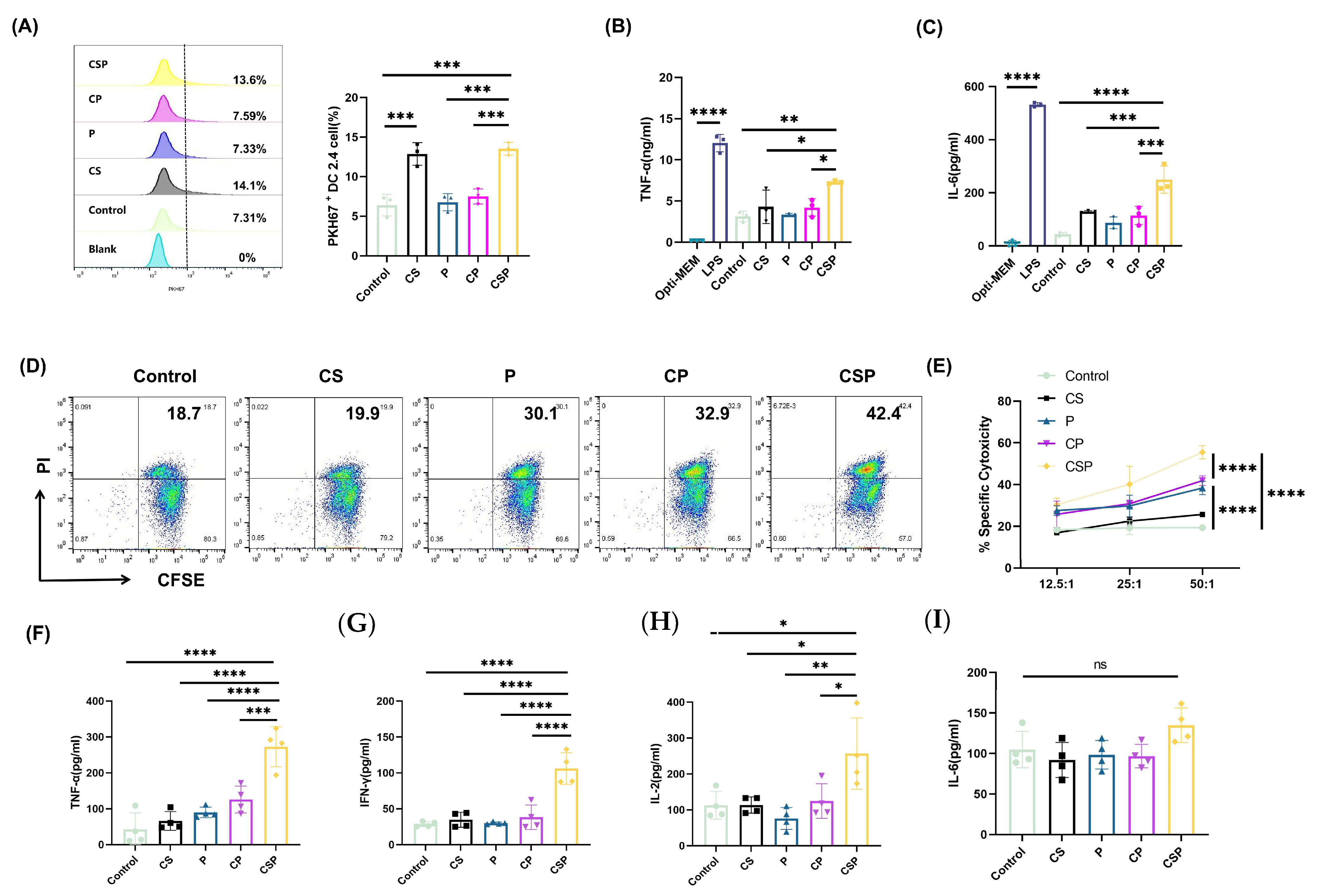
2.8. DC2.4 Cell Uptake of EVs
2.9. DC2.4 Cell Cytokine Release
2.10. Spleen Cell Isolation and Cytokine Analysis
2.11. In Vitro Cytotoxicity Assay
2.12. In Vivo Tumor Treatment Strategy
2.13. RNAseq
2.14. Flow Cytometry Analysis
2.15. In Vivo Safety Evaluation
2.16. Statistical Analysis
3. Results
3.1. Construction of the DNA Vaccine pCSP and Fusion Protein Validation
3.2. Preparation and Characterization of CSP-Modified EVs
3.3. CSP Modification Enhances EV Targeting to DCs
3.4. The pCSP Vaccine Enhances In Vitro Cytotoxicity and Immune Cell Activation
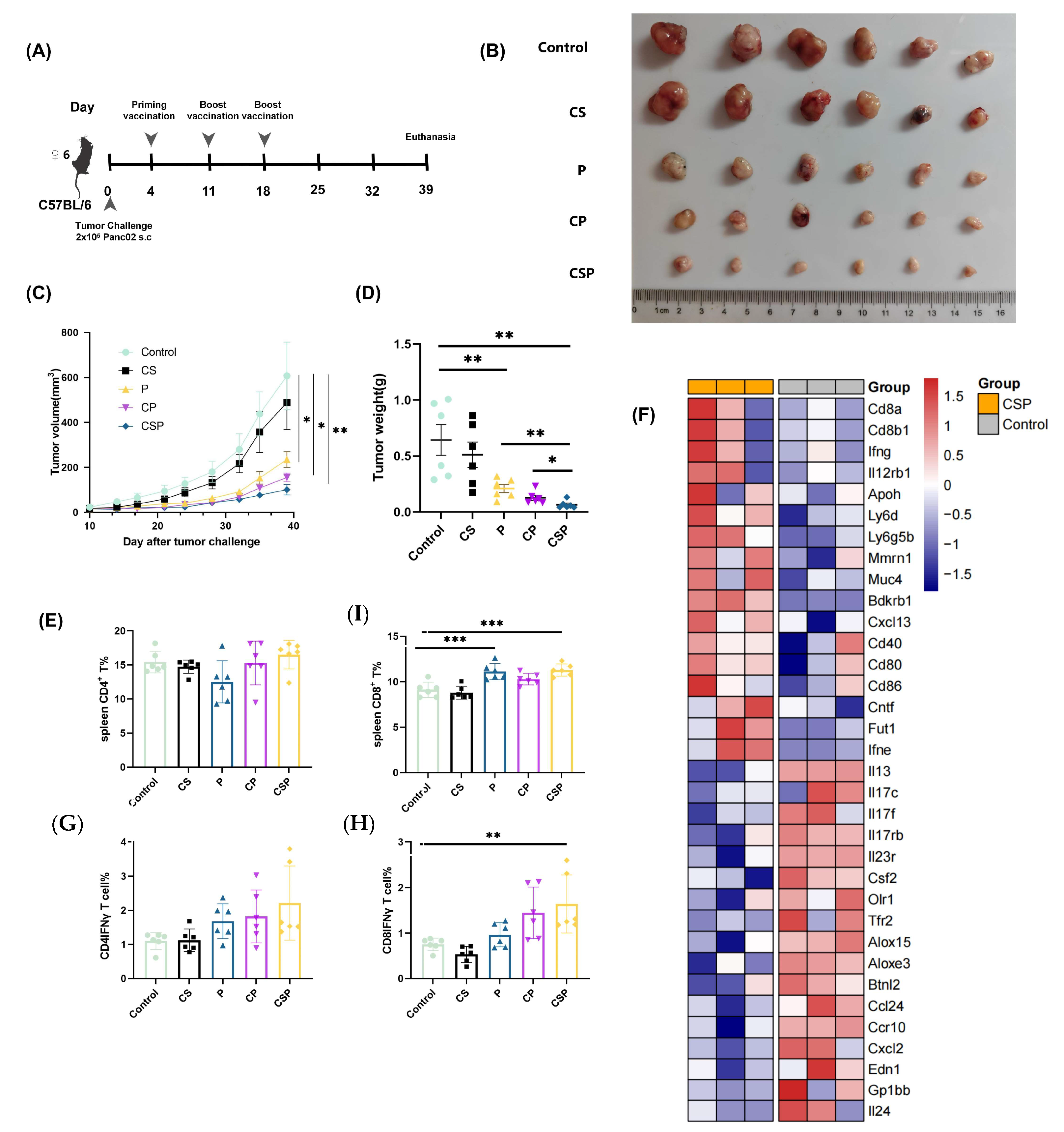
3.5. In Vivo Antitumor Effect of pCSP
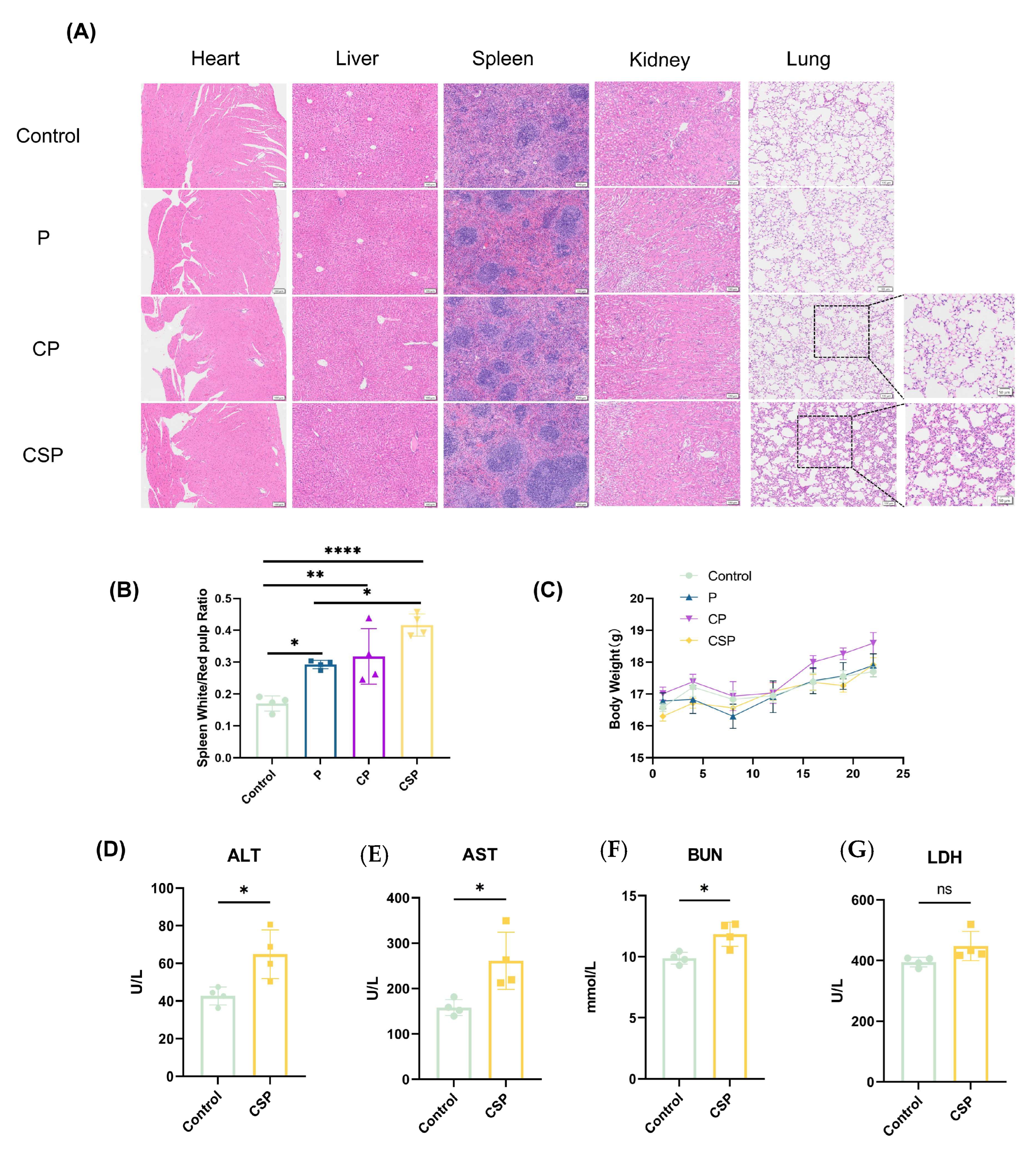
3.6. Safety Evaluation of the pCSP Vaccine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| ALT | Alanine aminotransferase |
| APCs | Antigen-presenting cells |
| AST | Aspartate aminotransferase |
| BUN | Blood urea nitrogen |
| CD63 | Cluster of differentiation 63 |
| CFSE | 5,6-carboxyfluorescein diacetate succinimidyl ester |
| DC | Dendritic cell |
| DC-SIGN | Dendritic cell-specific ICAM-3-grabbing non-integrin |
| EV | Extracellular vesicle |
| LDH | Lactate dehydrogenase |
| MANA | Mutation-associated neoantigens |
| PDAC | Pancreatic ductal adenocarcinoma |
| PI | Propidium iodide |
| RBD | Receptor-binding domain |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
References
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic Cancer Vaccines: Advancements, Challenges, and Prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Chai, D.; Shan, H.; Wang, G.; Zhang, Q.; Li, H.; Fang, L.; Song, J.; Liu, N.; Zhang, Q.; Yao, H.; et al. Combining DNA Vaccine and AIM2 in H1 Nanoparticles Exert Anti-Renal Carcinoma Effects via Enhancing Tumor-Specific Multi-Functional CD8+ T-Cell Responses. Mol. Cancer Ther. 2019, 18, 323–334. [Google Scholar] [CrossRef]
- Norell, H.; Poschke, I.; Charo, J.; Wei, W.Z.; Erskine, C.; Piechocki, M.P.; Knutson, K.L.; Bergh, J.; Lidbrink, E.; Kiessling, R. Vaccination with a Plasmid DNA Encoding HER-2/Neu Together with Low Doses of GM-CSF and IL-2 in Patients with Metastatic Breast Carcinoma: A Pilot Clinical Trial. J. Transl. Med. 2010, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.R.; Raviprakash, K. DNA Vaccine Delivery and Improved Immunogenicity. Curr. Issues Mol. Biol. 2017, 22, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA Vaccines: Current Preclinical and Clinical Developments and Future Perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards Personalized, Tumour-Specific, Therapeutic Vaccines for Cancer. Nat. Rev. Immunol. 2017, 18, 168–182. [Google Scholar] [CrossRef]
- Hu, Z.; Leet, D.E.; Allesøe, R.L.; Oliveira, G.; Li, S.; Luoma, A.M.; Liu, J.; Forman, J.; Huang, T.; Iorgulescu, J.B.; et al. Personal Neoantigen Vaccines Induce Persistent Memory T Cell Responses and Epitope Spreading in Patients with Melanoma. Nat. Med. 2021, 27, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen Vaccine Generates Intratumoral T Cell Responses in Phase Ib Glioblastoma Trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Kreiter, S.; Vormehr, M.; van de Roemer, N.; Diken, M.; Löwer, M.; Diekmann, J.; Boegel, S.; Schrörs, B.; Vascotto, F.; Castle, J.C.; et al. Mutant MHC Class II Epitopes Drive Therapeutic Immune Responses to Cancer. Nature 2015, 520, 692–696. [Google Scholar] [CrossRef]
- Cho, H.-I.; Celis, E. Design of Immunogenic and Effective Multi-Epitope DNA Vaccines for Melanoma. Cancer Immunol. Immunother. 2011, 61, 343–351. [Google Scholar] [CrossRef]
- Duperret, E.K.; Perales-Puchalt, A.; Stoltz, R.; Hiranjith, G.H.; Mandloi, N.; Barlow, J.; Chaudhuri, A.; Sardesai, N.Y.; Weiner, D.B. A Synthetic DNA, Multi-Neoantigen Vaccine Drives Predominately MHC Class I CD8+ T-Cell Responses, Impacting Tumor Challenge. Cancer Immunol. Res. 2019, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Gane, E.J.; Marron, T.U.; Perales-Linares, R.; Yan, J.; Cooch, N.; Shu, D.H.; Fertig, E.J.; Kagohara, L.T.; Bartha, G.; et al. Personalized Neoantigen Vaccine and Pembrolizumab in Advanced Hepatocellular Carcinoma: A Phase 1/2 Trial. Nat. Med. 2024, 30, 1044–1053. [Google Scholar] [CrossRef]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.K.P.; Bonam, S.R.; Kurapati, R.; Zheng, J.; Chai, D. The Next-Generation DNA Vaccine Platforms and Delivery Systems: Advances, Challenges and Prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef] [PubMed]
- Nchinda, G.; Amadu, D.; Trumpfheller, C.; Mizenina, O.; Überla, K.; Steinman, R.M. Dendritic Cell Targeted HIV Gag Protein Vaccine Provides Help to a DNA Vaccine Including Mobilization of Protective CD8+ T Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4281–4286. [Google Scholar] [CrossRef]
- Li, L.; Petrovsky, N. Molecular Mechanisms for Enhanced DNA Vaccine Immunogenicity. Expert Rev. Vaccines 2015, 15, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Kanuma, T.; Yamamoto, T.; Kobiyama, K.; Moriishi, E.; Masuta, Y.; Kusakabe, T.; Ozasa, K.; Kuroda, E.; Jounai, N.; Ishii, K.J. CD63-Mediated Antigen Delivery into Extracellular Vesicles via DNA Vaccination Results in Robust CD8+ T Cell Responses. J. Immunol. 2017, 198, 4707–4715. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Sung, B.H.; von Lersner, A.; Guerrero, J.; Krystofiak, E.S.; Inman, D.; Pelletier, R.; Zijlstra, A.; Ponik, S.M.; Weaver, A.M. A Live Cell Reporter of Exosome Secretion and Uptake Reveals Pathfinding Behavior of Migrating Cells. Nat. Commun. 2020, 11, 2092. [Google Scholar] [CrossRef]
- Verweij, F.J.; Bebelman, M.P.; Jimenez, C.R.; Garcia-Vallejo, J.J.; Janssen, H.; Neefjes, J.; Knol, J.C.; de Goeij-de Haas, R.; Piersma, S.R.; Baglio, S.R.; et al. Quantifying Exosome Secretion from Single Cells Reveals a Modulatory Role for GPCR Signaling. J. Cell. Biol. 2018, 217, 1129–1142. [Google Scholar] [CrossRef]
- Amraei, R.; Yin, W.; Napoleon, M.A.; Suder, E.L.; Berrigan, J.; Zhao, Q.; Olejnik, J.; Chandler, K.B.; Xia, C.; Feldman, J.; et al. CD209L/L-SIGN and CD209/DC-SIGN Act as Receptors for SARS-CoV-2. ACS Central Sci. 2021, 7, 1156–1165. [Google Scholar] [CrossRef]
- Simpson, J.D.; Ray, A.; Marcon, C.; dos Santos Natividade, R.; Dorrazehi, G.M.; Durlet, K.; Koehler, M.; Alsteens, D. Single-Molecule Analysis of SARS-CoV-2 Binding to C-Type Lectin Receptors. Nano Lett. 2023, 23, 1496–1504. [Google Scholar] [CrossRef]
- Engering, A.; Geijtenbeek, T.B.H.; van Vliet, S.J.; Wijers, M.; van Liempt, E.; Demaurex, N.; Lanzavecchia, A.; Fransen, J.; Figdor, C.G.; Piguet, V.; et al. The Dendritic Cell-Specific Adhesion Receptor DC-SIGN Internalizes Antigen for Presentation to T Cells. J. Immunol. 2002, 168, 2118–2126. [Google Scholar] [CrossRef]
- Karamitopoulou, E. Tumour Microenvironment of Pancreatic Cancer: Immune Landscape Is Dictated by Molecular and Histopathological Features. Br. J. Cancer 2019, 121, 5–14. [Google Scholar] [CrossRef]
- Ho, W.J.; Erbe, R.; Danilova, L.; Phyo, Z.; Bigelow, E.; Stein-O’Brien, G.; Thomas, D.L.; Charmsaz, S.; Gross, N.; Woolman, S.; et al. Multi-Omic Profiling of Lung and Liver Tumor Microenvironments of Metastatic Pancreatic Cancer Reveals Site-Specific Immune Regulatory Pathways. Genome Biol. 2021, 22, 154. [Google Scholar] [CrossRef]
- Yuan, S.; Natesan, R.; Sanchez-Rivera, F.J.; Li, J.; Bhanu, N.V.; Yamazoe, T.; Lin, J.H.; Merrell, A.J.; Sela, Y.; Thomas, S.K.; et al. Global Regulation of the Histone Mark H3K36me2 Underlies Epithelial Plasticity and Metastatic Progression. Cancer Discov. 2020, 10, 854–871. [Google Scholar] [CrossRef] [PubMed]
- Ruscetti, M.; Morris, J.P.; Mezzadra, R.; Russell, J.; Leibold, J.; Romesser, P.B.; Simon, J.; Kulick, A.; Ho, Y.-J.; Fennell, M.; et al. Senescence-Induced Vascular Remodeling Creates Therapeutic Vulnerabilities in Pancreas Cancer. Cell 2020, 181, 424–441.e21. [Google Scholar] [CrossRef]
- Kodysh, J.; Rubinsteyn, A. OpenVax: An Open-Source Computational Pipeline for Cancer Neoantigen Prediction. Methods Mol. Biol. 2020, 2120, 147–160. [Google Scholar] [CrossRef]
- Hundal, J.; Carreno, B.M.; Petti, A.A.; Linette, G.P.; Griffith, O.L.; Mardis, E.R.; Griffith, M. pVAC-Seq: A Genome-Guided in Silico Approach to Identifying Tumor Neoantigens. Genome Med. 2016, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Hundal, J.; Kiwala, S.; McMichael, J.; Miller, C.A.; Xia, H.; Wollam, A.T.; Liu, C.J.; Zhao, S.; Feng, Y.-Y.; Graubert, A.P.; et al. pVACtools: A Computational Toolkit to Identify and Visualize Cancer Neoantigens. Cancer Immunol. Res. 2020, 8, 409–420. [Google Scholar] [CrossRef]
- De Silva, N.S.; Siewiera, J.; Alkhoury, C.; Nader, G.P.F.; Nadalin, F.; de Azevedo, K.; Couty, M.; Izquierdo, H.M.; Bhargava, A.; Conrad, C.; et al. Nuclear Envelope Disruption Triggers Hallmarks of Aging in Lung Alveolar Macrophages. Nat. Aging 2023, 3, 1251–1268. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM Predicts Alpha and Beta Transmembrane Proteins Using Deep Neural Networks. bioRxiv 2022. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Chu, J.; Zhang, X.; Xu, C.; Liu, S.; Wan, Z.; Wang, J.; Zhang, L.; Liu, K.; et al. Anti-EGFR ScFv Functionalized Exosomes Delivering LPCAT1 Specific siRNAs for Inhibition of Lung Cancer Brain Metastases. J. Nanobiotechnol. 2024, 22, 159. [Google Scholar] [CrossRef]
- Breuer, K.; Foroushani, A.K.; Laird, M.R.; Chen, C.; Sribnaia, A.; Lo, R.; Winsor, G.L.; Hancock, R.E.W.; Brinkman, F.S.L.; Lynn, D.J. InnateDB: Systems Biology of Innate Immunity and beyond—Recent Updates and Continuing Curation. Nucleic Acids Res. 2012, 41, D1228–D1233. [Google Scholar] [CrossRef]
- Gupta, J.; Malik, Z.; Chaturvedi, M.; Mishra, M.; Mishra, S.K.; Grover, A.; Ray, A.K.; Chaturvedi, R. SARS CoV-2 Spike Protein Variants Exploit DC-SIGN/DC-SIGNR Receptor for Evolution and Severity: An in-Silico Insight. VirusDisease 2023, 34, 278–296. [Google Scholar] [CrossRef]
- Fritah, H.; Rovelli, R.; Chiang, C.L.-L.; Kandalaft, L.E. The Current Clinical Landscape of Personalized Cancer Vaccines. Cancer Treat. Rev. 2022, 106, 102383. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in Cancer Immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Isoda, F.; Matsumoto, N.; Watanabe, N.; Hiranuka, K.; Yamada, T.; Fujinami, N.; Shimomura, M.; Suzuki, T.; Nakatsura, T.; et al. Abstract 3552: Personalized Neoantigen Cancer Vaccine Assembled on DC Targeting Antibody Improves Cancer Immunity. Cancer Res. 2022, 82, 3552. [Google Scholar] [CrossRef]
- Melief, C.J.M. Cancer Immunotherapy by Dendritic Cells. Immunity 2008, 29, 372–383. [Google Scholar] [CrossRef]
- Nchinda, G.; Kuroiwa, J.; Oks, M.; Trumpfheller, C.; Park, C.G.; Huang, Y.; Hannaman, D.; Schlesinger, S.J.; Mizenina, O.; Nussenzweig, M.C.; et al. The Efficacy of DNA Vaccination Is Enhanced in Mice by Targeting the Encoded Protein to Dendritic Cells. J. Clin. Investig. 2008, 118, 1427–1436. [Google Scholar] [CrossRef]
- Gong, N.; Alameh, M.-G.; El-Mayta, R.; Xue, L.; Weissman, D.; Mitchell, M.J. Enhancing in Situ Cancer Vaccines Using Delivery Technologies. Nat. Rev. Drug Discov. 2024, 23, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Jiao, Y.-Y.; Ma, Y.; Peng, X.-L.; Fu, Y.-H.; Zhang, X.-J.; Zheng, Y.-B.; Zheng, Y.-P.; Hong, T.; He, J.-S. Enhanced Humoral and CD8+ T Cell Immunity in Mice Vaccinated by DNA Vaccine against Human Respiratory Syncytial Virus through Targeting the Encoded F Protein to Dendritic Cells. Int. Immunopharmacol. 2017, 46, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Koppel, E.A.; Van Gisbergen, K.P.J.M.; Geijtenbeek, T.B.H.; Van Kooyk, Y. Distinct Functions of DC-SIGN and Its Homologues L-SIGN (DC-SIGNR) and mSIGNR1 in Pathogen Recognition and Immune Regulation. Cell Microbiol. 2004, 7, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Rappocciolo, G.; Piazza, P.; Fuller, C.L.; Reinhart, T.A.; Watkins, S.C.; Rowe, D.T.; Jais, M.; Gupta, P.; Rinaldo, C.R. DC-SIGN on B Lymphocytes Is Required For Transmission of HIV-1 to T Lymphocytes. PLoS Pathog. 2006, 2, e70. [Google Scholar] [CrossRef][Green Version]
- Gu, H.; Chen, Q.; Yang, G.; He, L.; Fan, H.; Deng, Y.-Q.; Wang, Y.; Teng, Y.; Zhao, Z.; Cui, Y.; et al. Adaptation of SARS-CoV-2 in BALB/c Mice for Testing Vaccine Efficacy. Science 2020, 369, 1603–1607. [Google Scholar] [CrossRef]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Pironti, G.; Strachan, R.T.; Abraham, D.; Yu, S.M.-W.; Chen, M.; Chen, W.; Hanada, K.; Mao, L.; Watson, L.J.; Rockman, H.A. Circulating Exosomes Induced by Cardiac Pressure Overload Contain Functional Angiotensin II Type 1 Receptors. Circulation 2015, 131, 2120–2130. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular Vesicle in Vivo Biodistribution Is Determined by Cell Source, Route of Administration and Targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Salunkhe, S.; Basak, M.; Chitkara, D.; Mittal, A. Surface Functionalization of Exosomes for Target-Specific Delivery and in Vivo Imaging & Tracking: Strategies and Significance. J. Control. Release 2020, 326, 599–614. [Google Scholar] [CrossRef]
- Park, C.G.; Takahara, K.; Umemoto, E.; Yashima, Y.; Matsubara, K.; Matsuda, Y.; Clausen, B.E.; Inaba, K.; Steinman, R.M. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int. Immunol. 2001, 13, 1283–1290. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct Gene Transfer into Mouse Muscle in Vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Casares, S.; Inaba, K.; Brumeanu, T.-D.; Steinman, R.M.; Bona, C.A. Antigen Presentation by Dendritic Cells after Immunization with DNA Encoding a Major Histocompatibility Complex Class II–Restricted Viral Epitope. J. Exp. Med. 1997, 186, 1481–1486. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-Presentation by Dendritic Cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.-E.; Williamson, M.; Antón-Plágaro, C.; Shoemark, D.K.; Simón-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 Is a Host Factor for SARS-CoV-2 Infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, R.; Lepelletier, Y.; Lemarchandel, V.; Cambot, M.; Gaulard, P.; Hermine, O.; Roméo, P.-H. A Neuronal Receptor, Neuropilin-1, Is Essential for the Initiation of the Primary Immune Response. Nat. Immunol. 2002, 3, 477–482. [Google Scholar] [CrossRef]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The Protein Expression Profile of ACE2 in Human Tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Hao, P.; Xu, W.; Li, L.; Jiang, Y.; Xu, Z.; Chen, J.; Gao, Z.; Pang, Z.; Jin, N.; et al. A Vaccine of SARS-CoV-2 S Protein RBD Induces Protective Immunity. Int. J. Mol. Sci. 2022, 23, 13716. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Yuan, Z.; Wu, Z.; Yang, Q.; Ding, T.; Yu, K.; Dong, J. CD63-Mediated SARS-CoV-2 RBD Fusion Neoantigen DNA Vaccine Enhances Antitumor Immune Response in a Mouse Panc02 Model via EV-Targeted Delivery. Vaccines 2025, 13, 977. https://doi.org/10.3390/vaccines13090977
Liu G, Yuan Z, Wu Z, Yang Q, Ding T, Yu K, Dong J. CD63-Mediated SARS-CoV-2 RBD Fusion Neoantigen DNA Vaccine Enhances Antitumor Immune Response in a Mouse Panc02 Model via EV-Targeted Delivery. Vaccines. 2025; 13(9):977. https://doi.org/10.3390/vaccines13090977
Chicago/Turabian StyleLiu, Guang, Ziqing Yuan, Ziyi Wu, Qiyv Yang, Tingbo Ding, Ker Yu, and Jibin Dong. 2025. "CD63-Mediated SARS-CoV-2 RBD Fusion Neoantigen DNA Vaccine Enhances Antitumor Immune Response in a Mouse Panc02 Model via EV-Targeted Delivery" Vaccines 13, no. 9: 977. https://doi.org/10.3390/vaccines13090977
APA StyleLiu, G., Yuan, Z., Wu, Z., Yang, Q., Ding, T., Yu, K., & Dong, J. (2025). CD63-Mediated SARS-CoV-2 RBD Fusion Neoantigen DNA Vaccine Enhances Antitumor Immune Response in a Mouse Panc02 Model via EV-Targeted Delivery. Vaccines, 13(9), 977. https://doi.org/10.3390/vaccines13090977




