Immunomodulation of Patients with Canine Visceral Leishmaniasis at Different Stages: A 12-Month Follow-Up Study Using LaSap
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Inclusion and Exclusion Criteria and Randomization
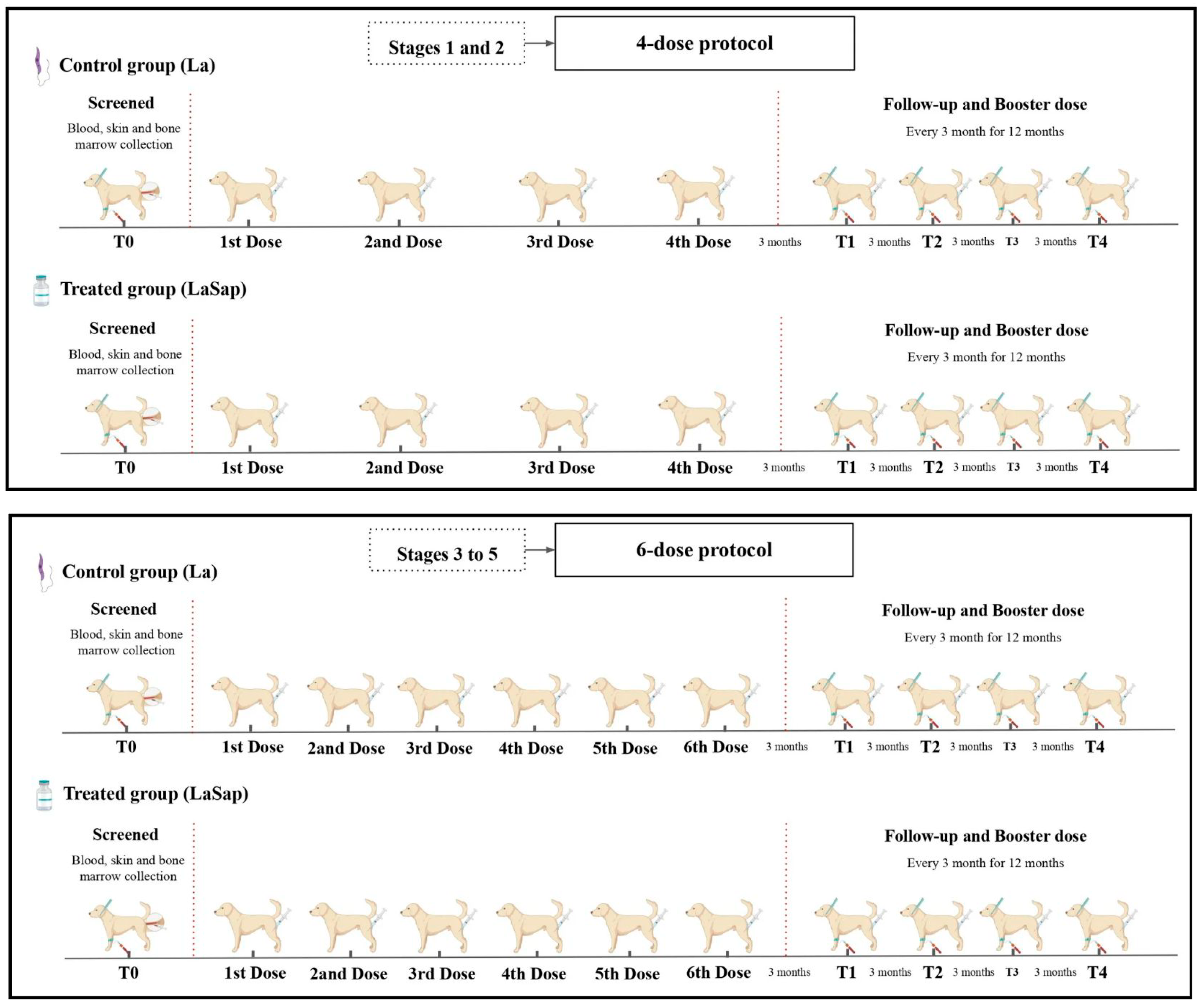
2.3. Experimental Design
2.4. Hematology and Biochemistry
2.5. ELISA
2.6. Isolation of Peripheral Blood Mononuclear Cells
2.7. ELISA Cytokine Assay
2.8. Parasite Load
2.9. Statistical Analysis
3. Results
3.1. Immunotherapy Improves the Clinical Profile of Patients with CVL
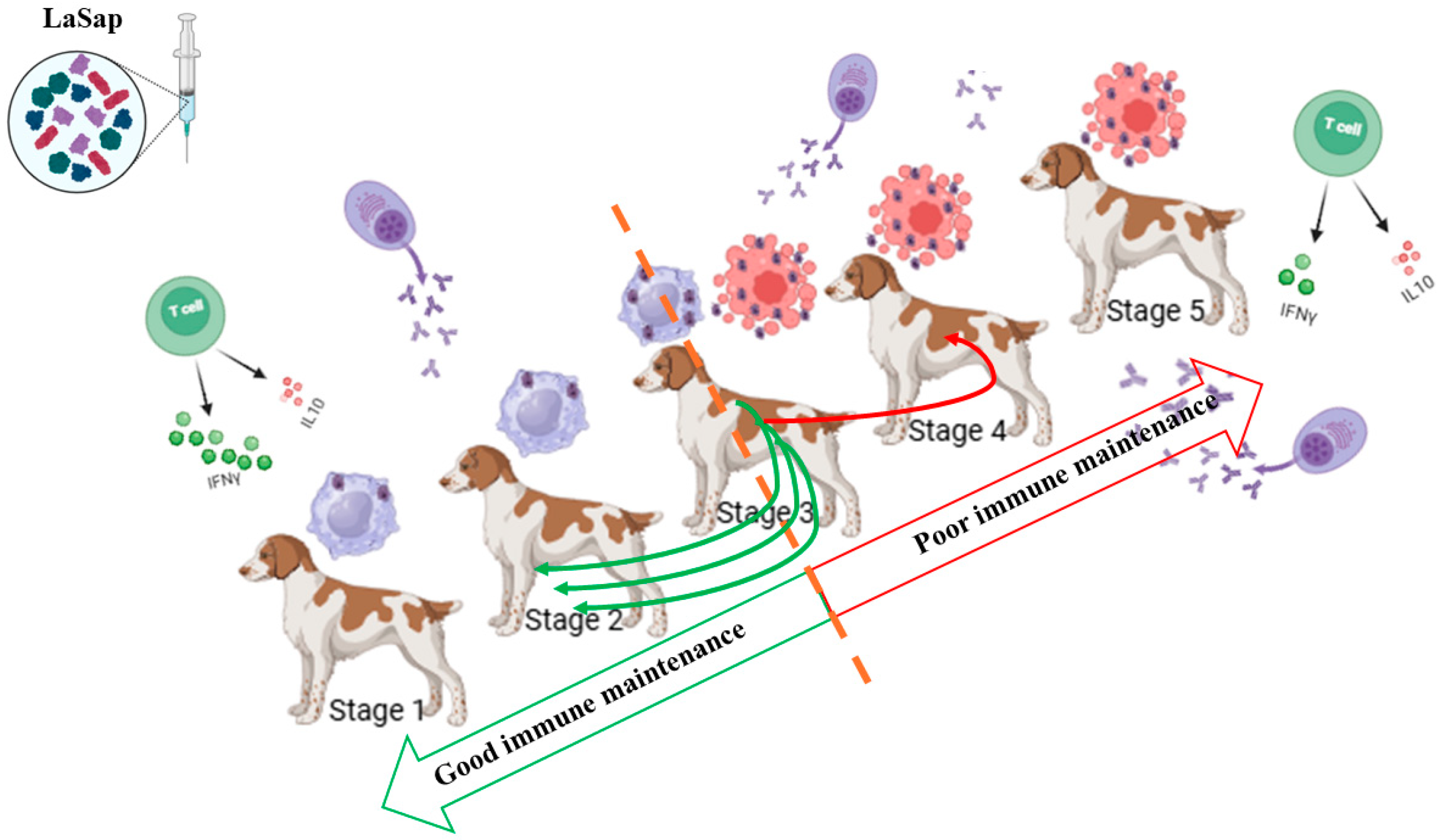
3.2. LaSap Immunotherapy Stabilizes Most Staged Patients
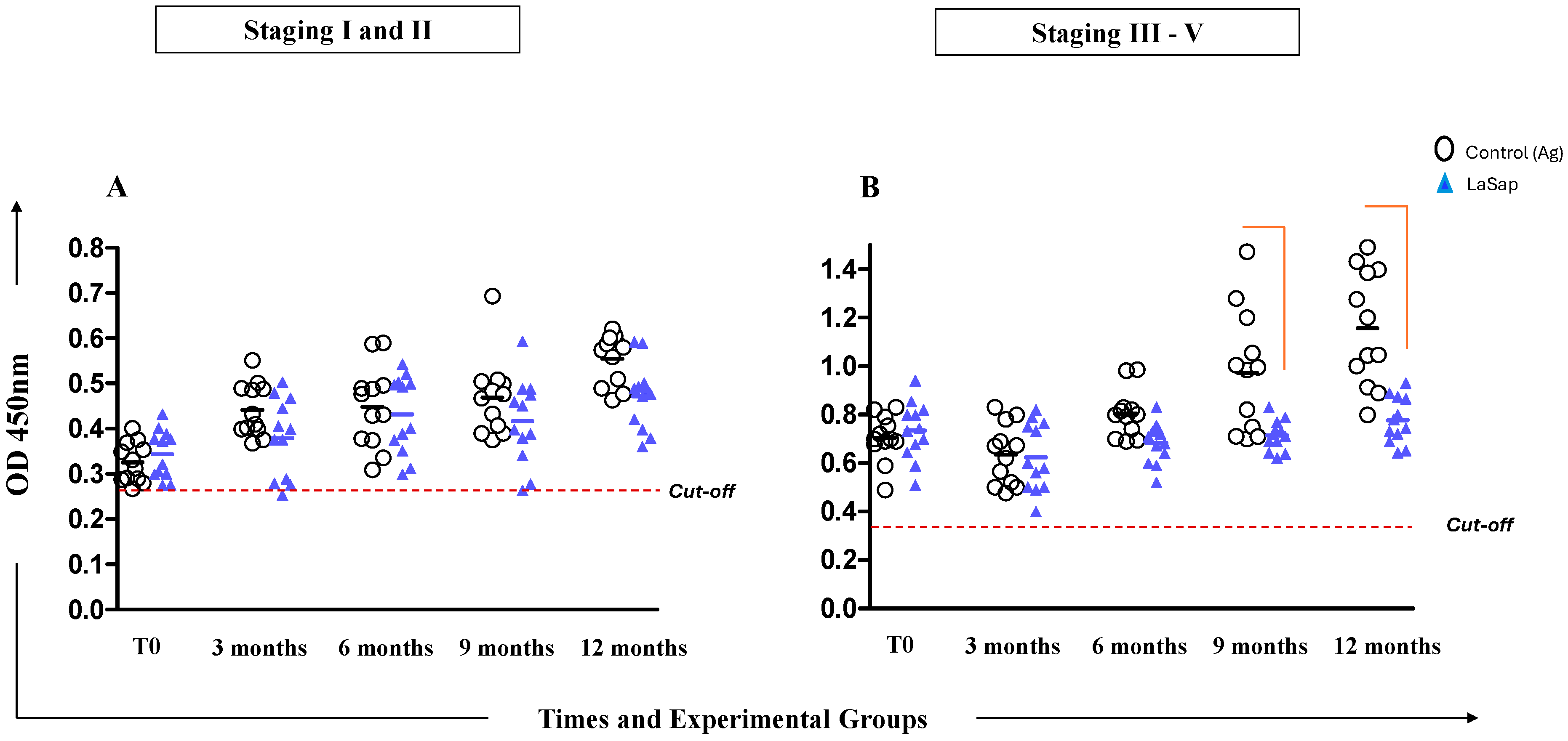
3.3. Dogs May Exhibit Variable Antibody Levels Throughout Treatment with LaSap
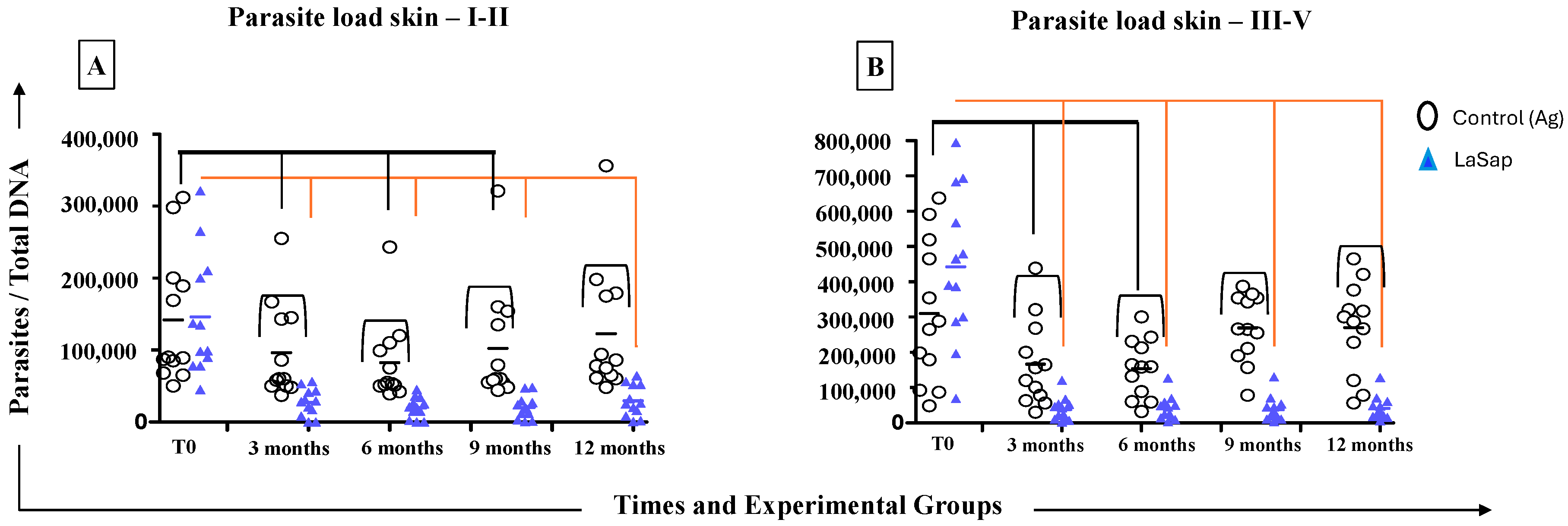
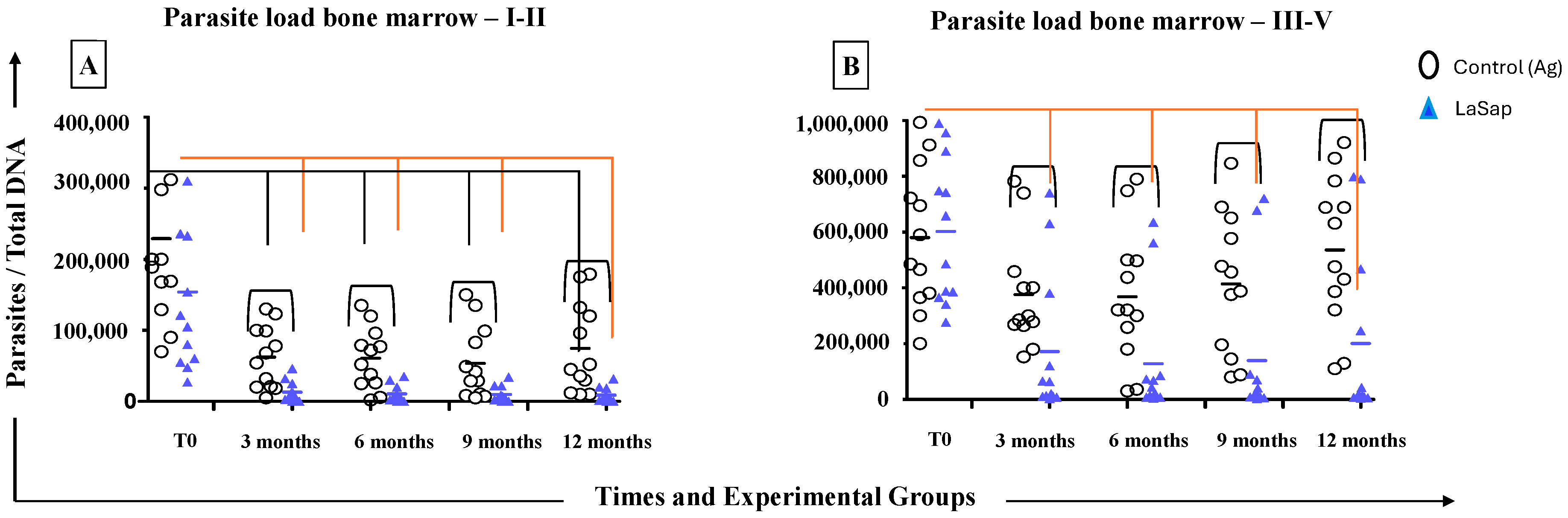
3.4. LaSap Reduces and Stabilizes Parasite Load in Most Treated Dogs
3.5. LaSap Stimulates Higher Levels of IFN-γ in Dogs Naturally Infected with L. infantum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVL | Canine visceral leishmaniasis |
| LaSap | L. amazonensis antigens plus saponin |
| WHO | World Health Organization |
| VL | Visceral leishmaniasis |
| La | L. amazonensis |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| PBMC | Peripheral blood mononuclear cells |
| SLiAg | Micrograms of soluble L. infantum antigen |
| SLaAg | Micrograms of soluble L amazonensis antigen |
| DNA polα | DNA polymerase |
References
- World Health Organization. Leishmaniasis 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 31 January 2025).
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Neglected Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- Oryan, A.; Akbari, M. Worldwide risk factors in leishmaniasis. Asian Pac. J. Trop. Med. 2016, 9, 925–932. [Google Scholar] [CrossRef]
- Nagi, N. Bangladesh eliminates visceral leishmaniasis. Lancet. Microbe 2024, 5, e420. [Google Scholar] [CrossRef]
- Postigo, J.A. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. Int. J. Antimicrob. Agents 2010, 36 (Suppl. 1), S62–S65. [Google Scholar] [CrossRef]
- Lainson, R.; Rangel, E.F. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: A review. Mem. Inst. Oswaldo Cruz. 2005, 100, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.D.; Rossi, C.N.; da Matta, V.L.; Tomokane, T.Y.; Corbett, C.E.; Secundino, N.F.; Pimenta, P.F.; Marcondes, M. Asymptomatic dogs are highly competent to transmit Leishmania (Leishmania) infantum chagasi to the natural vector. Vet. Parasitol. 2013, 196, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G., Jr.; Teva, A.; Dos-Santos, C.B.; Santos, F.N.; Pinto, I.D.; Fux, B.; Leite, G.R.; Falqueto, A. Field trial of efficacy of the Leish-tec® vaccine against canine leishmaniasis caused by Leishmania infantum in an endemic area with high transmission rates. PLoS ONE 2017, 12, e0185438. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; dos Santos Fernandes, C.E.; Araújo e Silva, E.; Brazil, R.P.; de Oliveira, A.G. Climatic factors and population density of Lutzomyia longipalpis (Lutz & Neiva, 1912) in an urban endemic area of visceral leishmaniasis in midwest Brazil. J. Vector Ecol. J. Soc. Vector Ecol. 2013, 38, 224–228. [Google Scholar] [CrossRef]
- Convit, J.; Ulrich, M.; Fernández, C.T.; Tapia, F.J.; Cáceres-Dittmar, G.; Castés, M.; Rondón, A.J. The clinical and immunological spectrum of American cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 444–448. [Google Scholar] [CrossRef]
- Mayrink, W.; Magalhaes, P.A.; Michalick, M.S.; da Costa, C.A.; Lima, A.d.O.; Melo, M.N.; Toledo, V.P.; Nascimento, E.; Dias, M.; Genaro, O. Immunotherapy as a treatment of American cutaneous leishmaniasis: Preliminary studies in Brazil. Parassitologia 1992, 34, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.deA.; Ituassu, L.T.; de Melo, M.N.; de Andrade, A.S. Evaluation of the conjunctival swab for canine visceral leishmaniasis diagnosis by PCR-hybridization in Minas Gerais State, Brazil. Vet. Parasitol. 2008, 152, 257–263. [Google Scholar] [CrossRef]
- Viana, K.F.; Lacerda, G.; Teixeira, N.S.; Rodrigues Cangussu, A.S.; Sousa Aguiar, R.W.; Giunchetti, R.C. Therapeutic vaccine of killed Leishmania amazonensis plus saponin reduced parasite burden in dogs naturally infected with Leishmania infantum. Vet. Parasitol. 2018, 254, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Clasta, R.B.; Rivas, A.V.; Souza, A.B.; Dos Santos, A.G.V.; Le Quesne, A.H.M.; Gonçalves, A.A.M.; Cangussu, A.S.R.; Giunchetti, R.C.; Viana, K.F. LaSap vaccine: Immunotherapy and immunochemotherapy associated with allopurinol in dogs naturally infected with Leishmania infantum. Parasite Immunol. 2024, 46, e13028. [Google Scholar] [CrossRef]
- Viana, K.F.; Fiuza, J.A.; Gannavaram, S.; Dey, R.; Selvapandiyan, A.; Bartholomeu, D.C.; da Silveira-Lemos, D.; Bueno, L.L.; Dutra, W.O.; Fujiwara, R.T.; et al. Application of rapid in vitro co-culture system of macrophages and T-cell subsets to assess the immunogenicity of dogs vaccinated with live attenuated Leishmania donovani centrin deleted parasites (LdCen-/-). Parasit. Vectors 2016, 9, 250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reis, A.B.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Giunchetti, R.C.; Carneiro, C.M.; Mayrink, W.; Tafuri, W.L.; Corrêa-Oliveira, R. Systemic and compartmentalized immune response in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 2009, 128, 87–95. [Google Scholar] [CrossRef]
- Moreno, J. Assessment of Vaccine-Induced Immunity Against Canine Visceral Leishmaniasis. Front. Vet. Sci. 2019, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.H. How effective is dog culling in controlling zoonotic visceral leishmaniasis? A critical evaluation of the science, politics and ethics behind this public health policy. Rev. Da Soc. Bras. Med. Trop. 2011, 44, 232–242. [Google Scholar] [CrossRef][Green Version]
- Ready, P.D. Leishmaniasis emergence and climate change. Rev. Sci. Tech. (Int. Off. Epizoot.) 2008, 27, 399–412. [Google Scholar] [CrossRef]
- Travi, B.L.; Adler, G.H.; Lozano, M.; Cadena, H.; Montoya-Lerma, J. Impact of habitat degradation on phlebotominae (Diptera: Psychodidae) of tropical dry forests in Northern Colombia. J. Med. Entomol. 2002, 39, 451–456. [Google Scholar] [CrossRef]
- Tazerji, S.S.; Nardini, R.; Safdar, M.; Shehata, A.A.; Duarte, P.M. An Overview of Anthropogenic Actions as Drivers for Emerging and Re-Emerging Zoonotic Diseases. Pathogens 2022, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Noli, C.; Saridomichelakis, M.N. An update on the diagnosis and treatment of canine leishmaniosis caused by Leishmania infantum (syn. L. chagasi). Vet. J. 2014, 202, 425–435. [Google Scholar] [CrossRef]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine Leishmaniasis: Update on Epidemiology, Diagnosis, Treatment, and Prevention. Vet. Sci. 2022, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.A.M.; Leite, J.C.; Resende, L.A.; Mariano, R.M.D.S.; Silveira, P.; Melo-Júnior, O.A.O.; Ribeiro, H.S.; de Oliveira, D.S.; Soares, D.F.; Santos, T.A.P.; et al. An Overview of Immunotherapeutic Approaches Against Canine Visceral Leishmaniasis: What Has Been Tested on Dogs and a New Perspective on Improving Treatment Efficacy. Front. Cell. Infect. Microbiol. 2019, 9, 427. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Di Filippo, L.; Ordeix, L.; Planellas, M.; Roura, X.; Altet, L.; Martínez-Orellana, P.; Montserrat, S. Early reduction of Leishmania infantum-specific antibodies and blood parasitemia during treatment in dogs with moderate or severe disease. Parasites Vectors 2016, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.M.; da Silva, S.M.; Menz, I.; Tabanez, P.; Nogueira, F.d.S.; Werkhaüser, M.; da Fonseca, A.L.; Dantas-Torres, F. Brasileish—A Study Group about Animal Leishmaniasis Control of visceral leishmaniasis in Brazil: Recommendations from Brasileish. Parasites Vectors 2013, 6, 8. [Google Scholar] [CrossRef]
- Sun, H.X.; Xie, Y.; Ye, Y.P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef]
- Araújo Verçosa, B.L.; Muniz-Junqueira, M.I.; Menezes-Souza, D.; Mourão Dias Magalhães, L.; Fujiwara, R.T.; Melo, M.N.; Vasconcelos, A.C. Enhanced apoptotic index, chemokines and inflammatory recruitment in renal tissues shows relationship with the clinical signs in Leishmania-infected dogs. Vet. Parasitol. 2021, 300, 109611. [Google Scholar] [CrossRef]
- Alves, A.F.; Pereira, R.A.; Rodrigues, M.A.; Campos, L.S.; do Carmo, D.D.; de Abreu Teles, P.P.; Andrade, H.M.; de Araújo, S.A.; Gomes, D.A.; Tafuri, W.L. Leishmania (L.) infantum BH401 strain induces classic renal lesions in dogs: Histological and confocal microscopy study. Exp. Parasitol. 2022, 242, 108342. [Google Scholar] [CrossRef] [PubMed]
- Roura, X.; Cortadellas, O.; Day, M.J.; Benali, S.L.; Zatelli, A.; Canine Leishmaniosis Working Group. Canine leishmaniosis and kidney disease: Q&A for an overall management in clinical practice. J. Small Anim. Pract. 2021, 62, 3. [Google Scholar] [CrossRef]
- Vasconcelos, T.C.; Bruno, S.F.; de Miranda, L.H.M.; Conceição-Silva, F.; Belo, V.S.; Figueiredo, F.B. Parasite load, iNOS and cytokine profiles, and histopathological aspects of Leishmania infantum infection in dogs with different clinical presentations. Scielo Braz. 2019, 49, e20180984. [Google Scholar] [CrossRef]
- Batista, L.F.S.; Torrecilha, R.B.P.; Silva, R.B.; Utsunomiya, Y.T.; Silva, T.B.F.; Tomokane, T.Y.; Pacheco, A.D.; Bosco, A.M.; Paulan, S.C.; Rossi, C.N.; et al. Chromosomal segments may explain the antibody response cooperation for canine leishmaniasis pathogenesis. Vet. Parasitol. 2020, 288, 109276. [Google Scholar] [CrossRef]
- Boggiatto, P.M.; Ramer-Tait, A.E.; Metz, K.; Kramer, E.E.; Gibson-Corley, K.; Mullin, K.; Hostetter, J.M.; Gallup, J.M.; Jones, D.E.; Petersen, C.A. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clin. Vaccine Immunol. CVI 2010, 17, 267–273. [Google Scholar] [CrossRef]
- Proverbio, D.; Spada, E.; Bagnagatti de Giorgi, G.; Perego, R.; Valena, E. Relationship between Leishmania IFAT titer and clinicopathological manifestations (clinical score) in dogs. BioMed Res. Int. 2014, 2014, 412808. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G.; The LeishVet Group. LeishVet guidelines for the practical management of canine leishmaniosis. Parasites Vectors 2011, 4, 86. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.B.; Giunchetti, R.C.; Carrillo, E.; Martins-Filho, O.A.; Moreno, J. Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Trends Parasitol. 2010, 26, 341–349. [Google Scholar] [CrossRef]
- Toepp, A.; Larson, M.; Wilson, G.; Grinnage-Pulley, T.; Bennett, C.; Leal-Lima, A.; Anderson, B.; Parrish, M.; Anderson, M.; Fowler, H.; et al. Randomized, controlled, double-blinded field trial to assess Leishmania vaccine effectiveness as immunotherapy for canine leishmaniosis. Vaccine 2018, 36, 6433–6441. [Google Scholar] [CrossRef]
- Roatt, B.M.; Aguiar-Soares, R.D.; Reis, L.E.; Cardoso, J.M.; Mathias, F.A.; de Brito, R.C.; da Silva, S.M.; Gontijo, N.F.; Ferreira, S.A.; Valenzuela, J.G.; et al. A Vaccine Therapy for Canine Visceral Leishmaniasis Promoted Significant Improvement of Clinical and Immune Status with Reduction in Parasite Burden. Front. Immunol. 2017, 8, 217. [Google Scholar] [CrossRef]
- Miró, G.; Segarra, S.; Cerón, J.J.; Ferrer, L.; Solano-Gallego, L.; Montell, L.; Costa, E.; Teichenne, J.; Mariné-Casadó, R.; GALILEI trial Group; et al. New immunomodulatory treatment protocol for canine leishmaniosis reduces parasitemia and proteinuria. PLoS Neglected Trop. Dis. 2024, 18, e0012712. [Google Scholar] [CrossRef]
- Barbiéri, C.L. Immunology of canine leishmaniasis. Parasite Immunol. 2006, 28, 329–337. [Google Scholar] [CrossRef]
- Carrillo, E.; Moreno, J. Cytokine profiles in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 2009, 128, 67–70. [Google Scholar] [CrossRef]
- Abbehusen, M.M.C.; Almeida, V.D.A.; Solcà, M.D.S.; Pereira, L.D.S.; Costa, D.J.; Gil-Santana, L.; Bozza, P.T.; Fraga, D.B.M.; Veras, P.S.T.; Dos-Santos, W.L.C.; et al. Clinical and immunopathological findings during long term follow-up in Leishmania infantum experimentally infected dogs. Sci. Rep. 2017, 7, 15914. [Google Scholar] [CrossRef] [PubMed]
- Samant, M.; Sahu, U.; Pandey, S.C.; Khare, P. Role of Cytokines in Experimental and Human Visceral Leishmaniasis. Front. Cell. Infect. Microbiol. 2021, 11, 624009. [Google Scholar] [CrossRef] [PubMed]
 , culture stimulated with LPS
, culture stimulated with LPS  , SLaAg
, SLaAg  and SLiAg
and SLiAg  . * and connecting lines indicate statistical differences within and outside the groups.
. * and connecting lines indicate statistical differences within and outside the groups.
 , culture stimulated with LPS
, culture stimulated with LPS  , SLaAg
, SLaAg  and SLiAg
and SLiAg  . * and connecting lines indicate statistical differences within and outside the groups.
. * and connecting lines indicate statistical differences within and outside the groups.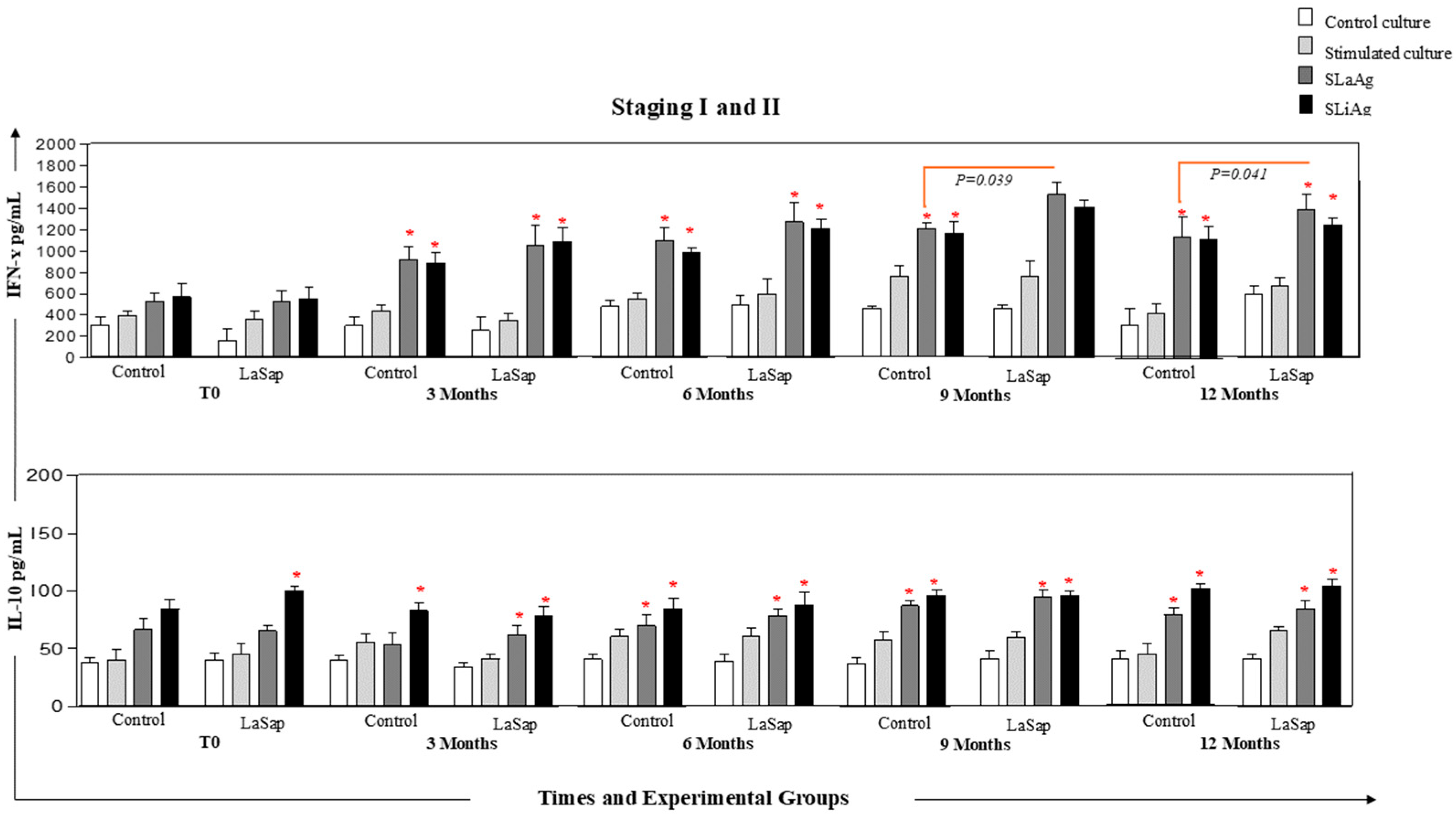
 , culture stimulated with LPS
, culture stimulated with LPS  , SLaAg
, SLaAg  and SLiAg
and SLiAg  . * and connecting lines indicate statistical differences within and outside the groups.
. * and connecting lines indicate statistical differences within and outside the groups.
 , culture stimulated with LPS
, culture stimulated with LPS  , SLaAg
, SLaAg  and SLiAg
and SLiAg  . * and connecting lines indicate statistical differences within and outside the groups.
. * and connecting lines indicate statistical differences within and outside the groups.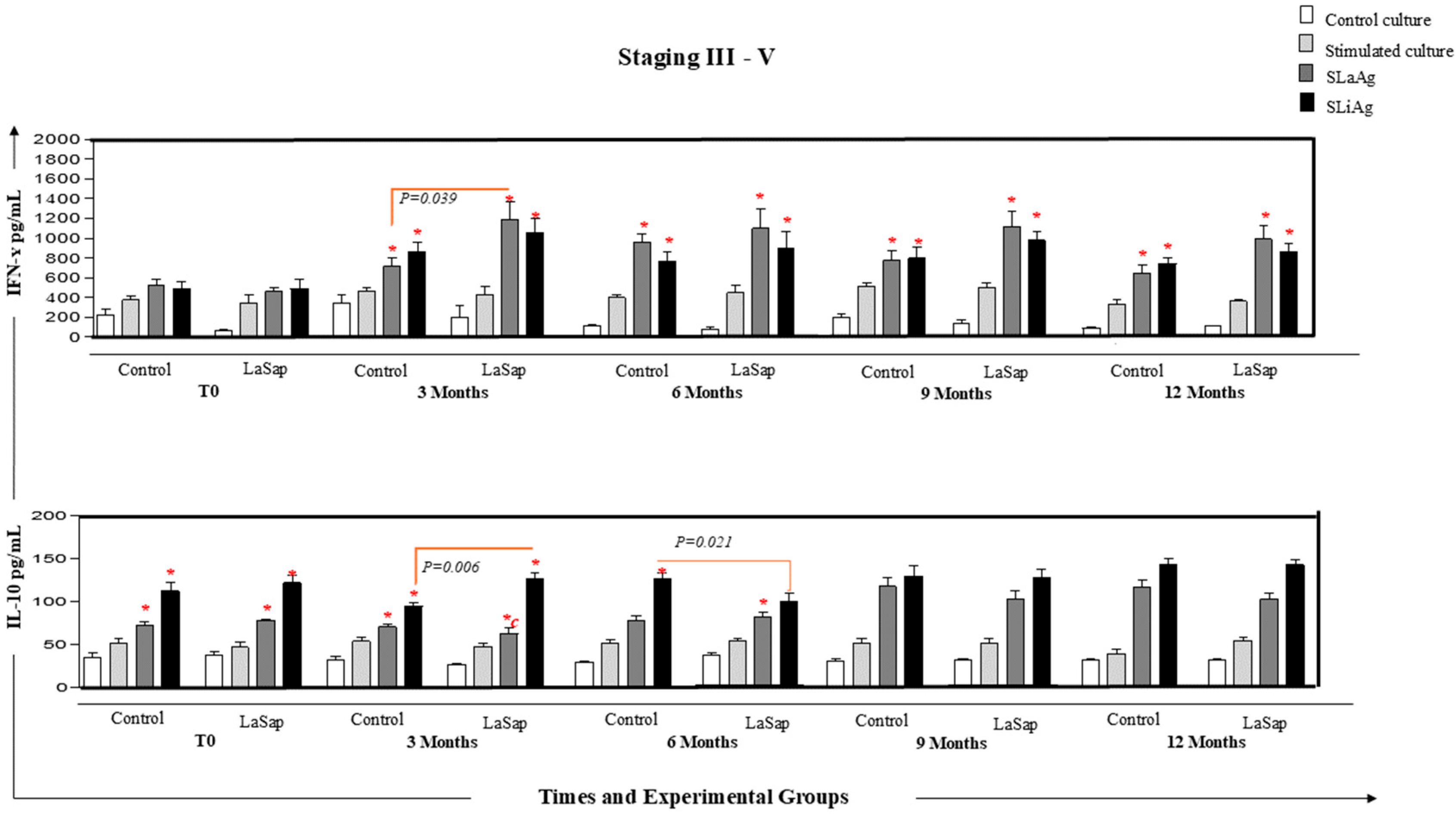
| Clinical Signs Staging 1 and 2 | 0 Time | 3 Months | 6 Months | 9 Months | 12 Months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| La | LaSap | La | LaSap | La | LaSap | La | LaSap | La | LaSap | |
| Alopecia/exfoliative dermatitis | 10/12 | 9/12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymphadenopathy | 8/12 | 7/12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lethargy | ||||||||||
| Cachexia | ||||||||||
| Epistaxis | ||||||||||
| Vasculitis | ||||||||||
| Arthritis | ||||||||||
| Clinical Signs Staging 3–5 | 0 Time | 3 Months | 6 Months | 9 Months | 12 Months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| La | LaSap | La | LaSap | La | LaSap | La | LaSap | La | LaSap | |
| Alopecia/exfoliative dermatitis | 8/12 | 10/12 | 0/12 | 0/12 | 2/12 | 0 | 4/12 | 1/12 | 5/12 | 1/12 |
| Lymphadenopathy | 10/12 | 12/12 | 4/12 | 0/12 | 4/12 | 1/12 | 5/12 | 1/12 | 5/12 | 2/12 |
| Lethargy | 4/12 | 5/12 | 0/12 | 0/12 | 1/12 | 0/12 | 1/12 | 0/12 | 2/12 | 1/12 |
| Cachexia | 3/12 | 4/12 | 0/12 | 0/12 | 1/12 | 0/12 | 1/12 | 0/12 | 1/12 | 0/12 |
| Epistaxis | 2/12 | 2/12 | 0/12 | 0/12 | 1/12 | 0/12 | 2/12 | 0/12 | 2/12 | 2/12 |
| Vasculitis | 6/12 | 6/12 | 3/12 | 0/12 | 3/12 | 0/12 | 4/12 | 1/12 | 5/12 | 2/12 |
| Arthritis | 1/12 | 2/12 | 0/12 | 0/12 | 1/12 | 0/12 | 1/12 | 1/12 | 1/12 | 1/12 |
| Clinical Signs Staging 1–2 | 0 Time | 3 Months | 6 Months | 9 Months | 12 Months | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| La | LaSap | La | LaSap | La | LaSap | La | LaSap | La | LaSap | ||
| Total leukocytes | 12,401 | 8068 | 12,891 | 8914 | 8819 | 10,463 | 10,250 | 10,604 | 8234 | 9214 | 6000–17,000 mm3 |
| Rods | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0–300 |
| Segmented | 7875 | 4228 | 7893 | 5495 | 4704 | 5580 | 4984 | 5833 | 4680 | 5022 | 3000–11,500 |
| Lymphocytes | 3570 | 2579 | 3891 | 2858 | 3072 | 3900 | 3921 | 3682 | 2954 | 3240 | 1000–4800 |
| Monocytes | 767 | 317 | 866 | 371 | 800 | 843 | 1024 | 810 | 498 | 672 | 150–1350 |
| Eosinophils | 222 | 186 | 239 | 188 | 243 | 140 | 321 | 279 | 102 | 280 | 100–1250 |
| Platelets | 346,000 | 315,000 | 345,000 | 329,000 | 322,000 | 354,000 | 319,000 | 377,000 | 320,000 | 350,000 | 200,000–500,000 mm3 |
| AST | 45 | 52 | 37 | 40 | 58 | 44 | 39 | 31 | 64 | 48 | 10–88 U/L |
| ALT | 50 | 42 | 33 | 28 | 57 | 39 | 61 | 46 | 52 | 37 | 10–88 U/L |
| Urea | 22 | 37 | 29 | 23 | 38 | 42 | 30 | 39 | 43 | 38 | 20–50 mg/dL |
| Creatinine | 0.9 | 1.0 | 1.2 | 1.1 | 0.89 | 1.0 | 1.1 | 1.2 | 1.0 | 0.8 | 0.5–1.4 mg/dL |
| Clinical Signs Staging 3–5 | 0 Time | 3 Months | 6 Months | 9 Months | 12 Months | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| La | LaSap | La | LaSap | La | LaSap | La | LaSap | La | LaSap | ||
| Total leukocytes | 7420 | 7968 | 9370 | 10,311 | 7250 | 9230 | 7821 | 10,590 | 6294 | 8760 | 6000–17,000 mm3 |
| Rods | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0–300 |
| Segmented | 4823 | 5557 | 6465 | 7320 | 4640 | 6398 | 4849 | 7200 | 4405 | 5606 | 3000–11,500 |
| Lymphocytes | 1946 | 1788 | 2010 | 2410 | 1620 | 1890 | 1972 | 2287 | 1200 | 2468 | 1000–4800 |
| Monocytes | 319 | 388 | 621 | 300 | 660 | 785 | 840 | 847 | 422 | 398 | 150–1350 |
| Eosinophils | 332 | 238 | 274 | 281 | 330 | 157 | 160 | 256 | 267 | 288 | 100–1250 |
| Platelets | 240,000 | 276,000 | 350,000 | 392,000 | 228,000 | 312,000 | 196,000 | 260,000 | 182,000 | 220,000 | 200,000–500,000 mm3 |
| AST | 65 | 74 | 61 | 65 | 69 | 57 | 76 | 60 | 85 | 63 | 10–88 U/L |
| ALT | 72 | 69 | 59 | 56 | 66 | 55 | 75 | 67 | 73 | 65 | 10–88 U/L |
| Urea | 50 | 52 | 36 | 38 | 40 | 35 | 47 | 44 | 55 | 50 | 20–50 mg/dL |
| Creatinine | 1.6 | 1.7 | 1.3 | 1.1 | 1.4 | 1.2 | 1.4 | 1.1 | 1.5 | 1.4 | 0.5–1.4 mg/dL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, K.F.; Souza, A.B.d.; Torres, S.; Garcia, M.C.E.; Rivas, A.V.; Cangussu, A.S.R.; Guedes da Silva, F.H.; Giunchetti, R.C. Immunomodulation of Patients with Canine Visceral Leishmaniasis at Different Stages: A 12-Month Follow-Up Study Using LaSap. Vaccines 2025, 13, 933. https://doi.org/10.3390/vaccines13090933
Viana KF, Souza ABd, Torres S, Garcia MCE, Rivas AV, Cangussu ASR, Guedes da Silva FH, Giunchetti RC. Immunomodulation of Patients with Canine Visceral Leishmaniasis at Different Stages: A 12-Month Follow-Up Study Using LaSap. Vaccines. 2025; 13(9):933. https://doi.org/10.3390/vaccines13090933
Chicago/Turabian StyleViana, Kelvinson Fernandes, Adrieli Barboza de Souza, Sara Torres, Maria Camila Escobar Garcia, Açucena Veleh Rivas, Alex Sander Rodrigues Cangussu, Francisca Hildemagna Guedes da Silva, and Rodolfo Cordeiro Giunchetti. 2025. "Immunomodulation of Patients with Canine Visceral Leishmaniasis at Different Stages: A 12-Month Follow-Up Study Using LaSap" Vaccines 13, no. 9: 933. https://doi.org/10.3390/vaccines13090933
APA StyleViana, K. F., Souza, A. B. d., Torres, S., Garcia, M. C. E., Rivas, A. V., Cangussu, A. S. R., Guedes da Silva, F. H., & Giunchetti, R. C. (2025). Immunomodulation of Patients with Canine Visceral Leishmaniasis at Different Stages: A 12-Month Follow-Up Study Using LaSap. Vaccines, 13(9), 933. https://doi.org/10.3390/vaccines13090933







