Immunogenicity and Antigenicity of the Recombinant Ectodomain of Rabies Virus Glycoprotein Containing the Human Collagen XVIII Trimerization Domain
Abstract
1. Introduction
2. Materials and Methods
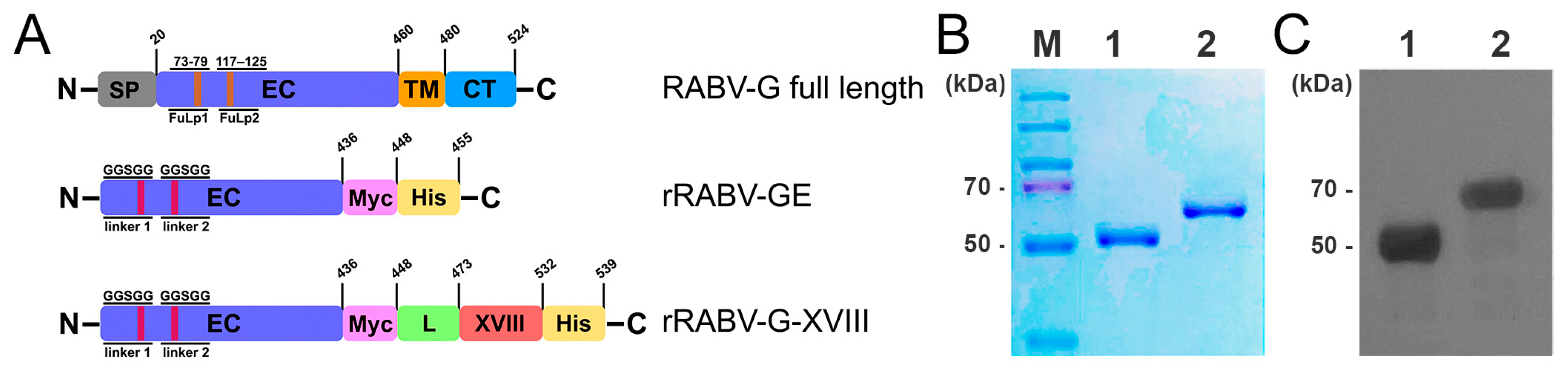
2.1. Construction of Candidate Vaccines
2.2. Recombinant Protein Expression and His-Tag Affinity Purification
2.3. Analysis of Proteins by Size Exclusion Chromatography
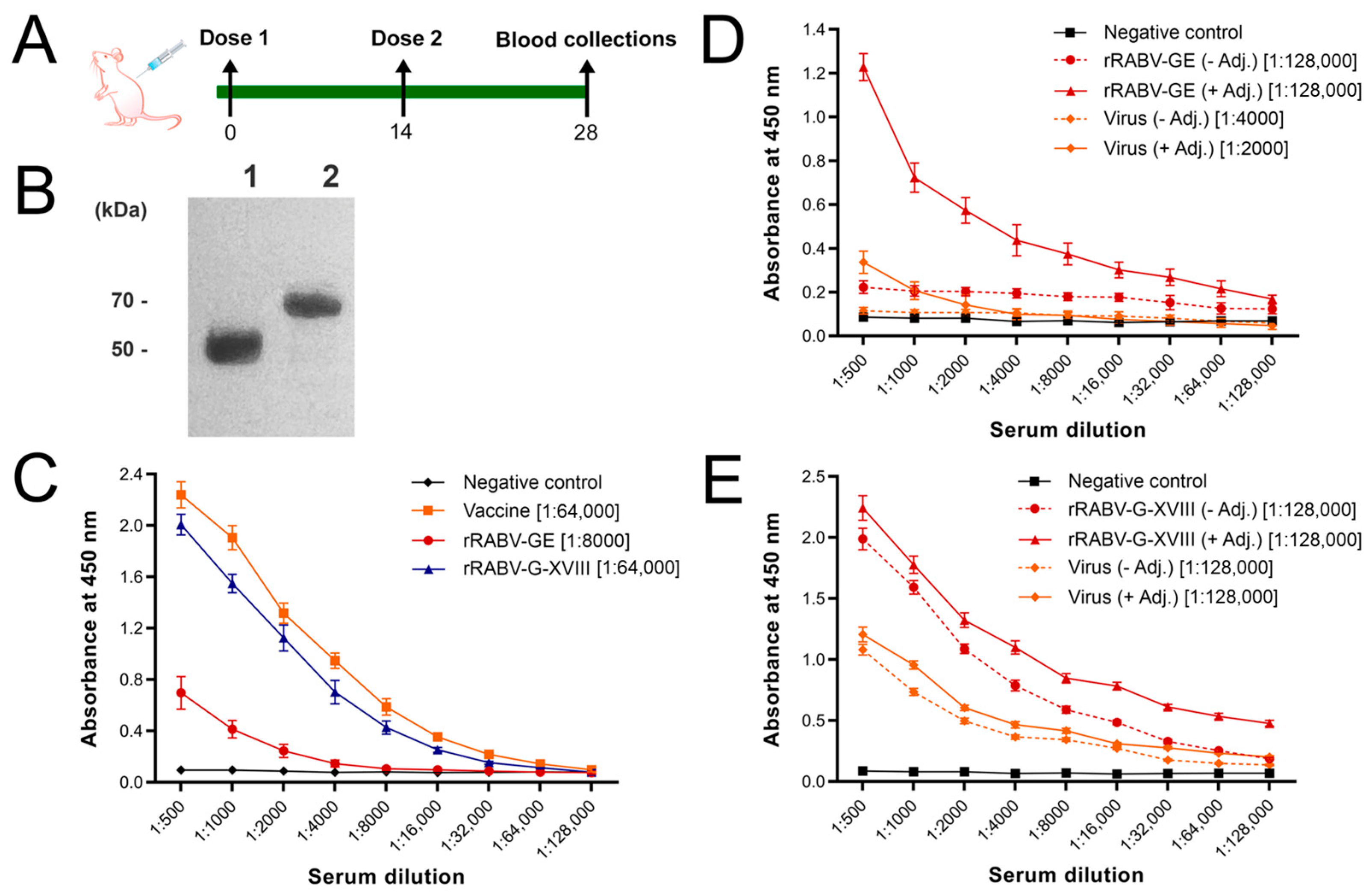
2.4. Immunization and Challenge of Mice
2.5. Enzyme-Linked Immunosorbent Assays
2.6. Neutralization Assay
3. Results
3.1. Production of Recombinant RABV-G Proteins
3.2. In Vivo Humoral Immune Responses
3.3. Protective Efficacy of Candidate Vaccines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, P.J.; Freitas-Astúa, J.; Bejerman, N.; Blasdell, K.R.; Breyta, R.; Dietzgen, R.G.; Fooks, A.R.; Kondo, H.; Kurath, G.; Kuzmin, I.V.; et al. ICTV virus taxonomy profile: Rhabdoviridae 2022. J. Gen. Virol. 2022, 103, 001689. [Google Scholar] [CrossRef]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the Global Burden of Endemic Canine Rabies. PLoS Neglect. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- Natesan, K.; Isloor, S.; Vinayagamurthy, B.; Ramakrishnaiah, S.; Doddamane, R.; Fooks, A.R. Developments in Rabies Vaccines: The Path Traversed from Pasteur to the Modern Era of Immunization. Vaccines 2023, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Bilal, A. Rabies is a Zoonotic Disease: A Literature Review. Occup. Med. Health Aff. 2021, 9, 334. [Google Scholar] [CrossRef]
- Astray, R.M.; Jorge, S.A.C.; Pereira, C.A. Rabies vaccine development by expression of recombinant viral glycoprotein. Arch. Virol. 2017, 162, 323–332. [Google Scholar] [CrossRef]
- Ertl, H.C.J. New Rabies Vaccines for Use in Humans. Vaccines 2019, 7, 54. [Google Scholar] [CrossRef]
- Cruz, E.T.; Romero, I.A.F.; Mendoza, J.G.L.; Sua’rez, S.O.; Gonza’lez, R.H.; Favela, F.B.; Torres, A.P.; Setie’n, J.A.A. Efficient postexposure prophylaxis against rabies by applying a four-dose DNA vaccine intranasally. Vaccine 2008, 26, 6936–6944. [Google Scholar] [CrossRef]
- Gaudin, Y.; Ruigrok, R.W.; Tuffereau, C.; Knossow, M.; Flamand, A. Rabies virus glycoprotein is a trimer. Virology 1992, 187, 627–632. [Google Scholar] [CrossRef]
- You, Y.; Yang, F.; Lin, S.; Chen, Z.; Shu, S.; Yu, Y.; He, B.; Cao, Y.; Lu, G. Rabies virus glycoprotein: Structure, function, and antivirals. hLife, 2025; in Press. [Google Scholar] [CrossRef]
- Singh, A.; Yadav, D.; Rai, K.M.; Srivastava, M.; Verma, P.C.; Singh, P.K.; Tuli, R. Enhanced Expression of Rabies Virus Surface G-Protein in Escherichia coli using SUMO Fusion. Protein J. 2012, 31, 68–74. [Google Scholar] [CrossRef]
- Qian, W.; Aguilar, F.; Wang, T.; Qiu, B. Secretion of truncated recombinant rabies virus glycoprotein with preserved antigenic properties using a co-expression system in Hansenula polymorpha. J. Microbiol. 2013, 51, 234–240. [Google Scholar] [CrossRef]
- Tiwari, S.; Mishra, D.K.; Roy, S.; Singh, A.; Singh, P.K.; Tuli, R. High level expression of a functionally active cholera toxin B: Rabies glycoprotein fusion protein in tobacco seeds. Plant Cell Rep. 2009, 28, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kang, H.; Min, K.; Kim, N.H.; Park, M.; Ouh, I.O.; Kim, H.H.; Song, J.Y.; Yang, D.K.; Sohn, E.J.; et al. Rabies virus glycoprotein produced in Nicotiana benthamiana is an immunogenic antigen in mice. Czech J. Genet. Plant Breed 2021, 57, 26–35. [Google Scholar] [CrossRef]
- Fu, Z.F.; Rupprecht, C.E.; Dietzschold, B.; Saikumar, P.; Niu, H.S.; Babka, I.; Wunner, W.H.; Koprowski, H. Oral vaccination of racoons (Procyon lotor) with baculovirus-expressed rabies virus glycoprotein. Vaccine 1993, 11, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Kankanamage, P.J.; Irie, T.; Shoji, J.; Tochikura, T.S. Further Characterization of the Rabies Virus Glycoproteins Produced by Virus-Infected and G cDNA-Transfected Cells Using a Monoclonal Antibody, #1-30-44, Which Recognizes an Acid-Sensitive Epitope. Microbiol. Immunol. 2003, 47, 337–349. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Yao, Q.; Gao, Z.; Cheng, Y.; Zhou, M.; Tang, Y.; Sun, L.; Dai, J.; Cao, G.; et al. AAV-expressed G protein induces robust humoral and cellular immune response and provides durable protection from rabies virus challenges in mice. Vet. Microbiol. 2020, 242, 108578. [Google Scholar] [CrossRef]
- Schnee, M.; Vogel, A.B.; Voss, D.; Petsch, B.; Baumhof, P.; Kramps, T.; Stitz, L. An mRNA Vaccine Encoding Rabies Virus Glycoprotein Induces Protection against Lethal Infection in Mice and Correlates of Protection in Adult and Newborn Pigs. PLoS Negl. Trop. Dis. 2016, 10, e0004746. [Google Scholar] [CrossRef]
- Boudko, S.; Sasaki, T.; Engel, J.; Lerch, T.F.; Nix, J.; Chapman, M.S.; Bächinger, H.P. Crystal Structure of Human Collagen XVIII Trimerization Domain: A Novel Collagen Trimerization Fold. J. Mol. Biol. 2009, 392, 787–802. [Google Scholar] [CrossRef]
- Yang, F.; Lin, S.; Ye, F.; Yang, J.; Qi, J.; Chen, Z.; Lin, X.; Wang, J.; Yue, D.; Cheng, Y.; et al. Structural Analysis of Rabies Virus Glycoprotein Reveals pH-Dependent Conformational Changes and Interactions with a Neutralizing Antibody. Cell Host Microbe 2020, 27, 441–453.e7. [Google Scholar] [CrossRef]
- Vallina, L.Á.; Martinez Á, C.; Pastor, N.S.; Alcober, L.S. Generation of Multifunctional and Multivalent Polypeptide Complexes with Collagen XVIII Trimerization Domain. EP2441776A1, 18 April 2019. [Google Scholar]
- Angel, M.C.; David, S.M.; Ana, B.T.; Maider, V.; Kelly, E.A.; Ana, A.S.; Sainz, P.; Laura, S.; Francisco, J.B.; Luis, A.V. Improved stability of multivalent antibodies containing the human collagen XV trimerization domain. In Mabs; Taylor & Francis: Abingdon-on-Thames, UK, 2012; Volume 4, pp. 226–232. [Google Scholar] [CrossRef]
- Lobstein, J.; Emrich, C.A.; Jeans, C.; Faulkner, M.; Riggs, P.; Berkmen, M. Microbial cell factories. SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microb. Cell Fact. 2012, 11, 753. [Google Scholar] [CrossRef]
- Tu, A.-H.T. Transformation of Escherichia coli made competent by calcium chloride protocol. ASM 2008, 1–10. [Google Scholar]
- Abdualiyeva, A.A.; Akhmetsadykov, N.N.; Valdovska, A.; Shanbaev, B.U. Improving the technology of obtaining an inactivated antirabic vaccine from CVS-11 strain. Res. J. Pharm. Technol. 2020, 13, 5929–5934. [Google Scholar] [CrossRef]
- Servat, A.; Wasniewski, M.; Cliquet, F. Cross-Protection of Inactivated Rabies Vaccines for Veterinary Use against Bat Lyssaviruses Occurring in Europe. Viruses 2019, 11, 936. [Google Scholar] [CrossRef]
- Wiktor, T.J.; Gyorgy, E.; Schlumberger, D.; Sokol, F.; Koprowski, H. Antigenic Properties of Rabies Virus Components. J. Immunol. 1973, 110, 269–276. [Google Scholar] [CrossRef]
- Weldon, W.C.; Wang, B.Z.; Martin, M.P.; Koutsonanos, D.G.; Skountzou, I.; Compans, R.W. Enhanced Immunogenicity of Stabilized Trimeric Soluble Influenza Hemagglutinin. PLoS ONE 2010, 5, e12466. [Google Scholar] [CrossRef]
- Grundner, C.; Li, Y.; Louder, M.; Mascola, J.; Yang, X.; Sodroski, J.; Wyatt, R. Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology 2005, 331, 33–46. [Google Scholar] [CrossRef]
- Guo, L.; Bi, W.; Wang, X.; Xu, W.; Yan, R.; Zhang, Y.; Zhao, K.; Li, Y.; Zhang, M.; Cai, X.; et al. Engineered trimeric ACE2 binds viral spike protein and locks it in “Three-up” conformation to potently inhibit SARS-CoV-2 infection. Cell Res. 2021, 31, 98–100. [Google Scholar] [CrossRef]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.W.; Balmert, S.C.; Carey, C.D.; Raj, V.S.; Epperly, M.W.; Klimstra, W.B.; Haagmans, B.L.; et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine 2020, 55, 102743. [Google Scholar] [CrossRef] [PubMed]
- Sliepen, K.; van Montfort, T.; Melchers, M.; Isik, G.; Sanders, R.W. Immunosilencing a Highly Immunogenic Protein Trimerization Domain. J. Biol. Chem. 2015, 290, 7436–7442. [Google Scholar] [CrossRef] [PubMed]
- Callaway, H.M.; Zyla, D.; Larrous, F.; de Melo, G.D.; Hastie, K.M.; Avalos, R.D.; Agarwal, A.; Corti, D.; Bourhy, H.; Saphire, E.O. Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Sci. Adv. 2022, 8, eabp9151. [Google Scholar] [CrossRef] [PubMed]
- Hellert, J.; Buchrieser, J.; Larrous, F.; Minola, A.; de Melo, G.D.; Soriaga, L.; England, P.; Haouz, A.; Telenti, A.; Schwartz, O.; et al. Structure of the prefusion-locking broadly neutralizing antibody RVC20 bound to the rabies virus glycoprotein. Nat. Commun. 2020, 11, 596. [Google Scholar] [CrossRef]
- Schnell, M.J.; McGettigan, J.P.; Wirblich, C.; Papaneri, A. The cell biology of rabies virus: Using stealth to reach the brain. Nat. Rev. Microbiol. 2010, 8, 51–61. [Google Scholar] [CrossRef]
- Koraka, P.; Bosch, B.J.; Cox, M.; Chubet, R.; van Amerongen, G.; Lövgren-Bengtsson, K.; Martina, B.E.; Roose, J.; Rottier, P.J.; Osterhaus, A.D. A recombinant rabies vaccine expressing the trimeric form of the glycoprotein confers enhanced immunogenicity and protection in outbred mice. Vaccine 2014, 32, 4644–4650. [Google Scholar] [CrossRef]
- Li, M.; Fang, E.; Wang, Y.; Shi, L.; Li, J.; Peng, Q.; Li, X.; Zhao, D.; Liu, X.; Liu, X.; et al. An mRNA vaccine against rabies provides strong and durable protection in mice. Front. Immunol. 2023, 14, 1288879. [Google Scholar] [CrossRef]
| Immunogen | Neutralization Titers |
| Vaccine serum | 1:512 |
| rRABV-GE (− Adjuvant) | ≤1:32 |
| rRABV-GE (+ Adjuvant) | 1:64 |
| rRABV-G-XVIII (− Adjuvant) | 1:512 |
| rRABV-G-XVIII (+ Adjuvant) | 1:512 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smekenov, I.; Bayandy, G.; Alybayev, S.; Baltakhozha, N.; Batanova, Z.; Akhmetsadykov, N.; Bissenbaev, A. Immunogenicity and Antigenicity of the Recombinant Ectodomain of Rabies Virus Glycoprotein Containing the Human Collagen XVIII Trimerization Domain. Vaccines 2025, 13, 971. https://doi.org/10.3390/vaccines13090971
Smekenov I, Bayandy G, Alybayev S, Baltakhozha N, Batanova Z, Akhmetsadykov N, Bissenbaev A. Immunogenicity and Antigenicity of the Recombinant Ectodomain of Rabies Virus Glycoprotein Containing the Human Collagen XVIII Trimerization Domain. Vaccines. 2025; 13(9):971. https://doi.org/10.3390/vaccines13090971
Chicago/Turabian StyleSmekenov, Izat, Gulshat Bayandy, Sanzhar Alybayev, Nuraiym Baltakhozha, Zhanat Batanova, Nurlan Akhmetsadykov, and Amangeldy Bissenbaev. 2025. "Immunogenicity and Antigenicity of the Recombinant Ectodomain of Rabies Virus Glycoprotein Containing the Human Collagen XVIII Trimerization Domain" Vaccines 13, no. 9: 971. https://doi.org/10.3390/vaccines13090971
APA StyleSmekenov, I., Bayandy, G., Alybayev, S., Baltakhozha, N., Batanova, Z., Akhmetsadykov, N., & Bissenbaev, A. (2025). Immunogenicity and Antigenicity of the Recombinant Ectodomain of Rabies Virus Glycoprotein Containing the Human Collagen XVIII Trimerization Domain. Vaccines, 13(9), 971. https://doi.org/10.3390/vaccines13090971





