Possible Anti-Pain Vaccines: A Narrative Review of Emerging Strategies and Clinical Prospects
Abstract
1. Introduction
2. Anti-Pain Vaccine Targets
2.1. Nerve Growth Factor (NGF)
2.2. Substance P (SP)
2.3. Calcitonin Gene-Related Peptide (CGRP)
2.4. Transient Receptor Potential Vanilloid-1 (TRPV1)
2.5. Voltage-Gated Sodium Channel Nav1.7 (Nav1.7)
2.6. Adjunct and Co-Therapy Strategies
3. Vaccine Platforms and Adjuvants
3.1. Peptide/Protein Conjugate Vaccines
3.2. Virus-like Particle (VLPs) Vaccines
3.3. DNA and mRNA Vaccines
3.4. Viral Vector Vaccines
3.5. Adjuvants
4. Safety Considerations
4.1. Preclinical Observations
4.2. Autoimmune Responses
4.3. Reversibility
4.4. Off-Target Effects and Cross-Reactivity
4.5. Immune Complex Deposition
4.6. Local and Systemic Reactogenicity
4.7. Safety in Special Populations
5. Regulatory and Ethical Issues
6. Future Directions
7. Public Perception and Education
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BSA | Bovine Serum Albumin |
| CGRP | Calcitonin Gene-Related Peptide |
| CNS | Central Nervous System |
| COVID-19 | Coronavirus Disease 2019 |
| CRPS | Complex Regional Pain Syndrome |
| CRM197 | Cross-Reactive Material 197 |
| DNA | Deoxyribonucleic Acid |
| HIV | Human Immunodeficiency Virus |
| IASP | International Association for the Study of Pain |
| IgG | Immunoglobulin G |
| IL-6 | Interleukin-6 |
| KLH | Keyhole Limpet Hemocyanin |
| mAbs | Monoclonal Antibodies |
| mRNA | Messenger Ribonucleic Acid |
| Nav1.7 | Voltage-Gated Sodium Channel Subtype 1.7 |
| NGF | Nerve Growth Factor |
| NK1 | Neurokinin-1 (Receptor) |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| PRRs | Pattern Recognition Receptors |
| Qβ | Qubevirus durum (a bacteriophage used as a VLP platform) |
| SCN9A | Sodium Voltage-Gated Channel Alpha Subunit 9 |
| SP | Substance P |
| THC | Delta-9-Tetrahydrocannabinol |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor-alpha |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| Tregs | Regulatory T Cells |
| TrkA | Tropomyosin Receptor Kinase A |
| TTX | Tetrodotoxin |
| VLP | Virus-Like Particle |
References
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2022, 163, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Denk, F.; Bennett, D.L.; McMahon, S.B. Nerve growth factor and pain mechanisms. Annu. Rev. Neurosci. 2017, 40, 307–325. [Google Scholar] [CrossRef]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—Successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef]
- Harrison, S.; Geppetti, P. Substance P. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef]
- Sommer, C.; Kress, M. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004, 361, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Kosek, E.; Cohen, M.; Baron, R.; Gebhart, G.F.; Mico, J.-A.; Rice, A.S.C.; Rief, W.; Sluka, K.A. Do we need a third mechanistic descriptor for chronic pain states? Pain 2016, 157, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, B.; Neeb, L.; Reuter, U. Monoclonal antibodies for the prevention of migraine. Expert Opin. Biol. Ther. 2019, 19, 1307–1317. [Google Scholar] [CrossRef]
- Zheng, S.; Hunter, D.J.; Xu, J.; Ding, C. Monoclonal antibodies for the treatment of osteoarthritis. Expert Opin. Biol. Ther. 2016, 16, 1529–1540. [Google Scholar] [CrossRef]
- Pecchi, E.; Priam, S.; Gosset, M.; Pigenet, A.; Sudre, L.; Laiguillon, M.C.; Berenbaum, F.; Houard, X. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: Possible involvement in osteoarthritis pain. Arthritis Res. Ther. 2014, 16, R16. [Google Scholar] [CrossRef]
- Berenbaum, F.; Blanco, F.J.; Guermazi, A.; Miki, K.; Yamabe, T.; Viktrup, L.; Junor, R.; Carey, W.; Brown, M.T.; West, C.R.; et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: Efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period. Ann. Rheum. Dis. 2020, 79, 800–810. [Google Scholar] [CrossRef]
- Lane, N.E.; Schnitzer, T.J.; Birbara, C.A.; Mokhtarani, M.; Shelton, D.L.; Smith, M.D.; Brown, M.T. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 2010, 363, 1521–1531. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Jaffal, S.; Khalil, R. Targeting nerve growth factor for pain relief: Pros and cons. Korean J. Pain 2024, 37, 288–298. [Google Scholar] [CrossRef]
- Balke, I.; Zeltins, A. Recent Advances in the Use of Plant Virus-Like Particles as Vaccines. Viruses 2020, 12, 270. [Google Scholar] [CrossRef]
- Bimonte, S.; Cascella, M.; Forte, C.A.; Esposito, G.; Cuomo, A. The Role of Anti-Nerve Growth Factor Monoclonal Antibodies in the Control of Chronic Cancer and Non-Cancer Pain. J. Pain Res. 2021, 14, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- von Loga, I.S.; El-Turabi, A.; Jostins, L.; Miotla-Zarebska, J.; Mackay-Alderson, J.; Zeltins, A.; Parisi, I.; Bachmann, M.F.; Vincent, T.L. Active immunisation targeting nerve growth factor attenuates chronic pain behaviour in murine osteoarthritis. Ann. Rheum. Dis. 2019, 78, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Bernard, N.J. NGF vaccine reduces pain. Nat. Rev. Rheumatol. 2019, 15, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, Y.X.; Huang, Y.; Guan, R.; Li, R.; Long, S.; Yang, M.; Yu, B.; Wang, G.Q.; Chen, P.; et al. The Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Single Subcutaneous Administration of a Novel Anti-NGF Monoclonal Antibody (AK115) in Healthy Participants: A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation Phase I Clinical Trial. Drug Des. Devel. Ther. 2025, 19, 3225–3235. [Google Scholar] [CrossRef]
- Humes, C.; Sic, A.; Knezevic, N.N. Substance P’s Impact on Chronic Pain and Psychiatric Conditions—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 5905. [Google Scholar] [CrossRef]
- Gonzalez, M.I.; Field, M.J.; Hughes, J.; Singh, L. Evaluation of selective NK1 receptor antagonist CI-1021 in animal models of inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2000, 294, 444–450. [Google Scholar] [CrossRef]
- Dionne, R.A.; Max, M.B.; Gordon, S.M.; Parada, S.; Sang, C.; Gracely, R.H.; Sethna, N.F.; MacLean, D.B. The substance P receptor antagonist CP-99,994 reduces acute postoperative pain. Clin. Pharmacol. Ther. 1998, 64, 562–568. [Google Scholar] [CrossRef]
- Hill, R. NK1 (substance P) receptor antagonists—Why are they not analgesic in humans? Trends Pharmacol. Sci. 2000, 21, 244–246. [Google Scholar] [CrossRef]
- Chizh, B.A.; Gohring, M.; Troster, A.; Quartey, G.K.; Schmelz, M.; Koppert, W. Effects of oral pregabalin and aprepitant on pain and central sensitization in the electrical hyperalgesia model in human volunteers. Br. J. Anaesth. 2007, 98, 246–254. [Google Scholar] [CrossRef]
- Sindrup, S.H.; Graf, A.; Sfikas, N. The NK1-receptor antagonist TKA731 in painful diabetic neuropathy: A randomised, controlled trial. Eur. J. Pain 2006, 10, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.J.; Wang, O.; Saper, J.R.; Stoltz, R.; Silberstein, S.D.; Mathew, N.T. Ineffectiveness of neurokinin-1 antagonist in acute migraine: A crossover study. Cephalalgia 1997, 17, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Barbeito Erba, L.H.; Trias Tejería, E.; Varela Piedra Buena, V.; Semiglia Repetto, G.G.; Filomeno Andriolo, A.E.; Semiglia Alvarez, C. Active Immunization for Reducing Osteoarthritic, Neuropathic, and Cancer Pain. Patent WO2023139542A1, 27 July 2023. [Google Scholar]
- Mehboob, R.; Oehme, P.; Pfaff, G. The role of Substance P in the defense line of the respiratory tract and neurological manifestations post COVID-19 infection. Front. Neurol. 2023, 14, 1052811. [Google Scholar] [CrossRef] [PubMed]
- Wattiez, A.S.; Sowers, L.P.; Russo, A.F. Calcitonin gene-related peptide (CGRP): Role in migraine pathophysiology and therapeutic targeting. Expert Opin. Ther. Targets 2020, 24, 91–100. [Google Scholar] [CrossRef]
- Iyengar, S.; Ossipov, M.H.; Johnson, K.W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 2017, 158, 543–559. [Google Scholar] [CrossRef]
- Tso, A.R.; Goadsby, P.J. Anti-CGRP Monoclonal Antibodies: The Next Era of Migraine Prevention? Curr. Treat. Options Neurol. 2017, 19, 27. [Google Scholar] [CrossRef]
- Dodick, D.W.; Ashina, M.; Brandes, J.L.; Kudrow, D.; Lanteri-Minet, M.; Osipova, V.; Palmer, K.; Picard, H.; Mikol, D.D.; Lenz, R.A. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018, 38, 1026–1037. [Google Scholar] [CrossRef]
- Dodart, J.-C. Migraine Vaccine Shows Comparable Efficacy to Monoclonal Antibodies for Migraine Relief: Jean-Cosme Dodart, PhD. Neurology Live, 2023, May 10. Gale OneFile: Health and Medicine. Available online: https://www.neurologylive.com/view/migraine-vaccine-shows-comparable-efficacy-monoclonal-antibodies-migraine-relief-jean-cosme-dodart (accessed on 25 August 2025).
- Boyd, J.D.; Wang, S.; Lin, H.W.; Hsieh, Y.T.; Sun, Y.S.; Thibodeaux, B.A.; Lu, H.; Sahni, J.; Wiggins, J.; Longo, M.S.; et al. Preclinical characterization of an active immunotherapy targeting calcitonin gene-related peptide. Commun. Med. 2025, 5, 145. [Google Scholar] [CrossRef]
- Messina, R.; Huessler, E.M.; Puledda, F.; Haghdoost, F.; Lebedeva, E.R.; Diener, H.C. Safety and tolerability of monoclonal antibodies targeting the CGRP pathway and gepants in migraine prevention: A systematic review and network meta-analysis. Cephalalgia 2023, 43, 3331024231152169. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, J.J.; Xie, Y.K.; Song, X.Z.; Sun, J.Y.; Zhang, B.L.; Qi, Y.K.; Xu, Z.Z.; Yang, F. Structure-guided peptide engineering of a positive allosteric modulator targeting the outer pore of TRPV1 for long-lasting analgesia. Nat. Commun. 2023, 14, 4. [Google Scholar] [CrossRef]
- Brito, R.; Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. TRPV1: A Potential Drug Target for Treating Various Diseases. Cells 2014, 3, 517–545. [Google Scholar] [CrossRef]
- Mitchell, K.; Lebovitz, E.E.; Keller, J.M.; Mannes, A.J.; Nemenov, M.I.; Iadarola, M.J. Nociception and inflammatory hyperalgesia evaluated in rodents using infrared laser stimulation after Trpv1 gene knockout or resiniferatoxin lesion. Pain 2014, 155, 733–745. [Google Scholar] [CrossRef]
- Iftinca, M.; Defaye, M.; Altier, C. TRPV1-targeted drugs in development for human pain conditions. Drugs 2021, 81, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Awad-Igbaria, Y.; Ben-Menashe, A.; Sakas, R.; Edelman, D.; Fishboom, T.; Shamir, A.; Francois-Soustiel, J.; Palzur, E. Novel insight into TRPV1-induced mitochondrial dysfunction in neuropathic pain. Brain 2025, 148, 2563–2578. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chung, M.K. Striving toward hyperthermia-free analgesia: Lessons from loss-of-function mutations of human TRPV1. J. Clin. Investig. 2023, 133, e167338. [Google Scholar] [CrossRef] [PubMed]
- Do, N.; Zuo, D.; Kim, M.; Kim, M.; Ha, H.J.; Blumberg, P.M.; Ann, J.; Hwang, S.W.; Lee, J. Discovery of Dual TRPA1 and TRPV1 Antagonists as Novel Therapeutic Agents for Pain. Pharmaceuticals 2024, 17, 1209. [Google Scholar] [CrossRef]
- Andrade, A.C.M.; Molina Esquivel, N.; Goldschmied Rossel, F.; Benso, B. TRPV1-target drugs for the treatment of orofacial pain. Front. Pharmacol. 2025, 16, 1568109. [Google Scholar] [CrossRef]
- Moiseenkova-Bell, V.Y.; Stanciu, L.A.; Serysheva, I.I.; Tobe, B.J.; Wensel, T.G. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 7451–7455. [Google Scholar] [CrossRef]
- Ghilardi, J.R.; Freeman, K.T.; Jimenez-Andrade, J.M.; Mantyh, W.G.; Bloom, A.P.; Kuskowski, M.A.; Mantyh, P.W. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer pain. Mol. Pain 2010, 6, 87. [Google Scholar] [CrossRef]
- Plevkova, J.; Poliacek, I.; Antosiewicz, J.; Adamkov, M.; Jakus, J.; Svirlochova, K.; Tatar, M. Intranasal TRPV1 agonist capsaicin challenge and its effect on c-fos expression in the guinea pig brainstem. Respir. Physiol. Neurobiol. 2010, 173, 11–15. [Google Scholar] [CrossRef]
- McDougall, J.J.; O’Brien, M.S. Analgesic potential of voltage gated sodium channel modulators for the management of pain. Curr. Opin. Pharmacol. 2024, 75, 102433. [Google Scholar] [CrossRef]
- Martina, M.; Banderali, U.; Yogi, A.; Arbabi Ghahroudi, M.; Liu, H.; Sulea, T.; Durocher, Y.; Hussack, G.; van Faassen, H.; Chakravarty, B.; et al. A Novel Antigen Design Strategy to Isolate Single-Domain Antibodies that Target Human Nav1.7 and Reduce Pain in Animal Models. Adv. Sci. 2024, 11, e2405432. [Google Scholar] [CrossRef] [PubMed]
- Eagles, D.A.; Chow, C.Y.; King, G.F. Fifteen years of NaV 1.7 channels as an analgesic target: Why has excellent in vitro pharmacology not translated into in vivo analgesic efficacy? Br. J. Pharmacol. 2022, 179, 3592–3611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; McArthur, J.R.; Azam, L.; Bulaj, G.; Olivera, B.M.; French, R.J.; Yoshikami, D. Synergistic and antagonistic interactions between tetrodotoxin and mu-conotoxin in blocking voltage-gated sodium channels. Channels 2009, 3, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Huang, J.; Fan, X.; Wang, K.; Jin, X.; Huang, G.; Li, J.; Pan, X.; Yan, N. Structural mapping of NaV1.7 antagonists. Nat. Commun. 2023, 14, 3224. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, J.; Gong, X.; Liu, D.; Han, Q.; Luo, X.; Chang, W.; Chen, G.; Im, S.T.; Kim, Y.H.; et al. Differential Inhibition of NaV1.7 and Neuropathic Pain by Hybridoma-Produced and Recombinant Monoclonal Antibodies that Target NaV1.7: Differential activities of Nav1.7-targeting monoclonal antibodies. Neurosci. Bull. 2018, 34, 22–41. [Google Scholar] [CrossRef]
- Angelidou, A.; Koster, J.A.; Sherman, A.C.; McLoughlin, C.; Lalwani, P.; Kelly, A.; Saeed, A.; McEnaney, K.; Baden, L.R.; Brogna, M.; et al. Product and trial design considerations on the path towards a vaccine to combat opioid overdose. npj Vaccines 2025, 10, 35. [Google Scholar] [CrossRef]
- Romano, I.G.; Johnson-Weaver, B.; Core, S.B.; Jamus, A.N.; Brackeen, M.; Blough, B.; Dey, S.; Huang, Y.; Staats, H.; Wetsel, W.C.; et al. Two doses of Qbeta virus like particle vaccines elicit protective antibodies against heroin and fentanyl. npj Vaccines 2025, 10, 57. [Google Scholar] [CrossRef]
- Raleigh, M.D.; Baruffaldi, F.; Peterson, S.J.; Le Naour, M.; Harmon, T.M.; Vigliaturo, J.R.; Pentel, P.R.; Pravetoni, M. A Fentanyl Vaccine Alters Fentanyl Distribution and Protects against Fentanyl-Induced Effects in Mice and Rats. J. Pharmacol. Exp. Ther. 2019, 368, 282–291. [Google Scholar] [CrossRef]
- Pravetoni, M.; Pentel, P.R.; Potter, D.N.; Chartoff, E.H.; Tally, L.; LeSage, M.G. Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS ONE 2014, 9, e101807. [Google Scholar] [CrossRef]
- Barrientos, R.C.; Bow, E.W.; Whalen, C.; Torres, O.B.; Sulima, A.; Beck, Z.; Jacobson, A.E.; Rice, K.C.; Matyas, G.R. Novel Vaccine That Blunts Fentanyl Effects and Sequesters Ultrapotent Fentanyl Analogues. Mol. Pharm. 2020, 17, 3447–3460. [Google Scholar] [CrossRef]
- Kosten, T.R. Vaccines as Immunotherapies for Substance Use Disorders. Am. J. Psychiatry 2024, 181, 362–371. [Google Scholar] [CrossRef]
- Hutchings, C.J.; Colussi, P.; Clark, T.G. Ion channels as therapeutic antibody targets. MAbs 2019, 11, 265–296. [Google Scholar] [CrossRef]
- Stephens, A.D.; Wilkinson, T. Discovery of therapeutic antibodies targeting complex multi-spanning membrane proteins. BioDrugs 2024, 38, 769–794. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Lee, J.C.; Blake, S.; Ellis, B.; Eubanks, L.M.; Janda, K.D. Broadly Neutralizing Synthetic Cannabinoid Vaccines. JACS Au 2021, 1, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Eiden, L.E.; Hernandez, V.S.; Jiang, S.Z.; Zhang, L. Neuropeptides and small-molecule amine transmitters: Cooperative signaling in the nervous system. Cell. Mol. Life Sci. 2022, 79, 492. [Google Scholar] [CrossRef]
- Micoli, F.; Adamo, R.; Costantino, P. Protein carriers for glycoconjugate vaccines: History, selection criteria, characterization and new trends. Molecules 2018, 23, 1451. [Google Scholar] [CrossRef]
- Hosztafi, S.; Galambos, A.R.; Koteles, I.; Karadi, D.A.; Furst, S.; Al-Khrasani, M. Opioid-Based Haptens: Development of Immunotherapy. Int. J. Mol. Sci. 2024, 25, 7781. [Google Scholar] [CrossRef]
- Facciola, A.; Visalli, G.; Lagana, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Zepeda-Cervantes, J.; Ramírez-Jarquín, J.O.; Vaca, L. Interaction between virus-like particles (VLPs) and pattern recognition receptors (PRRs) from dendritic cells (DCs): Toward better engineering of VLPs. Front. Immunol. 2020, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Bachmann, M.F. Virus-like particle vaccinology, from bench to bedside. Cell Mol. Immunol. 2022, 19, 993–1011. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Ghasemian, A.; Mahmoodi, S. Nucleic acid-based vaccine platforms against the coronavirus disease 19 (COVID-19). Arch. Microbiol. 2023, 205, 150. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Xu, J.; Wang, B.; Zhao, Z.; Wu, S.; Zhang, Z.; Liu, S.; Huo, N.; Zheng, W.; Chen, Y.; Gao, Z.; et al. Development of a novel adenovirus type 4 vector as a promising respiratory vaccine vehicle. Front. Immunol. 2025, 16, 1572081. [Google Scholar] [CrossRef]
- Li, Y.Z.; Ji, R.R. Gene therapy for chronic pain management. Cell Rep. Med. 2024, 5, 101756. [Google Scholar] [CrossRef]
- Guimaraes, L.E.; Baker, B.; Perricone, C.; Shoenfeld, Y. Vaccines, adjuvants and autoimmunity. Pharmacol. Res. 2015, 100, 190–209. [Google Scholar] [CrossRef]
- Ou, B.S.; Baillet, J.; Filsinger Interrante, M.V.; Adamska, J.Z.; Zhou, X.; Saouaf, O.M.; Yan, J.; Klich, J.H.; Jons, C.K.; Meany, E.L.; et al. Saponin nanoparticle adjuvants incorporating Toll-like receptor agonists drive distinct immune signatures and potent vaccine responses. Sci. Adv. 2024, 10, eadn7187. [Google Scholar] [CrossRef]
- Stone, A.E.; Scheuermann, S.E.; Haile, C.N.; Cuny, G.D.; Velasquez, M.L.; Linhuber, J.P.; Duddupudi, A.L.; Vigliaturo, J.R.; Pravetoni, M.; Kosten, T.A.; et al. Fentanyl conjugate vaccine by injected or mucosal delivery with dmLT or LTA1 adjuvants implicates IgA in protection from drug challenge. NPJ Vaccines 2021, 6, 69. [Google Scholar] [CrossRef]
- Hamid, F.A.; Marker, C.L.; Raleigh, M.D.; Khaimraj, A.; Winston, S.; Pentel, P.R.; Pravetoni, M. Pre-clinical safety and toxicology profile of a candidate vaccine to treat oxycodone use disorder. Vaccine 2022, 40, 3244–3252. [Google Scholar] [CrossRef]
- Raleigh, M.D.; Laudenbach, M.; Baruffaldi, F.; Peterson, S.J.; Roslawski, M.J.; Birnbaum, A.K.; Carroll, F.I.; Runyon, S.P.; Winston, S.; Pentel, P.R. Opioid dose-and route-dependent efficacy of oxycodone and heroin vaccines in rats. J. Pharmacol. Exp. Ther. 2018, 365, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Malerba, F.; Paoletti, F.; Cattaneo, A. NGF and proNGF Reciprocal Interference in Immunoassays: Open Questions, Criticalities, and Ways Forward. Front. Mol. Neurosci. 2016, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ma, D.; Zhu, X.; Wu, Z.; An, Q.; Zhao, J.; Gao, X.; Zhang, L. Roles of TRP and PIEZO receptors in autoimmune diseases. Expert Rev. Mol. Med. 2024, 26, e10. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, A.M.; Donner, A.; Miller, R.E. An update on targets for treating osteoarthritis pain: NGF and TRPV1. Curr. Treat. Options Rheumatol. 2020, 6, 129–145. [Google Scholar] [CrossRef]
- Weiss, J.; Pyrski, M.; Jacobi, E.; Bufe, B.; Willnecker, V.; Schick, B.; Zizzari, P.; Gossage, S.J.; Greer, C.A.; Leinders-Zufall, T. Loss-of-function mutations in sodium channel NaV1.7 cause anosmia. Nature 2011, 472, 186–190. [Google Scholar] [CrossRef]
- Chen, L.; Shao, C.; Li, J.; Zhu, F. Impact of Immunosenescence on vaccine immune responses and countermeasures. Vaccines 2024, 12, 1289. [Google Scholar] [CrossRef]
- Janković, S. Vaccination and autoimmune phenomena. Central Eur. J. Paed. 2017, 13, 12–23. [Google Scholar] [CrossRef]
- Finn, O.J.; Khleif, S.N.; Herberman, R.B. The FDA guidance on therapeutic cancer vaccines: The need for revision to include preventive cancer vaccines or for a new guidance dedicated to them. Cancer Prev. Res. 2015, 8, 1011–1016. [Google Scholar] [CrossRef]
- USA Food and Drug Administration. Clinical Considerations for Therapeutic Cancer Vaccines. 2011. Available online: https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Guidance-for-Industry--Clinical-Considerations-for-Therapeutic-Cancer-Vaccines.pdf (accessed on 13 August 2025).
- Raval, R.R.; Sharabi, A.B.; Walker, A.J.; Drake, C.G.; Sharma, P. Tumor immunology and cancer immunotherapy: Summary of the 2013 SITC primer. J. Immunother. Cancer 2014, 2, 14. [Google Scholar] [CrossRef]
- Rowbotham, M.C.; McDermott, M.P. Ethical considerations in the design, execution, and analysis of clinical trials of chronic pain treatments. Pain Rep. 2018, 4, e646. [Google Scholar] [CrossRef]
- Hodel, K.V.S.; Fiuza, B.S.D.; Conceição, R.S.; Aleluia, A.C.M.; Pitanga, T.N.; Fonseca, L.M.D.S.; Valente, C.O.; Minafra-Rezende, C.S.; Machado, B.A.S. Pharmacovigilance in vaccines: Importance, main aspects, perspectives, and challenges—A narrative review. Pharmaceuticals 2024, 17, 807. [Google Scholar] [CrossRef] [PubMed]
- Wartenweiler, V.; Chung, G.; Stewart, A.; Wenthur, C. Pharmacy stakeholder reports on ethical and logistical considerations in anti-opioid vaccine development. BMC Med. Ethics 2021, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Ladd, J.M.; Ungar, L.D. An Ethical Take on the “Stress Vaccine”. AMA J. Ethics 2012, 14, 60–67. [Google Scholar] [CrossRef]
- Phelps, C.E.; Navratilova, E.; Porreca, F. Cognition in the chronic pain experience: Preclinical insights. Trends Cogn. Sci. 2021, 25, 365–376. [Google Scholar] [CrossRef]
- Jia, T.; Pan, Y.; Li, J.; Wang, L. Strategies for active TNF-alpha vaccination in rheumatoid arthritis treatment. Vaccine 2013, 31, 4063–4068. [Google Scholar] [CrossRef]
- Reis, C.; Chambel, S.; Ferreira, A.; Cruz, C.D. Involvement of nerve growth factor (NGF) in chronic neuropathic pain—A systematic review. Rev. Neurosci. 2023, 34, 75–84. [Google Scholar] [CrossRef]
- Bouhassira, D.; Branders, S.; Attal, N.; Fernandes, A.M.; Demolle, D.; Barbour, J.; Ciampi de Andrade, D.; Pereira, A. Stratification of patients based on the Neuropathic Pain Symptom Inventory: Development and validation of a new algorithm. Pain 2021, 162, 1038–1046. [Google Scholar] [CrossRef]
- Schijns, V.; Majhen, D.; Van Der Ley, P.; Thakur, A.; Summerfield, A.; Berisio, R.; Nativi, C.; Fernández-Tejada, A.; Alvarez-Dominguez, C.; Gizurarson, S. Rational vaccine design in times of emerging diseases: The critical choices of immunological correlates of protection, vaccine antigen and immunomodulation. Pharmaceutics 2021, 13, 501. [Google Scholar] [CrossRef]
- Lamontagne, F.; Khatri, V.; St-Louis, P.; Bourgault, S.; Archambault, D. Vaccination strategies based on bacterial self-assembling proteins as antigen delivery nanoscaffolds. Vaccines 2022, 10, 1920. [Google Scholar] [CrossRef]
- Shirahama, T.; Muroya, D.; Matsueda, S.; Yamada, A.; Shichijo, S.; Naito, M.; Yamashita, T.; Sakamoto, S.; Okuda, K.; Itoh, K.; et al. A randomized phase II trial of personalized peptide vaccine with low dose cyclophosphamide in biliary tract cancer. Cancer Sci. 2017, 108, 838–845. [Google Scholar] [CrossRef]
- Brown, S.A.; Surman, S.L.; Sealy, R.; Jones, B.G.; Slobod, K.S.; Branum, K.; Lockey, T.D.; Howlett, N.; Freiden, P.; Flynn, P.; et al. Heterologous Prime-Boost HIV-1 Vaccination Regimens in Pre-Clinical and Clinical Trials. Viruses 2010, 2, 435–467. [Google Scholar] [CrossRef]
- Dalmia, N.; Ramsay, A.J. Prime-boost approaches to tuberculosis vaccine development. Expert Rev. Vaccines 2012, 11, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huh, Y.; Bortsov, A.; Diatchenko, L.; Ji, R.R. Immunotherapies in chronic pain through modulation of neuroimmune interactions. Pharmacol. Ther. 2023, 248, 108476. [Google Scholar] [CrossRef]
- Furlan, R. A tolerizing mRNA vaccine against autoimmunity? Mol. Ther. 2021, 29, 896–897. [Google Scholar] [CrossRef]
- Urbonaviciute, V.; Romero-Castillo, L.; Xu, B.; Luo, H.; Schneider, N.; Weisse, S.; Do, N.-N.; Oliveira-Coelho, A.; Fernandez Lahore, G.; Li, T. Therapy targeting antigen-specific T cells by a peptide-based tolerizing vaccine against autoimmune arthritis. Proc. Natl. Acad. Sci. USA 2023, 120, e2218668120. [Google Scholar] [CrossRef]
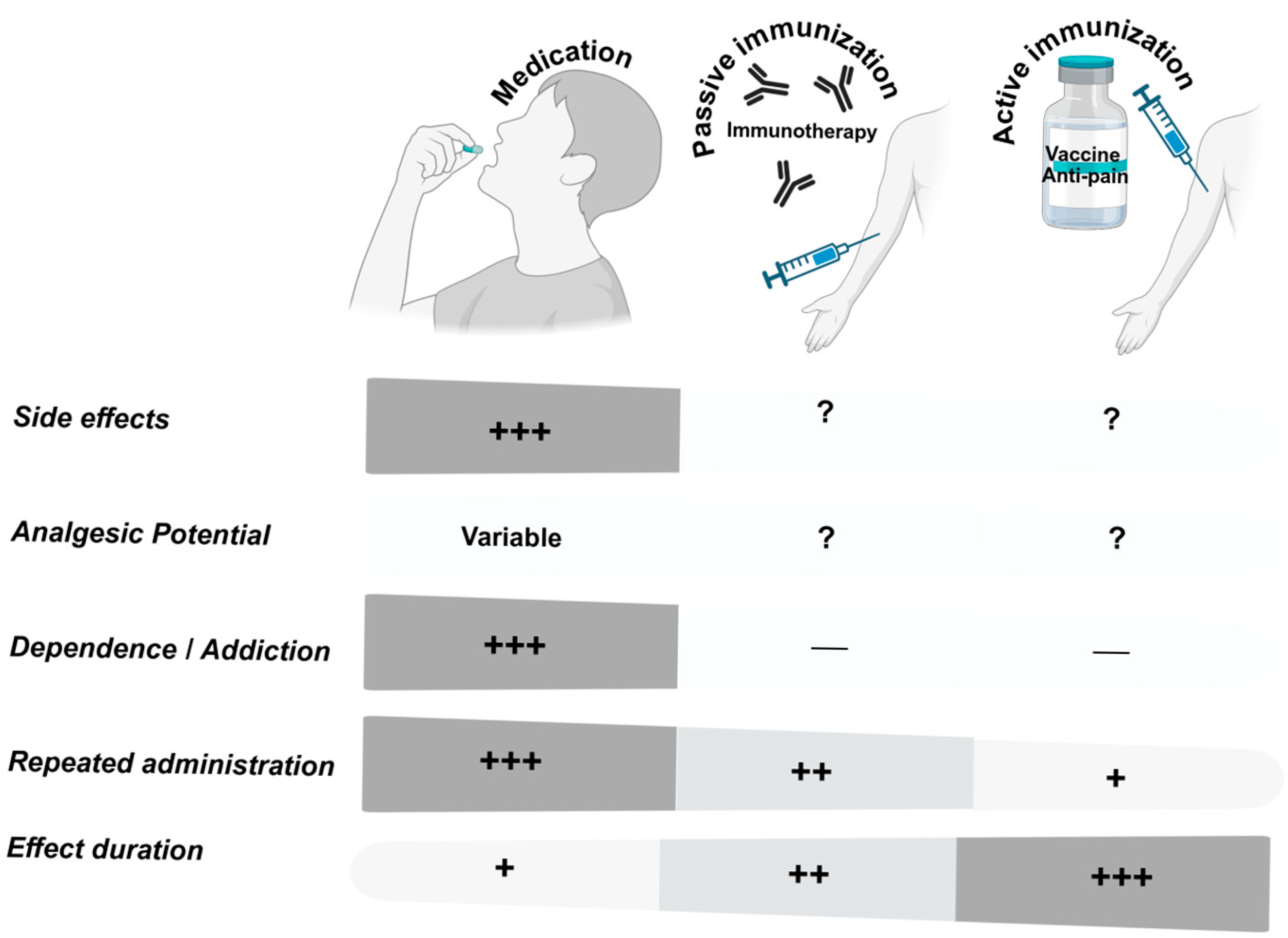
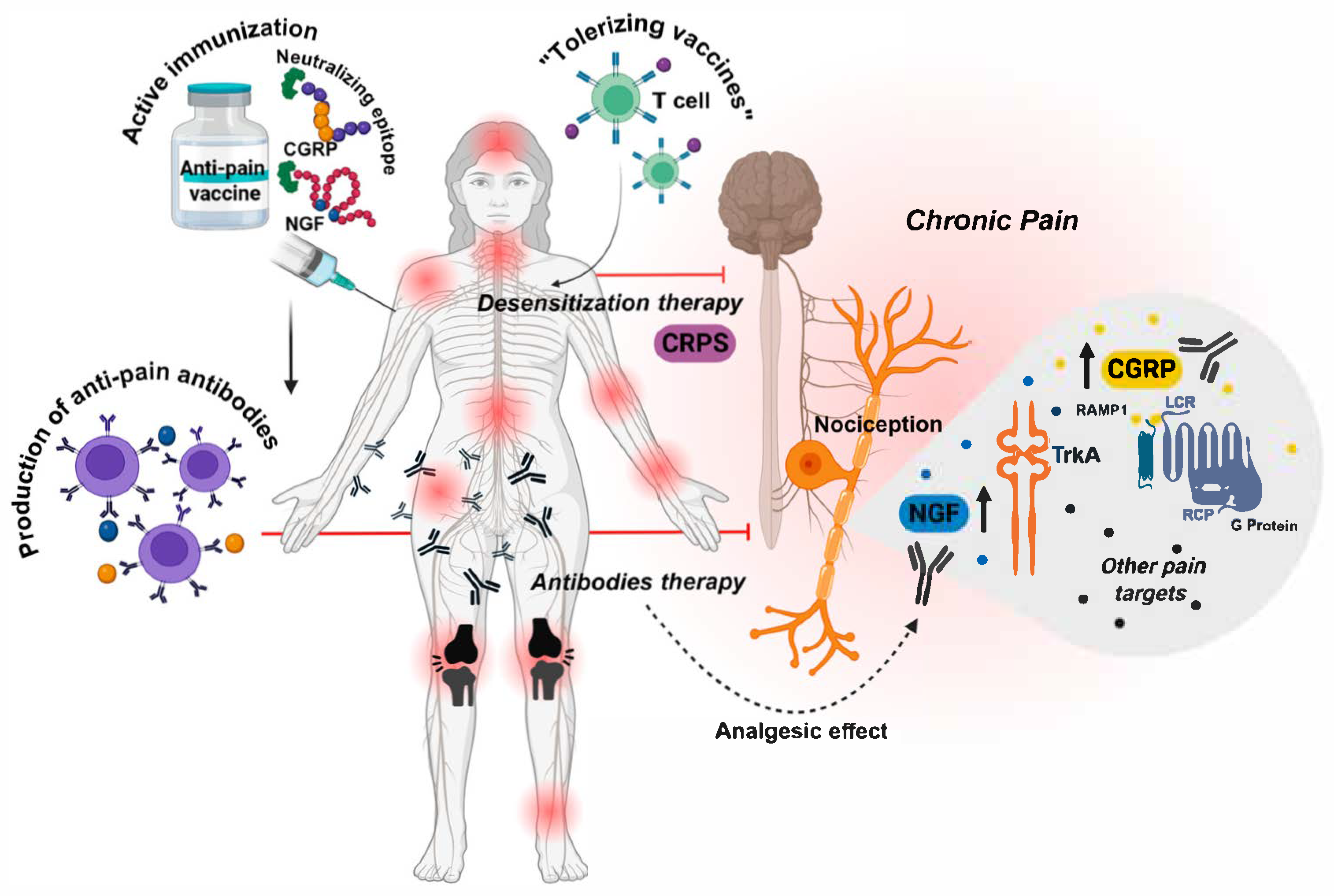
| Target | Preclinical Data | Clinical Data | Safety Issues | Platforms Used | Indications |
|---|---|---|---|---|---|
| NGF | VLP-NGF vaccine in mice showed high anti-NGF antibody titers, reversed pain behaviors in osteoarthritis model, no overt toxicity [23]. | Phase I trial completed; good safety and tolerability; long-term efficacy still unknown [25]. | Potential joint degeneration risk; long-term NGF blockade may affect repair mechanisms [17,86]. | Virus-like particles (VLP) using cucumber mosaic virus [23]. | Osteoarthritis, chronic low-back pain [17,23]. |
| Substance P | Patent-based canine study with dual NGF+SP vaccine; strong antibody response and improved mobility; no peer-reviewed rodent data [32]. | No human data; dual NGF+SP vaccine only tested in dogs (OA) [32]. | Dual-targeting may increase complexity; off-target modulation of SP’s anti-nociceptive roles is a concern [33]. | Recombinant fusion protein vaccine combining SP and NGF for dogs [32]. | Inflammatory/neuropathic pain (e.g., CRPS, fibromyalgia) [26,32]. |
| CGRP | UB-313 vaccine generated high-affinity antibodies in rodents and primates; reduced pain behavior in trigeminal pain models [39]. | UB-313 Phase 1 trial started 2023; interim data shows tolerability and antibody generation; efficacy pending [38]. | Possible cardiovascular effects with long-term CGRP suppression; needs monitoring [40]. | VLP platform UB-313, derived from bacteriophage VLPs [39]. | Migraine (primary); potential for cluster headaches [38]. |
| TRPV1 | No direct TRPV1 vaccine tested; nanobody approaches modulate TRPV1; capsaicin-BSA vaccine raised antibodies in guinea pigs [51]. | No clinical vaccine studies; hyperthermia remains a major safety barrier with TRPV1 targeting [46,47]. | Hyperthermia and sensory disruption due to TRPV1’s thermoregulation role [46,47]. | No vaccine yet; early-stage nanobody/immunogen work; capsaicin-BSA model [51]. | Neuropathic and inflammatory pain syndromes [44]. |
| Nav1.7 | Nanobodies and mAbs reduced pain in rodent models; no vaccine yet; theoretical models propose peptide/domain-specific immunization [53,56]. | No human data; theoretical risks include cross-reactivity and autoimmunity; no vaccine candidates yet [53,77]. | Autoimmunity and anosmia due to Nav1.7’s expression in sensory/olfactory neurons [77]. | Monoclonal/nanobody proof-of-concept; theoretical peptide/domain vaccines [53,56]. | Congenital or acquired pain disorders; chronic inflammatory pain [53]. |
| Opioids | Heroin/fentanyl/oxycodone vaccines showed protection in rodents; high antibody titers blocked analgesic and respiratory effects [59,62]. | Phase 1 oxycodone vaccine showed immunogenicity in OUD patients; reduced drug liking in high responders [63]. | Risk of impeding emergency analgesia; off-target immune complex disease not observed in short term [60,61]. | Hapten-protein conjugates with KLH or VLPs (e.g., QÎ2); alum/MPL adjuvants [59]. | Opioid use disorder; reducing abuse and overdose [58,59]. |
| Cannabinoids | Synthetic cannabinoid vaccines in mice raised antibodies against various analogues; reduced behavioral effects; no pain data [64]. | No clinical studies; potential for public health use in cannabis misuse rather than direct analgesia [64]. | May interfere with therapeutic use of cannabinoids in legitimate medical contexts [64]. | Synthetic cannabinoids conjugated to protein carriers; adjuvanted conjugate vaccines [64]. | Cannabis misuse; public health and addiction contexts [64]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, Y.C.; De-Sousa, L.P.; Murin, P.J.; Sadeghipour, H.; Daniel-Ribeiro, C.T. Possible Anti-Pain Vaccines: A Narrative Review of Emerging Strategies and Clinical Prospects. Vaccines 2025, 13, 909. https://doi.org/10.3390/vaccines13090909
Martins YC, De-Sousa LP, Murin PJ, Sadeghipour H, Daniel-Ribeiro CT. Possible Anti-Pain Vaccines: A Narrative Review of Emerging Strategies and Clinical Prospects. Vaccines. 2025; 13(9):909. https://doi.org/10.3390/vaccines13090909
Chicago/Turabian StyleMartins, Yuri Chaves, Luciana Pereira De-Sousa, Peyton J. Murin, Hamed Sadeghipour, and Cláudio Tadeu Daniel-Ribeiro. 2025. "Possible Anti-Pain Vaccines: A Narrative Review of Emerging Strategies and Clinical Prospects" Vaccines 13, no. 9: 909. https://doi.org/10.3390/vaccines13090909
APA StyleMartins, Y. C., De-Sousa, L. P., Murin, P. J., Sadeghipour, H., & Daniel-Ribeiro, C. T. (2025). Possible Anti-Pain Vaccines: A Narrative Review of Emerging Strategies and Clinical Prospects. Vaccines, 13(9), 909. https://doi.org/10.3390/vaccines13090909






