Oncolytic Herpes Simplex Virus Therapy: Latest Advances, Core Challenges, and Future Outlook
Abstract
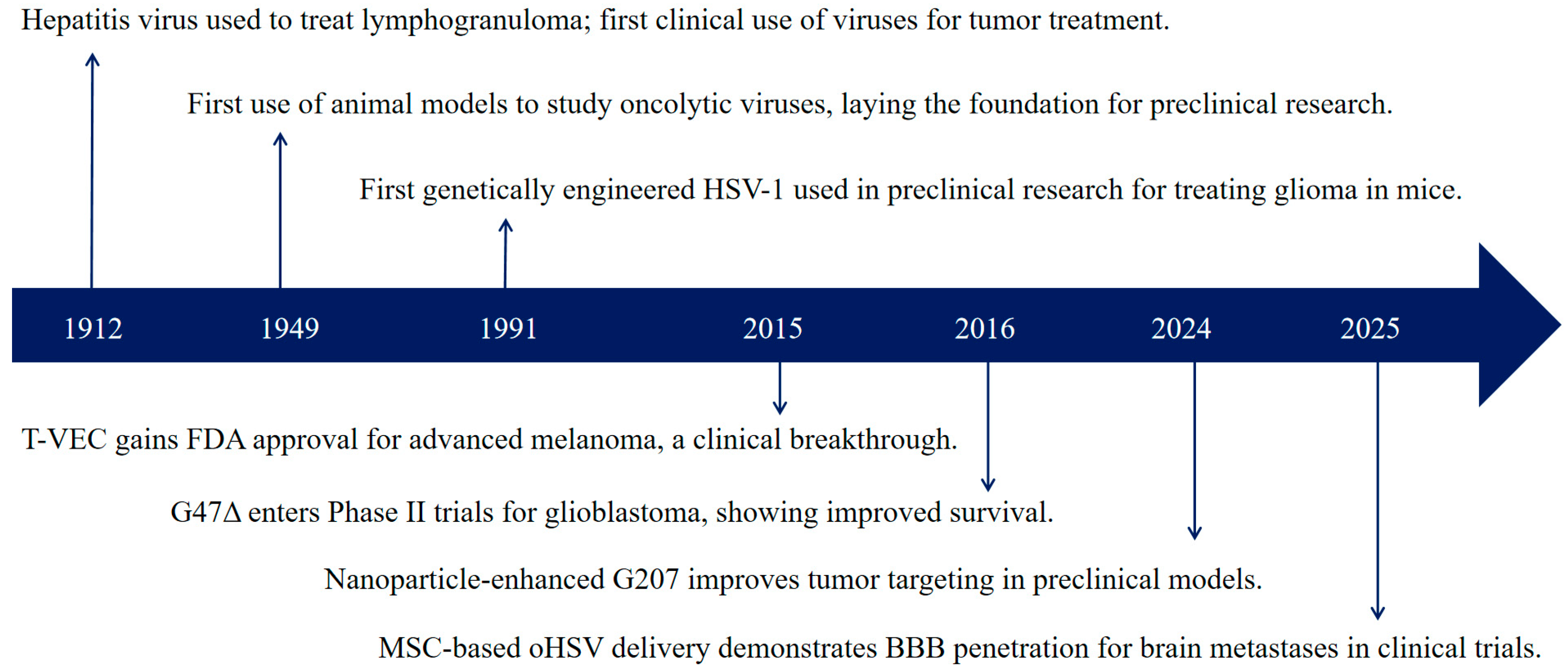
1. Introduction
2. Mechanisms and Advantages of oHSV
2.1. Core Anti-Tumor Mechanisms
2.2. Unique Advantages of HSV
| Type | Virus Type | Genome Size | Transgene Capacity | Infection Receptor | Cell Entry Mechanism | Replication Site | Advantages |
|---|---|---|---|---|---|---|---|
| dsDNA | Herpes Simplex Virus (HSV) [27] | 150 kb | High | HVEM, nectin1, nectin 2 [28] | Endocytosis; Penetration | Nucleus and Cytoplasm | Large genome, high manipulability |
| Adenovirus (Adv) [29] | 36 kb | Medium | CAR, CD46 | Endocytosis | Nucleus and Cytoplasm | Easy to prepare high-titer virus samples; easy genome manipulation | |
| Vaccinia Virus (VV) [30] | 190 kb | High | GAGs, EFC | Membrane fusion; Endocytosis | Cytoplasm | High virus propagation efficiency; short life cycle; allows insertion of large fragments | |
| dsRNA | Respiratory Enteric Orphan Virus (REO Virus) [31] | 16~27 kb | High | JAM-A | Receptor-mediated endocytosis | Cytoplasm | Suitable for intravenous injection; no dose-dependent toxicity |
| ssDNA | Parvovirus [32] | 5 kb | Low | Cyclin A, E2F | Receptor-mediated endocytosis | Nucleus | Tumor tropism, high replication efficiency |
| (+) ssRNA | Coxsackievirus (CVA) [33] | 7.5 kb | Low | CAR, ICAM1, DAF [34] | Micropinocytosis | Cytoplasm | Suitable for intravenous injection |
| Seneca Valley Virus (SVV) [35] | 7 kb | Low | ANTXR1 [36] | Receptor-mediated endocytosis | Cytoplasm | Non-pathogenic to human | |
| Poliovirus (PV) [37] | 7.5 kb | Medium | CD155 | Receptor-mediated endocytosis | Cytoplasm | Infection receptor widely expressed in malignant tumors | |
| (−) ssRNA | Measles Virus (MeV) [38] | 16 kb | Low | SLAM, CD46 | Membrane fusion | Cytoplasm | Tumor tropism |
| Sendai Virus (SeV) [39] | 15 kb | Low | Sialic acid | Membrane fusion | Cytoplasm | Tumor tropism; high safety | |
| Newcastle Disease Virus (NDV) [40] | 15 kb | Low | Sialic acid | Endocytosis; pH-independent fusion | Cytoplasm | Non-pathogenic to humans | |
| Vesicular Stomatitis Virus (VSV) [41] | 11 kb | Low | LDLR | Endocytosis | Cytoplasm | Short life cycle; non-pathogenic to humans |
3. Research Progress
3.1. Preclinical Advances
3.1.1. Cytokine Genes
| Target Gene | Name | Year | Application Method | Tumor Model | ROA | Preclinical Outcome |
|---|---|---|---|---|---|---|
| GM-CSF | OncoVEX mGM-CSF [43] (HSV-1) | 2023 | CTLA-4 and PD-1 antibody; 1 × 106 PFU | B16F10 | i.t. | Reduced lung metastases, prolonged animal survival. |
| RP1-19 [47] (HSV-1) | 2020 | CTLA-4 and PD-1 antibody; 5 × 105 PFU | TBP-B79 | i.t. | Triple combination therapies (PD-1 and CTLA-4 blockade) enhanced antitumor effects. | |
| OH2 [48,49,50,51] (HSV-2) | 2024 | 1 × 106 CCID50/mL | U87, GL261 | i.c. | Reduced tumor growth, prolonged animal survival. | |
| 2022 | 1 × 106, 1 × 105, 1 × 104 CCID50/mL | CT26 | i.t. | Significant antitumor activity and favorable tolerance | ||
| 2022 | SIRPα antibody; 2 × 106 PFU | CT26 | i.t. | Induction of regional cytokine storm (mainly IL-6). | ||
| 2019 | 2 × 107 CCID50/mL | HT-29, CT26 | i.t. | OH2 is safe. | ||
| OX40L | OV-mOX40L [60] (HSV-1) | 2023 | IL-6 and PD-1 antibody; 2 × 106 PFU | KPC | i.t. | Improved immunosuppressive microenvironment. |
| IL-12, IL-15, PD-L1B | VG161 [59,62] (HSV-1) | 2020 | 5 × 105, 5 × 106 PFU | CT26, A20, LS174T | i.t. | Induced robust oncolysis and anti-tumor immune response. |
| 2023 | Paclitaxel; 1 × 107 PFU | EMT-6 | i.t. | Reduced breast cancer growth and metastasis. | ||
| IL-12, IL-15/IL-15Rα | VG2025 [58] (HSV-1) | 2023 | 1 × 106 PFU | A549 | i.t. | Robust antitumor immune response. |
| IL-12/IL-15/GM-CSF/PD-1 antibody/IL-7, CCL19 | oHSV2-IL12, -IL15, -GM-CSF, -PD1v, -IL7 × CCL19 [44] (HSV-2) | 2022 | 1 × 107 PFU | 4T1, CT26 | i.t. | Combination therapy had better anti-tumor effect. |
| IL-12, GM-CSF | Δ6/GM/IL12 [45] (HSV-1) | 2021 | 1 × 107 PFU | B16F10 | i.t. | The anti-tumor immune response was enhanced. |
| R-123 [46] (HSV-1) | 2020 | PD-1 antibody; 1 × 108 PFU | HER2-LLC1 | i.t. | Reduced tumor metastasis. | |
| IL-12 | R-115 [52,53] (HSV-1) | 2018 | 2 × 109 PFU | HER2-LLC1 | i.p. | Improved immunosuppressive microenvironment. |
| 2019 | 2 × 106, 1 × 108 PFU | mHGGpdgf-hHER2 | i.t. | Reduced tumor growth, improved median survival time. | ||
| M002 [54,55,56,57] (HSV-1) | 2017 | 1 × 107 PFU | SARC | i.t. | Improved immunosuppressive microenvironment. | |
| 2018 | 1 × 107 PFU | X21415, D456, GBM-12, UAB1016 | i.t. | Prolonged animal survival. | ||
| 2014 | XRT; 1 × 107 PFU | HuH6, G401, SK-NEP-1 | i.t. | Reduced tumor growth, prolonged animal survival. | ||
| 2013 | XRT; 1 × 107 PFU | SK-N-AS, SK-N-BE, Neuro-2a | i.t. | Reduced tumor growth, prolonged animal survival. | ||
| C5252 [63] (HSV-1) | 2024 | 5 × 106 PFU | U87 | i.t. | Safe antitumor activity. | |
| CXCL11, IL-12 | O-HSV1211 [64] (HSV-1) | 2023 | 1 × 107 PFU | MC38 | i.t. | Reduced tumor growth. |
3.1.2. Tumor Suppressor Genes
3.1.3. Anti-Angiogenic Factor Genes
3.1.4. Tumor Antibody-Associated Genes
3.1.5. Viral Native Genes
3.2. Clinical Advances
3.2.1. Malignant Brain Tumors
3.2.2. Skin and Soft Tissue Sarcomas
3.2.3. Mucosal Epithelial Tumors
4. Technical Challenges and Solutions
4.1. Targeted Delivery
4.2. Administration Routes
4.3. Quality Control
5. Conclusions and Future Directions
5.1. Conclusions
5.2. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Hoster, H.A.; Zanes, R.P., Jr.; Von Haam, E. Studies in Hodgkin’s Syndrome; the Association of Viral Hepatitis and Hodgkin’s Disease; a Preliminary Report. Cancer Res. 1949, 9, 473–480. [Google Scholar]
- Moore, A.E. The Destructive Effect of the Virus of Russian Far East Encephalitis on the Transplantable Mouse Sarcoma 180. Cancer 1949, 2, 525–534. [Google Scholar] [CrossRef]
- Martuza, R.L.; Malick, A.; Markert, J.M.; Ruffner, K.L.; Coen, D.M. Experimental Therapy of Human Glioma by Means of a Genetically Engineered Virus Mutant. Science 1991, 252, 854–856. [Google Scholar] [CrossRef]
- Ledford, H. Cancer-Fighting Viruses Win Approval. Nature 2015, 526, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, X.; Sander, M.; Zhang, H.; Yan, G.; Lin, Y. Oncolytic Viro-Immunotherapy: An Emerging Option in the Treatment of Gliomas. Front. Immunol. 2021, 12, 721830. [Google Scholar] [CrossRef] [PubMed]
- Totsch, S.K.; Ishizuka, A.S.; Kang, K.D.; Gary, S.E.; Rocco, A.; Fan, A.E.; Zhou, L.; Valdes, P.A.; Lee, S.; Li, J.; et al. Combination Immunotherapy with Vaccine and Oncolytic Hsv Virotherapy Is Time Dependent. Mol. Cancer Ther. 2024, 23, 1273–1281. [Google Scholar] [CrossRef]
- Young, C.C.; Narsinh, K.H.; Chen, S.R.; Ansari, S.A.; Hetts, S.W.; Lang, F.F.; Wintermark, M.; Kan, P.T. State of Practice: A Report from the Inaugural Snis Neurointerventional Oncology Summit. AJNR Am. J. Neuroradiol. 2025, ajnr.A8902. [Google Scholar] [CrossRef]
- Wei, D.; Xu, J.; Liu, X.Y.; Chen, Z.N.; Bian, H. Fighting Cancer with Viruses: Oncolytic Virus Therapy in China. Hum. Gene Ther. 2018, 29, 151–159. [Google Scholar] [CrossRef]
- Xia, Z.J.; Chang, J.H.; Zhang, L.; Jiang, W.Q.; Guan, Z.Z.; Liu, J.W.; Zhang, Y.; Hu, X.H.; Wu, G.H.; Wang, H.Q.; et al. Phase Iii Randomized Clinical Trial of Intratumoral Injection of E1b Gene-Deleted Adenovirus (H101) Combined with Cisplatin-Based Chemotherapy in Treating Squamous Cell Cancer of Head and Neck or Esophagus. Ai Zheng 2004, 23, 1666–1670. [Google Scholar] [PubMed]
- Lee, A. Nadofaragene Firadenovec: First Approval. Drugs 2023, 83, 353–357. [Google Scholar] [CrossRef]
- Su, Y.; Su, C.; Qin, L. Current Landscape and Perspective of Oncolytic Viruses and Their Combination Therapies. Transl. Oncol. 2022, 25, 101530. [Google Scholar] [CrossRef]
- Lawler, S.E.; Speranza, M.C.; Cho, C.F.; Chiocca, E.A. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017, 3, 841–849. [Google Scholar] [CrossRef]
- Bronger, H.; Singer, J.; Windmüller, C.; Reuning, U.; Zech, D.; Delbridge, C.; Dorn, J.; Kiechle, M.; Schmalfeldt, B.; Schmitt, M.; et al. Cxcl9 and Cxcl10 Predict Survival and Are Regulated by Cyclooxygenase Inhibition in Advanced Serous Ovarian Cancer. Br. J. Cancer 2016, 115, 553–563. [Google Scholar] [CrossRef]
- Muscolini, M.; Tassone, E.; Hiscott, J. Oncolytic Immunotherapy: Can’t Start a Fire without a Spark. Cytokine Growth Factor Rev. 2020, 56, 94–101. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic Viruses: A New Class of Immunotherapy Drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Tsuji, T.; Nakamori, M.; Iwahashi, M.; Nakamura, M.; Ojima, T.; Iida, T.; Katsuda, M.; Hayata, K.; Ino, Y.; Todo, T.; et al. An Armed Oncolytic Herpes Simplex Virus Expressing Thrombospondin-1 Has an Enhanced in Vivo Antitumor Effect against Human Gastric Cancer. Int. J. Cancer 2013, 132, 485–494. [Google Scholar] [CrossRef]
- Zhang, W.; Fulci, G.; Wakimoto, H.; Cheema, T.A.; Buhrman, J.S.; Jeyaretna, D.S.; Stemmer Rachamimov, A.O.; Rabkin, S.D.; Martuza, R.L. Combination of Oncolytic Herpes Simplex Viruses Armed with Angiostatin and Il-12 Enhances Antitumor Efficacy in Human Glioblastoma Models. Neoplasia 2013, 15, 591–599. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, G.; Nie, X.; Mi, R.; Zhu, G.; Jia, W.; Liu, F. Enhanced Antitumor Efficacy of an Oncolytic Herpes Simplex Virus Expressing an Endostatin-Angiostatin Fusion Gene in Human Glioblastoma Stem Cell Xenografts. PLoS ONE 2014, 9, e95872. [Google Scholar] [CrossRef]
- Goodwin, J.M.; Schmitt, A.D.; McGinn, C.M.; Fuchs, B.C.; Kuruppu, D.; Tanabe, K.K.; Lanuti, M. Angiogenesis Inhibition Using an Oncolytic Herpes Simplex Virus Expressing Endostatin in a Murine Lung Cancer Model. Cancer Investig. 2012, 30, 243–250. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Bell, J.C.; Hwang, T.H.; Kirn, D.H.; Burke, J. The Emerging Therapeutic Potential of the Oncolytic Immunotherapeutic Pexa-Vec (Jx-594). Oncolytic Virother. 2015, 4, 25–31. [Google Scholar] [CrossRef]
- Omarova, S.; Cannon, A.; Weiss, W.; Bruccoleri, A.; Puccio, J. Genital Herpes Simplex Virus-an Updated Review. Adv. Pediatr. 2022, 69, 149–162. [Google Scholar] [CrossRef]
- Ahmad, I.; Wilson, D.W. Hsv-1 Cytoplasmic Envelopment and Egress. Int. J. Mol. Sci. 2020, 21, 5969. [Google Scholar] [CrossRef]
- Roizman, B.; Whitley, R.J. An Inquiry into the Molecular Basis of Hsv Latency and Reactivation. Annu. Rev. Microbiol. 2013, 67, 355–374. [Google Scholar] [CrossRef]
- Corey, L.; Wald, A. Maternal and Neonatal Herpes Simplex Virus Infections. N. Engl. J. Med. 2009, 361, 1376–1385. [Google Scholar] [CrossRef]
- Bailer, S.M.; Funk, C.; Riedl, A.; Ruzsics, Z. Herpesviral Vectors and Their Application in Oncolytic Therapy, Vaccination, and Gene Transfer. Virus Genes 2017, 53, 741–748. [Google Scholar] [CrossRef]
- Chiu, M.; Armstrong, E.J.L.; Jennings, V.; Foo, S.; Crespo-Rodriguez, E.; Bozhanova, G.; Patin, E.C.; McLaughlin, M.; Mansfield, D.; Baker, G.; et al. Combination Therapy with Oncolytic Viruses and Immune Checkpoint Inhibitors. Expert Opin. Biol. Ther. 2020, 20, 635–652. [Google Scholar] [CrossRef]
- Ma, W.; He, H.; Wang, H. Oncolytic Herpes Simplex Virus and Immunotherapy. BMC Immunol. 2018, 19, 40. [Google Scholar] [CrossRef]
- Huang, Y.; Song, Y.; Li, J.; Lv, C.; Chen, Z.S.; Liu, Z. Receptors and Ligands for Herpes Simplex Viruses: Novel Insights for Drug Targeting. Drug Discov. Today 2022, 27, 185–195. [Google Scholar] [CrossRef]
- Hensen, L.C.M.; Hoeben, R.C.; Bots, S.T.F. Adenovirus Receptor Expression in Cancer and Its Multifaceted Role in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2020, 21, 6828. [Google Scholar] [CrossRef]
- Wu, D.; Lou, Y.C.; Chang, W.; Tzou, D.M. Nmr Assignments of Vaccinia Virus Protein A28: An Entry-Fusion Complex Component. Biomol. NMR Assign. 2021, 15, 117–120. [Google Scholar] [CrossRef]
- Danthi, P.; Holm, G.H.; Stehle, T.; Dermody, T.S. Reovirus Receptors, Cell Entry, and Proapoptotic Signaling. Adv. Exp. Med. Biol. 2013, 790, 42–71. [Google Scholar]
- Blechacz, B.; Russell, S.J. Parvovirus Vectors: Use and Optimisation in Cancer Gene Therapy. Expert Rev. Mol. Med. 2004, 6, 1–24. [Google Scholar] [CrossRef]
- Inal, J.M.; Jorfi, S. Coxsackievirus B Transmission and Possible New Roles for Extracellular Vesicles. Biochem. Soc. Trans. 2013, 41, 299–302. [Google Scholar] [CrossRef]
- Selinka, H.C.; Wolde, A.; Sauter, M.; Kandolf, R.; Klingel, K. Virus-Receptor Interactions of Coxsackie B Viruses and Their Putative Influence on Cardiotropism. Med. Microbiol. Immunol. 2004, 193, 127–131. [Google Scholar] [CrossRef]
- Burke, M.J. Oncolytic Seneca Valley Virus: Past Perspectives and Future Directions. Oncolytic Virother. 2016, 5, 81–89. [Google Scholar] [CrossRef]
- Corbett, V.; Hallenbeck, P.; Rychahou, P.; Chauhan, A. Evolving Role of Seneca Valley Virus and Its Biomarker Tem8/Antxr1 in Cancer Therapeutics. Front. Mol. Biosci. 2022, 9, 930207. [Google Scholar] [CrossRef]
- Buijs, P.R.; Verhagen, J.H.; van Eijck, C.H.; van den Hoogen, B.G. Oncolytic Viruses: From Bench to Bedside with a Focus on Safety. Hum. Vaccin. Immunother. 2015, 11, 1573–1584. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Yadava, P.K. Measles Virus: Background and Oncolytic Virotherapy. Biochem. Biophys. Rep. 2018, 13, 58–62. [Google Scholar] [CrossRef]
- Saga, K.; Kaneda, Y. Oncolytic Sendai Virus-Based Virotherapy for Cancer: Recent Advances. Oncolytic Virother. 2015, 4, 141–147. [Google Scholar]
- Huang, F.; Dai, C.; Zhang, Y.; Zhao, Y.; Wang, Y.; Ru, G. Development of Molecular Mechanisms and Their Application on Oncolytic Newcastle Disease Virus in Cancer Therapy. Front. Mol. Biosci. 2022, 9, 889403. [Google Scholar] [CrossRef]
- Ammayappan, A.; Peng, K.W.; Russell, S.J. Characteristics of Oncolytic Vesicular Stomatitis Virus Displaying Tumor-Targeting Ligands. J. Virol. 2013, 87, 13543–13555. [Google Scholar] [CrossRef]
- Dinarello, C.A. Historical Insights into Cytokines. Eur. J. Immunol. 2007, 37 (Suppl. S1), S34–S45. [Google Scholar] [CrossRef]
- Estrada, J.; Zhan, J.; Mitchell, P.; Werner, J.; Beltran, P.J.; DeVoss, J.; Qing, J.; Cooke, K.S. Oncovex(Mgm-Csf)Expands Tumor Antigen-Specific Cd8+ T-Cell Response in Preclinical Models. J. Immunother Cancer 2023, 11, e006374. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, S.; Cai, L.; Duan, H.; Li, Y.; Yang, J.; Wang, Y.; Liu, B.; Dong, S.; Fang, Z.; et al. A Novel Cocktail Therapy Based on Quintuplet Combination of Oncolytic Herpes Simplex Virus-2 Vectors Armed with Interleukin-12, Interleukin-15, Gm-Csf, Pd1v, and Il-7 × Ccl19 Results in Enhanced Antitumor Efficacy. Virol. J. 2022, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Moon, D.; Kong, S.J.; Lee, Y.S.; Yoo, Y.; Kim, S.; Kim, C.; Chon, H.J.; Kim, J.H.; Choi, K.J. Antitumor Effects of Il-12 and Gm-Csf Co-Expressed in an Engineered Oncolytic Hsv-1. Gene Ther. 2021, 28, 186–198. [Google Scholar] [CrossRef]
- De Lucia, M.; Cotugno, G.; Bignone, V.; Garzia, I.; Nocchi, L.; Langone, F.; Petrovic, B.; Sasso, E.; Pepe, S.; Froechlich, G.; et al. Retargeted and Multi-Cytokine-Armed Herpes Virus Is a Potent Cancer Endovaccine for Local and Systemic Anti-Tumor Treatment. Mol. Ther. Oncolytics 2020, 19, 253–264. [Google Scholar] [CrossRef]
- Crespo-Rodriguez, E.; Bergerhoff, K.; Bozhanova, G.; Foo, S.; Patin, E.C.; Whittock, H.; Buus, R.; Haider, S.; Muirhead, G.; Thway, K.; et al. Combining Braf Inhibition with Oncolytic Herpes Simplex Virus Enhances the Immune-Mediated Antitumor Therapy of Braf-Mutant Thyroid Cancer. J. Immunother Cancer 2020, 8, e000698. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Wu, Z.; Hu, H.; Jin, J.; Hu, Y.; Dong, Y.; Zou, J.; Mao, Z.; Shi, X.; et al. Preclinical Safety Evaluation of Oncolytic Herpes Simplex Virus Type 2. Hum. Gene Ther. 2019, 30, 651–660. [Google Scholar] [CrossRef]
- Kong, D.; Yang, Z.; Li, G.; Wu, Q.; Gu, Z.; Wan, D.; Zhang, Q.; Zhang, X.; Cheng, S.; Liu, B.; et al. Sirpα Antibody Combined with Oncolytic Virus Oh2 Protects against Tumours by Activating Innate Immunity and Reprogramming the Tumour Immune Microenvironment. BMC Med. 2022, 20, 376. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Ji, Q.; Fang, A.; Song, L.; Xu, X.; Lin, Y.; Peng, Y.; Yu, J.; Xie, L.; et al. Oh2 Oncolytic Virus: A Novel Approach to Glioblastoma Intervention through Direct Targeting of Tumor Cells and Augmentation of Anti-Tumor Immune Responses. Cancer Lett. 2024, 589, 216834. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Liu, B.; Hu, S.; Guo, F.; Zhong, Y.; Cai, Q.; Zhang, S.; Qian, Y.; Wang, J.; Zhou, F. A Novel Oncolytic Virus Induces a Regional Cytokine Storm and Safely Eliminates Malignant Ascites of Colon Cancer. Cancer Med. 2022, 11, 4297–4309. [Google Scholar] [CrossRef]
- Alessandrini, F.; Menotti, L.; Avitabile, E.; Appolloni, I.; Ceresa, D.; Marubbi, D.; Campadelli-Fiume, G.; Malatesta, P. Eradication of Glioblastoma by Immuno-Virotherapy with a Retargeted Oncolytic Hsv in a Preclinical Model. Oncogene 2019, 38, 4467–4479. [Google Scholar] [CrossRef]
- Leoni, V.; Vannini, A.; Gatta, V.; Rambaldi, J.; Sanapo, M.; Barboni, C.; Zaghini, A.; Nanni, P.; Lollini, P.L.; Casiraghi, C.; et al. A Fully-Virulent Retargeted Oncolytic Hsv Armed with Il-12 Elicits Local Immunity and Vaccine Therapy Towards Distant Tumors. PLoS Pathog. 2018, 14, e1007209. [Google Scholar] [CrossRef]
- Friedman, G.K.; Bernstock, J.D.; Chen, D.; Nan, L.; Moore, B.P.; Kelly, V.M.; Youngblood, S.L.; Langford, C.P.; Han, X.; Ring, E.K.; et al. Enhanced Sensitivity of Patient-Derived Pediatric High-Grade Brain Tumor Xenografts to Oncolytic Hsv-1 Virotherapy Correlates with Nectin-1 Expression. Sci. Rep. 2018, 8, 13930. [Google Scholar] [CrossRef]
- Ring, E.K.; Li, R.; Moore, B.P.; Nan, L.; Kelly, V.M.; Han, X.; Beierle, E.A.; Markert, J.M.; Leavenworth, J.W.; Gillespie, G.Y.; et al. Newly Characterized Murine Undifferentiated Sarcoma Models Sensitive to Virotherapy with Oncolytic Hsv-1 M002. Mol. Ther. Oncolytics 2017, 7, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Megison, M.L.; Gillory, L.A.; Stewart, J.E.; Nabers, H.C.; Mroczek-Musulman, E.; Waters, A.M.; Coleman, J.M.; Kelly, V.; Markert, J.M.; Gillespie, G.Y.; et al. Preclinical Evaluation of Engineered Oncolytic Herpes Simplex Virus for the Treatment of Pediatric Solid Tumors. PLoS ONE 2014, 9, e86843. [Google Scholar] [CrossRef]
- Gillory, L.A.; Megison, M.L.; Stewart, J.E.; Mroczek-Musulman, E.; Nabers, H.C.; Waters, A.M.; Kelly, V.; Coleman, J.M.; Markert, J.M.; Gillespie, G.Y.; et al. Preclinical Evaluation of Engineered Oncolytic Herpes Simplex Virus for the Treatment of Neuroblastoma. PLoS ONE 2013, 8, e77753. [Google Scholar] [CrossRef] [PubMed]
- Chouljenko, D.V.; Murad, Y.M.; Lee, I.F.; Delwar, Z.; Ding, J.; Liu, G.; Liu, X.; Bu, X.; Sun, Y.; Samudio, I.; et al. Targeting Carcinoembryonic Antigen-Expressing Tumors Using a Novel Transcriptional and Translational Dual-Regulated Oncolytic Herpes Simplex Virus Type 1. Mol. Ther. Oncolytics 2023, 28, 334–348. [Google Scholar] [CrossRef]
- Chouljenko, D.V.; Ding, J.; Lee, I.F.; Murad, Y.M.; Bu, X.; Liu, G.; Delwar, Z.; Sun, Y.; Yu, S.; Samudio, I.; et al. Induction of Durable Antitumor Response by a Novel Oncolytic Herpesvirus Expressing Multiple Immunomodulatory Transgenes. Biomedicines 2020, 8, 484. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, F.; Ma, Q.; Du, M.; Wang, H.; Zhu, Y.; Deng, L.; Gao, W.; Wang, C.; Liu, Y.; et al. Ox40l-Armed Oncolytic Virus Boosts T-Cell Response and Remodels Tumor Microenvironment for Pancreatic Cancer Treatment. Theranostics 2023, 13, 4016–4029. [Google Scholar] [CrossRef]
- Rosewell Shaw, A.; Suzuki, M. Oncolytic Viruses Partner with T-Cell Therapy for Solid Tumor Treatment. Front. Immunol. 2018, 9, 2103. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shen, Y.; Yi, M.; Zhang, C.; Zhao, B.; Zhong, G.; Weiyang, L.; Xue, D.; Leng, Q.; Ding, J.; et al. Combination of Novel Oncolytic Herpesvirus with Paclitaxel as an Efficient Strategy for Breast Cancer Therapy. J. Med. Virol. 2023, 95, e28768. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, X.; Chen, X.; Liu, Y.; Huang, Y.; Cheng, Y.; Ren, P.; Zhao, J.; Zhou, G.G. Enhanced Therapeutic Efficacy for Glioblastoma Immunotherapy with an Oncolytic Herpes Simplex Virus Armed with Anti-Pd-1 Antibody and Il-12. Mol. Ther. Oncol. 2024, 32, 200799. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Yu, J.; Wan, Y.; Zhang, C.; Zhang, H.; Cao, Y. Construction of an Il12 and Cxcl11 Armed Oncolytic Herpes Simplex Virus Using the Crispr/Cas9 System for Colon Cancer Treatment. Virus Res. 2023, 323, 198979. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Yu, X.; Li, T.; Xia, Y.; Lei, J.; Wang, Y.; Zhang, L. Herpes Simplex Virus Type 1 Vp22-Mediated Intercellular Delivery of Pten Increases the Antitumor Activity of Pten in Esophageal Squamous Cell Carcinoma Cells in Vitro and in Vivo. Oncol. Rep. 2016, 35, 3034–3040. [Google Scholar] [CrossRef]
- Russell, L.; Swanner, J.; Jaime-Ramirez, A.C.; Wang, Y.; Sprague, A.; Banasavadi-Siddegowda, Y.; Yoo, J.Y.; Sizemore, G.M.; Kladney, R.; Zhang, J.; et al. Pten Expression by an Oncolytic Herpesvirus Directs T-Cell Mediated Tumor Clearance. Nat. Commun. 2018, 9, 5006. [Google Scholar] [CrossRef]
- Sahu, U.; Mullarkey, M.P.; Pei, G.; Zhao, Z.; Hong, B.; Kaur, B. Ohsv-P10 Reduces Glioma Stem Cell Enrichment after Oncolytic Hsv Therapy. Mol. Ther. Oncolytics 2023, 29, 30–41. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Wang, X.; Liu, X.; Ma, Z. Oncolytic Property of Hsv-1 Recombinant Viruses Carrying the P53 Gene. Zhonghua Yi Xue Za Zhi 2016, 96, 370–374. [Google Scholar]
- Nyberg, P.; Xie, L.; Kalluri, R. Endogenous Inhibitors of Angiogenesis. Cancer Res. 2005, 65, 3967–3979. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Gordan, S.; Lux, A. Fcγr Dependent Mechanisms of Cytotoxic, Agonistic, and Neutralizing Antibody Activities. Trends Immunol. 2015, 36, 325–336. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Passaro, C.; Alayo, Q.; De Laura, I.; McNulty, J.; Grauwet, K.; Ito, H.; Bhaskaran, V.; Mineo, M.; Lawler, S.E.; Shah, K.; et al. Arming an Oncolytic Herpes Simplex Virus Type 1 with a Single-Chain Fragment Variable Antibody against Pd-1 for Experimental Glioblastoma Therapy. Clin. Cancer Res. 2019, 25, 290–299. [Google Scholar] [CrossRef]
- Huang, S.; Hu, H.; Tang, G.; Liu, K.; Luo, Z.; Zeng, W. An Oncolytic Herpes Simplex Virus Type 1 Strain Expressing a Single-Chain Variable Region Antibody Fragment against Pd-1 and a Pi3k Inhibitor Synergize to Elicit Antitumor Immunity in Ovarian Cancer. Arch. Virol. 2023, 168, 128. [Google Scholar] [CrossRef]
- Ju, F.; Luo, Y.; Lin, C.; Jia, X.; Xu, Z.; Tian, R.; Lin, Y.; Zhao, M.; Chang, Y.; Huang, X.; et al. Oncolytic Virus Expressing Pd-1 Inhibitors Activates a Collaborative Intratumoral Immune Response to Control Tumor and Synergizes with Ctla-4 or Tim-3 Blockade. J. Immunother Cancer 2022, 10, e004762. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lv, J.; Zhu, W.; Tian, C.; Li, J.; Liu, J.; Zhou, H.; Sun, C.; Hu, Z.; Li, X. The Combination Therapy of Oncolytic Hsv-1 Armed with Anti-Pd-1 Antibody and Il-12 Enhances Anti-Tumor Efficacy. Transl. Oncol. 2022, 15, 101287. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, J.; Zhou, H.; Li, J.; Sun, C.; Zhu, W.; Yin, Y.; Li, X. Enhanced Anti-Tumor Response Elicited by a Novel Oncolytic Hsv-1 Engineered with an Anti-Pd-1 Antibody. Cancer Lett. 2021, 518, 49–58. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, X.; Feng, L.; Yang, Z.; Zhou, L.; Duan, X.; Cheng, S.; Zhang, W.; Liu, B.; Zhang, K. Enhanced Therapeutic Efficacy of a Novel Oncolytic Herpes Simplex Virus Type 2 Encoding an Antibody against Programmed Cell Death 1. Mol. Ther. Oncolytics 2019, 15, 201–213. [Google Scholar] [CrossRef]
- Delwar, Z.; Tatsiy, O.; Chouljenko, D.V.; Lee, I.F.; Liu, G.; Liu, X.; Bu, L.; Ding, J.; Singh, M.; Murad, Y.M.; et al. Prophylactic Vaccination and Intratumoral Boost with Her2-Expressing Oncolytic Herpes Simplex Virus Induces Robust and Persistent Immune Response against Her2-Positive Tumor Cells. Vaccines 2023, 11, 1805. [Google Scholar] [CrossRef]
- Gianni, T.; Leoni, V.; Sanapo, M.; Parenti, F.; Bressanin, D.; Barboni, C.; Zaghini, A.; Campadelli-Fiume, G.; Vannini, A. Genotype of Immunologically Hot or Cold Tumors Determines the Antitumor Immune Response and Efficacy by Fully Virulent Retargeted Ohsv. Viruses 2021, 13, 1747. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Leoni, V.; Sanapo, M.; Gianni, T.; Giordani, G.; Gatta, V.; Barboni, C.; Zaghini, A.; Campadelli-Fiume, G. Immunotherapeutic Efficacy of Retargeted Ohsvs Designed for Propagation in an Ad Hoc Cell Line. Cancers 2021, 13, 266. [Google Scholar] [CrossRef]
- Froechlich, G.; Caiazza, C.; Gentile, C.; D’Alise, A.M.; De Lucia, M.; Langone, F.; Leoni, G.; Cotugno, G.; Scisciola, V.; Nicosia, A.; et al. Integrity of the Antiviral Sting-Mediated DNA Sensing in Tumor Cells Is Required to Sustain the Immunotherapeutic Efficacy of Herpes Simplex Oncolytic Virus. Cancers 2020, 12, 3407. [Google Scholar] [CrossRef]
- Leoni, V.; Petrovic, B.; Gianni, T.; Gatta, V.; Campadelli-Fiume, G. Simultaneous Insertion of Two Ligands in Gd for Cultivation of Oncolytic Herpes Simplex Viruses in Noncancer Cells and Retargeting to Cancer Receptors. J. Virol. 2018, 92, e02132-17. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the Erbb Signalling Network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Mascia, F.; Cataisson, C.; Lee, T.C.; Threadgill, D.; Mariani, V.; Amerio, P.; Chandrasekhara, C.; Souto Adeva, G.; Girolomoni, G.; Yuspa, S.H.; et al. Egfr Regulates the Expression of Keratinocyte-Derived Granulocyte/Macrophage Colony-Stimulating Factor in Vitro and in Vivo. J. Investig. Dermatol. 2010, 130, 682–693. [Google Scholar] [CrossRef]
- Tian, L.; Xu, B.; Chen, Y.; Li, Z.; Wang, J.; Zhang, J.; Ma, R.; Cao, S.; Hu, W.; Chiocca, E.A.; et al. Specific Targeting of Glioblastoma with an Oncolytic Virus Expressing a Cetuximab-Ccl5 Fusion Protein Via Innate and Adaptive Immunity. Nat. Cancer 2022, 3, 1318–1335. [Google Scholar] [CrossRef]
- Appolloni, I.; Alessandrini, F.; Menotti, L.; Avitabile, E.; Marubbi, D.; Piga, N.; Ceresa, D.; Piaggio, F.; Campadelli-Fiume, G.; Malatesta, P. Specificity, Safety, Efficacy of Egfrviii-Retargeted Oncolytic Hsv for Xenotransplanted Human Glioblastoma. Viruses 2021, 13, 1677. [Google Scholar] [CrossRef]
- He, S.; Han, J. Manipulation of Host Cell Death Pathways by Herpes Simplex Virus. Curr. Top. Microbiol. Immunol. 2023, 442, 85–103. [Google Scholar] [PubMed]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral Oncolytic Herpes Virus G47∆ for Residual or Recurrent Glioblastoma: A Phase 2 Trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, P.K.; Wakimoto, H.; Martuza, R.L.; Kaufman, H.L.; Rabkin, S.D.; Saha, D. Oncolytic Herpes Simplex Virus Expressing Il-2 Controls Glioblastoma Growth and Improves Survival. J. Immunother Cancer 2024, 12, e008880. [Google Scholar] [CrossRef]
- Saha, D.; Rabkin, S.D.; Martuza, R.L. Temozolomide Antagonizes Oncolytic Immunovirotherapy in Glioblastoma. J. Immunother Cancer 2020, 8, e000345. [Google Scholar] [CrossRef]
- Ghouse, S.M.; Nguyen, H.M.; Bommareddy, P.K.; Guz-Montgomery, K.; Saha, D. Oncolytic Herpes Simplex Virus Encoding Il12 Controls Triple-Negative Breast Cancer Growth and Metastasis. Front. Oncol. 2020, 10, 384. [Google Scholar] [CrossRef]
- Saha, D.; Wakimoto, H.; Peters, C.W.; Antoszczyk, S.J.; Rabkin, S.D.; Martuza, R.L. Combinatorial Effects of Vegfr Kinase Inhibitor Axitinib and Oncolytic Virotherapy in Mouse and Human Glioblastoma Stem-Like Cell Models. Clin. Cancer Res. 2018, 24, 3409–3422. [Google Scholar] [CrossRef]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Oncolytic Herpes Simplex Virus Immunovirotherapy in Combination with Immune Checkpoint Blockade to Treat Glioblastoma. Immunotherapy 2018, 10, 779–786. [Google Scholar] [CrossRef]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017, 32, 253–267.e255. [Google Scholar] [CrossRef]
- Antoszczyk, S.; Spyra, M.; Mautner, V.F.; Kurtz, A.; Stemmer-Rachamimov, A.O.; Martuza, R.L.; Rabkin, S.D. Treatment of Orthotopic Malignant Peripheral Nerve Sheath Tumors with Oncolytic Herpes Simplex Virus. Neuro Oncol. 2014, 16, 1057–1066. [Google Scholar] [CrossRef]
- Tazzyman, S.; Stewart, G.R.; Yeomans, J.; Linford, A.; Lath, D.; Conner, J.; Muthana, M.; Chantry, A.D.; Lawson, M.A. Hsv1716 Prevents Myeloma Cell Regrowth When Combined with Bortezomib in Vitro and Significantly Reduces Systemic Tumor Growth in Mouse Models. Viruses 2023, 15, 603. [Google Scholar] [CrossRef]
- Bozhanova, G.; Hassan, J.; Appleton, L.; Jennings, V.; Foo, S.; McLaughlin, M.; Chan Wah Hak, C.M.; Patin, E.C.; Crespo-Rodriguez, E.; Baker, G.; et al. Cd4 T Cell Dynamics Shape the Immune Response to Combination Oncolytic Herpes Virus and Braf Inhibitor Therapy for Melanoma. J. Immunother Cancer 2022, 10, e004410. [Google Scholar] [CrossRef]
- Kwan, A.; Winder, N.; Atkinson, E.; Al-Janabi, H.; Allen, R.J.; Hughes, R.; Moamin, M.; Louie, R.; Evans, D.; Hutchinson, M.; et al. Macrophages Mediate the Antitumor Effects of the Oncolytic Virus Hsv1716 in Mammary Tumors. Mol. Cancer Ther. 2021, 20, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Hutzen, B.; Chen, C.Y.; Wang, P.Y.; Sprague, L.; Swain, H.M.; Love, J.; Conner, J.; Boon, L.; Cripe, T.P. Tgf-Β Inhibition Improves Oncolytic Herpes Viroimmunotherapy in Murine Models of Rhabdomyosarcoma. Mol. Ther. Oncolytics 2017, 7, 17–26. [Google Scholar] [CrossRef]
- Currier, M.A.; Sprague, L.; Rizvi, T.A.; Nartker, B.; Chen, C.Y.; Wang, P.Y.; Hutzen, B.J.; Franczek, M.R.; Patel, A.V.; Chaney, K.E.; et al. Aurora a Kinase Inhibition Enhances Oncolytic Herpes Virotherapy through Cytotoxic Synergy and Innate Cellular Immune Modulation. Oncotarget 2017, 8, 17412–17427. [Google Scholar] [CrossRef] [PubMed]
- Cockle, J.V.; Brüning-Richardson, A.; Scott, K.J.; Thompson, J.; Kottke, T.; Morrison, E.; Ismail, A.; Carcaboso, A.M.; Rose, A.; Selby, P.; et al. Oncolytic Herpes Simplex Virus Inhibits Pediatric Brain Tumor Migration and Invasion. Mol. Ther. Oncolytics 2017, 5, 75–86. [Google Scholar] [CrossRef]
- Braidwood, L.; Learmonth, K.; Graham, A.; Conner, J. Potent Efficacy Signals from Systemically Administered Oncolytic Herpes Simplex Virus (Hsv1716) in Hepatocellular Carcinoma Xenograft Models. J. Hepatocell Carcinoma 2014, 1, 149–161. [Google Scholar][Green Version]
- Friedman, G.K.; Moore, B.P.; Nan, L.; Kelly, V.M.; Etminan, T.; Langford, C.P.; Xu, H.; Han, X.; Markert, J.M.; Beierle, E.A.; et al. Pediatric Medulloblastoma Xenografts Including Molecular Subgroup 3 and Cd133+ and Cd15+ Cells Are Sensitive to Killing by Oncolytic Herpes Simplex Viruses. Neuro Oncol. 2016, 18, 227–235. [Google Scholar] [CrossRef]
- Song, T.J.; Haddad, D.; Adusumilli, P.; Kim, T.; Stiles, B.; Hezel, M.; Socci, N.D.; Gönen, M.; Fong, Y. Molecular Network Pathways and Functional Analysis of Tumor Signatures Associated with Development of Resistance to Viral Gene Therapy. Cancer Gene Ther. 2012, 19, 38–48. [Google Scholar] [CrossRef]
- Thomas, S.; Kuncheria, L.; Roulstone, V.; Kyula, J.N.; Mansfield, D.; Bommareddy, P.K.; Smith, H.; Kaufman, H.L.; Harrington, K.J.; Coffin, R.S. Development of a New Fusion-Enhanced Oncolytic Immunotherapy Platform Based on Herpes Simplex Virus Type 1. J. Immunother Cancer 2019, 7, 214. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Li, M.; Su, X.; Tian, Y.; Wang, P.; Zhou, X.; Jin, G.; Liu, F. Oncolytic Hsv-1 Suppresses Cell Invasion through Downregulating Sp1 in Experimental Glioblastoma. Cell. Signal. 2023, 103, 110581. [Google Scholar] [CrossRef]
- Eissa, I.R.; Naoe, Y.; Bustos-Villalobos, I.; Ichinose, T.; Tanaka, M.; Zhiwen, W.; Mukoyama, N.; Morimoto, T.; Miyajima, N.; Hitoki, H.; et al. Genomic Signature of the Natural Oncolytic Herpes Simplex Virus Hf10 and Its Therapeutic Role in Preclinical and Clinical Trials. Front. Oncol. 2017, 7, 149. [Google Scholar] [CrossRef]
- Takano, G.; Esaki, S.; Goshima, F.; Enomoto, A.; Hatano, Y.; Ozaki, H.; Watanabe, T.; Sato, Y.; Kawakita, D.; Murakami, S.; et al. Oncolytic Activity of Naturally Attenuated Herpes-Simplex Virus Hf10 against an Immunocompetent Model of Oral Carcinoma. Mol. Ther. Oncolytics 2021, 20, 220–227. [Google Scholar] [CrossRef]
- Esaki, S.; Goshima, F.; Ozaki, H.; Takano, G.; Hatano, Y.; Kawakita, D.; Ijichi, K.; Watanabe, T.; Sato, Y.; Murata, T.; et al. Oncolytic Activity of Hf10 in Head and Neck Squamous Cell Carcinomas. Cancer Gene Ther. 2020, 27, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ichinose, T.; Naoe, Y.; Matsumura, S.; Villalobos, I.B.; Eissa, I.R.; Yamada, S.; Miyajima, N.; Morimoto, D.; Mukoyama, N.; et al. Combination of Cetuximab and Oncolytic Virus Canerpaturev Synergistically Inhibits Human Colorectal Cancer Growth. Mol. Ther. Oncolytics 2019, 13, 107–115. [Google Scholar] [CrossRef]
- Tanaka, R.; Goshima, F.; Esaki, S.; Sato, Y.; Murata, T.; Nishiyama, Y.; Watanabe, D.; Kimura, H. The Efficacy of Combination Therapy with Oncolytic Herpes Simplex Virus Hf10 and Dacarbazine in a Mouse Melanoma Model. Am. J. Cancer Res. 2017, 7, 1693–1703. [Google Scholar] [PubMed]
- Hotta, Y.; Kasuya, H.; Bustos, I.; Naoe, Y.; Ichinose, T.; Tanaka, M.; Kodera, Y. Curative Effect of Hf10 on Liver and Peritoneal Metastasis Mediated by Host Antitumor Immunity. Oncolytic Virother. 2017, 6, 31–38. [Google Scholar] [PubMed]
- Yamamura, K.; Kasuya, H.; Sahin, T.T.; Tan, G.; Hotta, Y.; Tsurumaru, N.; Fukuda, S.; Kanda, M.; Kobayashi, D.; Tanaka, C.; et al. Combination Treatment of Human Pancreatic Cancer Xenograft Models with the Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Erlotinib and Oncolytic Herpes Simplex Virus Hf10. Ann. Surg. Oncol. 2014, 21, 691–698. [Google Scholar] [CrossRef]
- Liu, T.C.; Wakimoto, H.; Martuza, R.L.; Rabkin, S.D. Herpes Simplex Virus Us3(-) Mutant as Oncolytic Strategy and Synergizes with Phosphatidylinositol 3-Kinase-Akt Targeting Molecular Therapeutics. Clin. Cancer Res. 2007, 13, 5897–5902. [Google Scholar] [CrossRef]
- Nanni, P.; Gatta, V.; Menotti, L.; De Giovanni, C.; Ianzano, M.; Palladini, A.; Grosso, V.; Dall’ora, M.; Croci, S.; Nicoletti, G.; et al. Preclinical Therapy of Disseminated Her-2+ Ovarian and Breast Carcinomas with a Her-2-Retargeted Oncolytic Herpesvirus. PLoS Pathog. 2013, 9, e1003155. [Google Scholar] [CrossRef]
- Zhu, W.; Lv, J.; Xie, X.; Tian, C.; Liu, J.; Zhou, H.; Sun, C.; Li, J.; Hu, Z.; Li, X. The Oncolytic Virus Vt09x Optimizes Immune Checkpoint Therapy in Low Immunogenic Melanoma. Immunol. Lett. 2022, 241, 15–22. [Google Scholar] [CrossRef]
- Workenhe, S.T.; Ketela, T.; Moffat, J.; Cuddington, B.P.; Mossman, K.L. Genome-Wide Lentiviral Shrna Screen Identifies Serine/Arginine-Rich Splicing Factor 2 as a Determinant of Oncolytic Virus Activity in Breast Cancer Cells. Oncogene 2016, 35, 2465–2474. [Google Scholar] [CrossRef]
- Czarnywojtek, A.; Borowska, M.; Dyrka, K.; Van Gool, S.; Sawicka-Gutaj, N.; Moskal, J.; Kościński, J.; Graczyk, P.; Hałas, T.; Lewandowska, A.M.; et al. Glioblastoma Multiforme: The Latest Diagnostics and Treatment Techniques. Pharmacology 2023, 108, 423–431. [Google Scholar] [CrossRef]
- Mineta, T.; Rabkin, S.D.; Yazaki, T.; Hunter, W.D.; Martuza, R.L. Attenuated Multi-Mutated Herpes Simplex Virus-1 for the Treatment of Malignant Gliomas. Nat. Med. 1995, 1, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Fukuhara, H.; Todo, T. Oncolytic Virus Therapy in Japan: Progress in Clinical Trials and Future Perspectives. Jpn J. Clin. Oncol. 2019, 49, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Ino, Y.; Ohtsu, H.; Shibahara, J.; Tanaka, M. A Phase I/Ii Study of Triple-Mutated Oncolytic Herpes Virus G47∆ in Patients with Progressive Glioblastoma. Nat. Commun. 2022, 13, 4119. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Lam, S.S.; Peng, W.; Aghbashlo, M.; Tabatabaei, M.; Guillemin, G.J. Oncolytic Viruses as a Promising Therapeutic Strategy against the Detrimental Health Impacts of Air Pollution: The Case of Glioblastoma Multiforme. Semin. Cancer Biol. 2022, 86, 1122–1142. [Google Scholar] [CrossRef]
- Kambara, H.; Okano, H.; Chiocca, E.A.; Saeki, Y. An Oncolytic Hsv-1 Mutant Expressing Icp34.5 under Control of a Nestin Promoter Increases Survival of Animals Even When Symptomatic from a Brain Tumor. Cancer Res. 2005, 65, 2832–2839. [Google Scholar] [CrossRef]
- Streby, K.A.; Geller, J.I.; Currier, M.A.; Warren, P.S.; Racadio, J.M.; Towbin, A.J.; Vaughan, M.R.; Triplet, M.; Ott-Napier, K.; Dishman, D.J.; et al. Intratumoral Injection of Hsv1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients. Clin. Cancer Res. 2017, 23, 3566–3574. [Google Scholar] [CrossRef]
- Estevez-Ordonez, D.; Chagoya, G.; Salehani, A.; Atchley, T.J.; Laskay, N.M.B.; Parr, M.S.; Elsayed, G.A.; Mahavadi, A.K.; Rahm, S.P.; Friedman, G.K.; et al. Immunovirotherapy for the Treatment of Glioblastoma and Other Malignant Gliomas. Neurosurg. Clin. N. Am. 2021, 32, 265–281. [Google Scholar] [CrossRef]
- Friedman, G.K.; Nan, L.; Haas, M.C.; Kelly, V.M.; Moore, B.P.; Langford, C.P.; Xu, H.; Han, X.; Beierle, E.A.; Markert, J.M.; et al. Γ134.5-Deleted Hsv-1-Expressing Human Cytomegalovirus Irs1 Gene Kills Human Glioblastoma Cells as Efficiently as Wild-Type Hsv-1 in Normoxia or Hypoxia. Gene Ther. 2015, 22, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Bernstock, J.D.; Bag, A.K.; Fiveash, J.; Kachurak, K.; Elsayed, G.; Chagoya, G.; Gessler, F.; Valdes, P.A.; Madan-Swain, A.; Whitley, R.; et al. Design and Rationale for First-in-Human Phase 1 Immunovirotherapy Clinical Trial of Oncolytic Hsv G207 to Treat Malignant Pediatric Cerebellar Brain Tumors. Hum. Gene Ther. 2020, 31, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.C.; Cassady, K.A.; Cody, J.J.; Parker, J.N.; Price, K.H.; Coleman, J.M.; Peggins, J.O.; Noker, P.E.; Powers, N.W.; Grimes, S.D.; et al. Evaluation of the Safety and Biodistribution of M032, an Attenuated Herpes Simplex Virus Type 1 Expressing Hil-12, after Intracerebral Administration to Aotus Nonhuman Primates. Hum. Gene Ther. Clin. Dev. 2014, 25, 16–27. [Google Scholar] [CrossRef]
- Patel, D.M.; Foreman, P.M.; Nabors, L.B.; Riley, K.O.; Gillespie, G.Y.; Markert, J.M. Design of a Phase I Clinical Trial to Evaluate M032, a Genetically Engineered Hsv-1 Expressing Il-12, in Patients with Recurrent/Progressive Glioblastoma Multiforme, Anaplastic Astrocytoma, or Gliosarcoma. Hum. Gene Ther. Clin. Dev. 2016, 27, 69–78. [Google Scholar] [CrossRef]
- Killock, D. Skin Cancer: T-Vec Oncolytic Viral Therapy Shows Promise in Melanoma. Nat. Rev. Clin. Oncol. 2015, 12, 438. [Google Scholar] [CrossRef]
- Poh, A. First Oncolytic Viral Therapy for Melanoma. Cancer Discov. 2016, 6, 6. [Google Scholar] [CrossRef]
- Cavalcante, L.; Chowdhary, A.; Sosman, J.A.; Chandra, S. Combining Tumor Vaccination and Oncolytic Viral Approaches with Checkpoint Inhibitors: Rationale, Pre-Clinical Experience, and Current Clinical Trials in Malignant Melanoma. Am. J. Clin. Dermatol. 2018, 19, 657–670. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kim, D.W.; DeRaffele, G.; Mitcham, J.; Coffin, R.S.; Kim-Schulze, S. Local and Distant Immunity Induced by Intralesional Vaccination with an Oncolytic Herpes Virus Encoding Gm-Csf in Patients with Stage Iiic and Iv Melanoma. Ann. Surg. Oncol. 2010, 17, 718–730. [Google Scholar] [CrossRef]
- Spitler, L.E.; Weber, R.W.; Allen, R.E.; Meyer, J.; Cruickshank, S.; Garbe, E.; Lin, H.Y.; Soong, S.J. Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor (Gm-Csf, Sargramostim) Administered for 3 Years as Adjuvant Therapy of Stages Ii(T4), Iii, and Iv Melanoma. J. Immunother. 2009, 32, 632–637. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.; Ross, M.; Puzanov, I.; Milhem, M.; Collichio, F.; Delman, K.A.; Amatruda, T.; Zager, J.S.; Cranmer, L.; Hsueh, E.; et al. Patterns of Clinical Response with Talimogene Laherparepvec (T-Vec) in Patients with Melanoma Treated in the Optim Phase Iii Clinical Trial. Ann. Surg. Oncol. 2016, 23, 4169–4177. [Google Scholar] [CrossRef]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Randomized, Open-Label Phase Ii Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination with Ipilimumab Versus Ipilimumab Alone in Patients with Advanced, Unresectable Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1658–1667. [Google Scholar] [CrossRef]
- Everett, A.S.; Pavlidakey, P.G.; Contreras, C.M.; De Los Santos, J.F.; Kim, J.Y.; McKee, S.B.; Kaufman, H.L.; Conry, R.M. Chronic Granulomatous Dermatitis Induced by Talimogene Laherparepvec Therapy of Melanoma Metastases. J. Cutan Pathol. 2018, 45, 48–53. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Li, Y.; Zhou, Q.; Yao, R.; Wu, Z.; Hu, H.; Fang, Z.; Dong, S.; Cai, Q.; et al. Nk Cell Tumor Therapy Modulated by Uv-Inactivated Oncolytic Herpes Simplex Virus Type 2 and Checkpoint Inhibitors. Transl. Res. 2022, 240, 64–86. [Google Scholar] [CrossRef]
- Wang, X.; Tian, H.; Chi, Z.; Si, L.; Sheng, X.; Hu, H.; Gu, X.; Li, S.; Li, C.; Lian, B.; et al. Oncolytic Virus Oh2 Extends Survival in Patients with Pd-1 Pretreated Melanoma: Phase Ia/Ib Trial Results and Biomarker Insights. J. Immunother Cancer 2025, 13, e010662. [Google Scholar] [CrossRef]
- Hecht, J.R.; Raman, S.S.; Chan, A.; Kalinsky, K.; Baurain, J.F.; Jimenez, M.M.; Garcia, M.M.; Berger, M.D.; Lauer, U.M.; Khattak, A.; et al. Phase Ib Study of Talimogene Laherparepvec in Combination with Atezolizumab in Patients with Triple Negative Breast Cancer and Colorectal Cancer with Liver Metastases. ESMO Open 2023, 8, 100884. [Google Scholar] [CrossRef]
- Runcie, K.; Bracero, Y.; Samouha, A.; Manji, G.; Remotti, H.E.; Gonda, T.A.; Saenger, Y. Phase I Study of Intratumoral Injection of Talimogene Laherparepvec for the Treatment of Advanced Pancreatic Cancer. Oncologist 2025, 30, oyae200. [Google Scholar] [CrossRef]
- Harrington, K.J.; Kong, A.; Mach, N.; Chesney, J.A.; Fernandez, B.C.; Rischin, D.; Cohen, E.E.W.; Radcliffe, H.S.; Gumuscu, B.; Cheng, J.; et al. Talimogene Laherparepvec and Pembrolizumab in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (Masterkey-232): A Multicenter, Phase 1b Study. Clin. Cancer Res. 2020, 26, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, J.; Tang, J.; Hu, S.; Luo, S.; Luo, Z.; Zhou, F.; Tan, S.; Ying, J.; Chang, Q.; et al. Intratumoral Oh2, an Oncolytic Herpes Simplex Virus 2, in Patients with Advanced Solid Tumors: A Multicenter, Phase I/Ii Clinical Trial. J. Immunother Cancer 2021, 9, e002224. [Google Scholar] [CrossRef] [PubMed]
- Farassati, F.; Yang, A.D.; Lee, P.W. Oncogenes in Ras Signalling Pathway Dictate Host-Cell Permissiveness to Herpes Simplex Virus 1. Nat. Cell Biol. 2001, 3, 745–750. [Google Scholar] [CrossRef]
- Smith, K.D.; Mezhir, J.J.; Bickenbach, K.; Veerapong, J.; Charron, J.; Posner, M.C.; Roizman, B.; Weichselbaum, R.R. Activated Mek Suppresses Activation of Pkr and Enables Efficient Replication and in Vivo Oncolysis by Deltagamma(1)34.5 Mutants of Herpes Simplex Virus 1. J. Virol. 2006, 80, 1110–1120. [Google Scholar] [CrossRef]
- Ye, Z.Q.; Zou, C.L.; Chen, H.B.; Lv, Q.Y.; Wu, R.Q.; Gu, D.N. Folate-Conjugated Herpes Simplex Virus for Retargeting to Tumor Cells. J. Gene Med. 2020, 22, e3177. [Google Scholar] [CrossRef] [PubMed]
- Zottel, A.; Videtič Paska, A.; Jovčevska, I. Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials 2019, 12, 1588. [Google Scholar] [CrossRef]
- Farhat, W.; Yeung, V.; Kahale, F.; Parekh, M.; Cortinas, J.; Chen, L.; Ross, A.E.; Ciolino, J.B. Doxorubicin-Loaded Extracellular Vesicles Enhance Tumor Cell Death in Retinoblastoma. Bioengineering 2022, 9, 671. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First Results on Survival from a Large Phase 3 Clinical Trial of an Autologous Dendritic Cell Vaccine in Newly Diagnosed Glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef]
- Blass, E.; Ott, P.A. Advances in the Development of Personalized Neoantigen-Based Therapeutic Cancer Vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef]
- Howard, F.H.N.; Al-Janabi, H.; Patel, P.; Cox, K.; Smith, E.; Vadakekolathu, J.; Pockley, A.G.; Conner, J.; Nohl, J.F.; Allwood, D.A.; et al. Nanobugs as Drugs: Bacterial Derived Nanomagnets Enhance Tumor Targeting and Oncolytic Activity of Hsv-1 Virus. Small 2022, 18, e2104763. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Ferraris, C.; Cavalli, R.; Panciani, P.P.; Battaglia, L. Overcoming the Blood-Brain Barrier: Successes and Challenges In developing Nanoparticle-Mediated Drug Delivery Systems for the Treatment of Brain Tumours. Int. J. Nanomed. 2020, 15, 2999–3022. [Google Scholar] [CrossRef]
- Ghasemi Darestani, N.; Gilmanova, A.I.; Al-Gazally, M.E.; Zekiy, A.O.; Ansari, M.J.; Zabibah, R.S.; Jawad, M.A.; Al-Shalah, S.A.J.; Rizaev, J.A.; Alnassar, Y.S.; et al. Mesenchymal Stem Cell-Released Oncolytic Virus: An Innovative Strategy for Cancer Treatment. Cell Commun. Signal 2023, 21, 43. [Google Scholar] [CrossRef]
- Ghaleh, H.E.G.; Vakilzadeh, G.; Zahiri, A.; Farzanehpour, M. Investigating the Potential of Oncolytic Viruses for Cancer Treatment Via Msc Delivery. Cell Commun. Signal 2023, 21, 228. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, J.; Li, Y.; Feng, C.; Shao, C.; Shi, Y.; Fang, J. Engineered Mesenchymal Stem/Stromal Cells against Cancer. Cell Death Dis. 2025, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, Z.; Hashemi, M.; Mahdipour, E.; Nourani, H.; Abnous, K.; Ramezani, M. Mesenchymal Stem Cells Engineered by Modified Polyethylenimine Polymer for Targeted Cancer Gene Therapy, in Vitro and in Vivo. Biotechnol. Prog. 2020, 36, e3025. [Google Scholar] [CrossRef]
- Jacobs, S.A.; Roobrouck, V.D.; Verfaillie, C.M.; Van Gool, S.W. Immunological Characteristics of Human Mesenchymal Stem Cells and Multipotent Adult Progenitor Cells. Immunol. Cell Biol. 2013, 91, 32–39. [Google Scholar] [CrossRef]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human Mesenchymal Stem Cells Modulate B-Cell Functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef]
- Franquesa, M.; Hoogduijn, M.J.; Bestard, O.; Grinyó, J.M. Immunomodulatory Effect of Mesenchymal Stem Cells on B Cells. Front. Immunol. 2012, 3, 212. [Google Scholar] [CrossRef]
- Najar, M.; Raicevic, G.; Fayyad-Kazan, H.; Bron, D.; Toungouz, M.; Lagneaux, L. Mesenchymal Stromal Cells and Immunomodulation: A Gathering of Regulatory Immune Cells. Cytotherapy 2016, 18, 160–171. [Google Scholar] [CrossRef]
- Ali, S.; Xia, Q.; Muhammad, T.; Liu, L.; Meng, X.; Bars-Cortina, D.; Khan, A.A.; Huang, Y.; Dong, L. Glioblastoma Therapy: Rationale for a Mesenchymal Stem Cell-Based Vehicle to Carry Recombinant Viruses. Stem Cell Rev. Rep. 2022, 18, 523–543. [Google Scholar] [CrossRef]
- Leoni, V.; Gatta, V.; Palladini, A.; Nicoletti, G.; Ranieri, D.; Dall’Ora, M.; Grosso, V.; Rossi, M.; Alviano, F.; Bonsi, L.; et al. Systemic Delivery of Her2-Retargeted Oncolytic-Hsv by Mesenchymal Stromal Cells Protects from Lung and Brain Metastases. Oncotarget 2015, 6, 34774–34787. [Google Scholar] [CrossRef]
- Mosallaei, M.; Simonian, M.; Ehtesham, N.; Karimzadeh, M.R.; Vatandoost, N.; Negahdari, B.; Salehi, R. Genetically Engineered Mesenchymal Stem Cells: Targeted Delivery of Immunomodulatory Agents for Tumor Eradication. Cancer Gene Ther. 2020, 27, 854–868. [Google Scholar] [CrossRef]
- Duebgen, M.; Martinez-Quintanilla, J.; Tamura, K.; Hingtgen, S.; Redjal, N.; Wakimoto, H.; Shah, K. Stem Cells Loaded with Multimechanistic Oncolytic Herpes Simplex Virus Variants for Brain Tumor Therapy. J. Natl. Cancer Inst. 2014, 106, dju090. [Google Scholar] [CrossRef]
- Du, W.; Seah, I.; Bougazzoul, O.; Choi, G.; Meeth, K.; Bosenberg, M.W.; Wakimoto, H.; Fisher, D.; Shah, K. Stem Cell-Released Oncolytic Herpes Simplex Virus Has Therapeutic Efficacy in Brain Metastatic Melanomas. Proc. Natl. Acad. Sci. USA 2017, 114, E6157–E6165. [Google Scholar] [CrossRef]
- Mahasa, K.J.; de Pillis, L.; Ouifki, R.; Eladdadi, A.; Maini, P.; Yoon, A.R.; Yun, C.O. Mesenchymal Stem Cells Used as Carrier Cells of Oncolytic Adenovirus Results in Enhanced Oncolytic Virotherapy. Sci. Rep. 2020, 10, 425. [Google Scholar] [CrossRef]
- Montoto-Meijide, R.; Meijide-Faílde, R.; Díaz-Prado, S.M.; Montoto-Marqués, A. Mesenchymal Stem Cell Therapy in Traumatic Spinal Cord Injury: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 11719. [Google Scholar] [CrossRef]
- Piekarska, K.; Urban-Wójciuk, Z.; Kurkowiak, M.; Pelikant-Małecka, I.; Schumacher, A.; Sakowska, J.; Spodnik, J.H.; Arcimowicz, Ł.; Zielińska, H.; Tymoniuk, B.; et al. Mesenchymal Stem Cells Transfer Mitochondria to Allogeneic Tregs in an Hla-Dependent Manner Improving Their Immunosuppressive Activity. Nat. Commun. 2022, 13, 856. [Google Scholar] [CrossRef]
- Qi, Z.; Long, X.; Liu, J.; Cheng, P. Glioblastoma Microenvironment and Its Reprogramming by Oncolytic Virotherapy. Front. Cell Neurosci. 2022, 16, 819363. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Shettigar, M.; Kaufman, H.L. Integrating Oncolytic Viruses in Combination Cancer Immunotherapy. Nat. Rev. Immunol. 2018, 18, 498–513. [Google Scholar] [CrossRef]
- Roy, D.G.; Bell, J.C. Cell Carriers for Oncolytic Viruses: Current Challenges and Future Directions. Oncolytic Virother. 2013, 2, 47–56. [Google Scholar]
- Zheng, M.; Huang, J.; Tong, A.; Yang, H. Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol. Ther. Oncolytics 2019, 15, 234–247. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel Co-Option in Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 469–493. [Google Scholar] [CrossRef]
- Huang, D.; Jin, Y.H.; Weng, H.; Huang, Q.; Zeng, X.T.; Wang, X.H. Combination of Intravesical Bacille Calmette-Guérin and Chemotherapy Vs. Bacille Calmette-Guérin Alone in Non-Muscle Invasive Bladder Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 121. [Google Scholar] [CrossRef]
- Goradel, N.H.; Baker, A.T.; Arashkia, A.; Ebrahimi, N.; Ghorghanlu, S.; Negahdari, B. Oncolytic Virotherapy: Challenges and Solutions. Curr. Probl. Cancer 2021, 45, 100639. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Han, D.; Tang, B.; Ma, J. Delivery and Biosafety of Oncolytic Virotherapy. Front. Oncol. 2020, 10, 475. [Google Scholar] [CrossRef]
- Howard, F.; Muthana, M. Designer Nanocarriers for Navigating the Systemic Delivery of Oncolytic Viruses. Nanomedicine 2020, 15, 93–110. [Google Scholar] [CrossRef]
- Rossmeisl, J.H. Novel Treatments for Brain Tumors. Vet. Clin. N. Am. Small Anim. Pract. 2025, 55, 81–94. [Google Scholar] [CrossRef]
- Italiya, K.S.; Mullins-Dansereau, V.; Geoffroy, K.; Gilchrist, V.H.; Alain, T.; Bourgeois-Daigneault, M.C.; Yu, F. Ultrasound and Microbubble Mediated Delivery of Virus-Sensitizing Drugs Improves in Vitro Oncolytic Virotherapy against Breast Cancer Cells. Ultrasound Med. Biol. 2025, 51, 1124–1133. [Google Scholar] [CrossRef]
- Shmulevitz, M.; Gujar, S.A.; Ahn, D.G.; Mohamed, A.; Lee, P.W. Reovirus Variants with Mutations in Genome Segments S1 and L2 Exhibit Enhanced Virion Infectivity and Superior Oncolysis. J. Virol. 2012, 86, 7403–7413. [Google Scholar] [CrossRef]
- Ungerechts, G.; Bossow, S.; Leuchs, B.; Holm, P.S.; Rommelaere, J.; Coffey, M.; Coffin, R.; Bell, J.; Nettelbeck, D.M. Moving Oncolytic Viruses into the Clinic: Clinical-Grade Production, Purification, and Characterization of Diverse Oncolytic Viruses. Mol. Ther. Methods Clin. Dev. 2016, 3, 16018. [Google Scholar] [CrossRef]
- Mendoza, E.J.; Manguiat, K.; Wood, H.; Drebot, M. Two Detailed Plaque Assay Protocols for the Quantification of Infectious Sars-Cov-2. Curr. Protoc. Microbiol. 2020, 57, ecpmc105. [Google Scholar] [CrossRef] [PubMed]
- Gujar, S.; Pol, J.G.; Kumar, V.; Lizarralde-Guerrero, M.; Konda, P.; Kroemer, G.; Bell, J.C. Tutorial: Design, Production and Testing of Oncolytic Viruses for Cancer Immunotherapy. Nat. Protoc. 2024, 19, 2540–2570. [Google Scholar] [CrossRef]
- Onnockx, S.; Baldo, A.; Pauwels, K. Oncolytic Viruses: An Inventory of Shedding Data from Clinical Trials and Elements for the Environmental Risk Assessment. Vaccines 2023, 11, 1448. [Google Scholar] [CrossRef]
- Eisenman, D.; Swindle, S. Fda Guidance on Shedding and Environmental Impact in Clinical Trials Involving Gene Therapy Products. Appl. Biosaf. 2022, 27, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Damerval, M.; Fagnoni-Legat, C.; Louvrier, A.; Fischer, S.; Limat, S.; Clairet, A.L.; Nerich, V.; Madelaine, I.; Kroemer, M. Atmp Environmental Exposure Assessment in European Healthcare Settings: A Systematic Review of the Literature. Front. Med. 2021, 8, 713047. [Google Scholar] [CrossRef]
- Salazar-Fontana, L.I. A Regulatory Risk-Based Approach to Atmp/Cgt Development: Integrating Scientific Challenges with Current Regulatory Expectations. Front. Med. 2022, 9, 855100. [Google Scholar] [CrossRef]
- Chowaniec, H.; Ślubowska, A.; Mroczek, M.; Borowczyk, M.; Braszka, M.; Dworacki, G.; Dobosz, P.; Wichtowski, M. New Hopes for the Breast Cancer Treatment: Perspectives on the Oncolytic Virus Therapy. Front. Immunol. 2024, 15, 1375433. [Google Scholar] [CrossRef]
- Ferguson, M.S.; Lemoine, N.R.; Wang, Y. Systemic Delivery of Oncolytic Viruses: Hopes and Hurdles. Adv. Virol. 2012, 2012, 805629. [Google Scholar] [CrossRef]
- Stoermer, K.A.; Morrison, T.E. Complement and Viral Pathogenesis. Virology 2011, 411, 362–373. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Gao, R.; Lv, S.; Chen, A.; Huang, J.; Wang, L.; Feng, Y.; Feng, J.; Liu, B.; Lei, J.; et al. Cancer Cell Employs a Microenvironmental Neural Signal Trans-Activating Nucleus-Mitochondria Coordination to Acquire Stemness. Signal Transduct. Target Ther. 2023, 8, 275. [Google Scholar] [CrossRef]
- Yun, C.O. Overcoming the Extracellular Matrix Barrier to Improve Intratumoral Spread and Therapeutic Potential of Oncolytic Virotherapy. Curr. Opin. Mol. Ther. 2008, 10, 356–361. [Google Scholar] [PubMed]
- Ebrahimi, S.; Makvandi, M.; Abbasi, S.; Azadmanesh, K.; Teimoori, A. Developing Oncolytic Herpes Simplex Virus Type 1 through Ul39 Knockout by Crispr-Cas9. Iran J. Basic Med. Sci. 2020, 23, 937–944. [Google Scholar]
- Hu, Z.; Liu, W.; Liu, J.; Zhou, H.; Sun, C.; Guo, X.; Zhu, C.; Shao, M.; Wang, S.; Wei, L.; et al. The Anti-Tumor Efficacy of a Recombinant Oncolytic Herpes Simplex Virus Mediated Crispr/Cas9 Delivery Targeting in Hpv16-Positive Cervical Cancer. Antivir. Res. 2024, 232, 106035. [Google Scholar] [CrossRef]

| Drug Name | Year of Approval | Viral Vector | Therapeutic Target | Indications | Company |
|---|---|---|---|---|---|
| Rigvir [8] | 2004 | ECHO virus | / | Melanoma, colorectal cancer | Latima, Riga, Latvia |
| H101 (Oncorine) [9] | 2005 | Human adenovirus-5 | E1B-55kDa, E3-19kDa | Head and neck cancer | Sunway Biotech, Shanghai, China |
| T-VEC (Lmlygic) [4] | 2015 | Herpes simplex virus-1 | Deletion of ICP34.5, ICP47; insertion of hGM-CSF | Advanced melanoma | Amgen, Thousand Oaks, CA, USA |
| G47Δ (Delytact) [5] | 2021 | Herpes simplex virus-1 | Deletion of ICP34.5, ICP47; insertion of LacZ | Malignant glioma, primary brain tumor | Daiichi Sankyo, Tokyo, Japan |
| Adstiladrin [10] | 2022 | Non-replicating adenovirus | Insertion of IFN-α2b | Non-muscle invasive bladder cancer | Ferring, Parsippany, NJ, USA |
| Target Gene | Name | Year | Application Method | Tumor Model | ROA | Preclinical Outcome |
|---|---|---|---|---|---|---|
| PTEN | HSV-P10 [67] (HSV-1) | 2018 | 1 × 105 PFU | DB7 U87ΔEGFR | i.t. | Overcame tumor immune escape. |
| oHSV-P10 [68] (HSV-1) | 2023 | 2 × 105 PFU | GBM-12 005 GSCs | i.t. | Reduced tumor growth. | |
| PTEN-VP22 [66] (HSV-1) | 2016 | 100 μg | Eca-109 | i.t. | Increased the antitumor activity of PTEN. | |
| P53 | MH1004 [69] (HSV-1) | 2016 | 2 × 106 PFU | B16-F10 | i.t. | Reduced tumor growth, prolonged animal survival. |
| Target Gene | Name | Year | Application Method | Tumor Model | ROA | Preclinical Outcome |
|---|---|---|---|---|---|---|
| TSP-1 | T-TSP-1 [16] (HSV-1) | 2013 | 1 × 107 PFU | TMK-1, MKN1 | i.t. | Reduced tumor angiogenesis. |
| Angiostatin | G47Δ-mAngio [17] (HSV-1) | 2013 | G47Δ-mIL12; 1 × 106 PFU | GSCs, U87 | i.t. | Reduced tumor growth. |
| Endostatin | HSV-Endo [19] (HSV-1) | 2012 | 1 × 107 PFU | L1C2 | i.t. | Reduced vascular density, incomplete regression. |
| VAE [18] (HSV-1) | 2014 | 5 × 104 PFU | GBM-SCs | i.t. | Reduced tumor growth. |
| Target Gene | Name [77,78] | Year | Application Method | Tumor Model | ROA | Preclinical Outcome |
|---|---|---|---|---|---|---|
| PD-1 antibody | NG34scFvPD-1 [73,74] (HSV-1) | 2019 | 1.5 × 106 PFU | GL261, CT2A | i.t. | Induced durable antitumor response. |
| 2023 | PI3K inhibitor; 1 × 106 PFU | ID8 | i.t. | Reduced tumor growth, prolonged animal survival. | ||
| YST-OVH [75] (HSV-1) | 2022 | 1 × 107 PFU | Hepa1-6 | i.t. | Antitumor immunity and safe. | |
| HSV-aPD-1 [77] (HSV-1) | 2021 | 1 × 107 PFU | MC38, B16-F10 | i.t. | Reduced tumor growth. | |
| VT1903M [76] (HSV-1) | 2022 | 1 × 107 PFU | CT26 | i.t. | Reduced tumor growth. | |
| oHSV2-aPD1 [78] (HSV-2) | 2019 | 2 × 105 PFU | B16R | i.t. | Induced durable antitumor response. | |
| HER2 antibody | VG22401 [79] (HSV-1) | 2023 | 1 × 107 PFU | CT26 | i.t. | Enhanced antitumor immunity and efficacy. |
| R337 [80] (HSV-1) | 2021 | 1 × 107, 1.5 × 107, 5 × 107 PFU | CT26-HER2 | i.t. | Enhanced antitumor immunity. | |
| R-335 [81] (HSV-1) | 2021 | 1 × 108 PFU | HER2-LLC1 | i.t. | Improved immunosuppressive microenvironment. | |
| R-LM113 [82] (HSV-1) | 2020 | PD-1 antibody; 1 × 108 PFU | HER2-LLC1 | i.t. | Sting provides fundamental contributions to immunotherapeutic efficacy. | |
| R87 [83] (HSV-1) | 2018 | 1 × 108 PFU | HER2-LLC1 | i.t. | Targeted HER2+ cancer cells. | |
| EGFR antibody | OV-Cmab-mCCL5 [86] (HSV-1) | 2022 | 2 × 105 PFU | CT2A-hEGFR | i.t. | Reduced tumor growth, prolonged animal survival. |
| R-613 [87] (HSV-1) | 2021 | 1 × 109 PFU | GBM | i.t. | Increased animal median survival time. |
| Target Gene | Mechanism of Action | Name | Year | Application Method | Tumor Model | ROA | Preclinical Outcome |
|---|---|---|---|---|---|---|---|
| gD (US6) | Binds to HVEM/nectin-1, promotes membrane fusion | R-LM249 [116] (HSV-1) | 2013 | 2 × 107, 1 × 108 PFU | SK-OV-3, MDA-MB-453 | i.p. | Reduced tumor growth, 95% reduction of neoplastic nodules. |
| ICP34.5 (RL1) | Reduces neurotoxicity; enhances tumor infection specificity | HSV1716 [97,98,99,100,101,102,103] (HSV-1) | 2014 | 2 × 106, 1 × 106 PFU | HuH7, HepG2 | i.t. i.v. | Reduced tumor growth, prolonged animal survival. |
| 2017 | 5 × 106 PFU | DIPG | i.t. | Inhibited brain tumor migration and invasion. | |||
| 2017 | A8301; 1 × 108 PFU | RMS | i.t. | Prolonged animal survival, some complete responses. | |||
| 2017 | Alisertib; 1 × 107 PFU | S462TY, SK-N-AS | i.t. | Reduced tumor growth, prolonged animal survival. | |||
| 2021 | 1 × 106 PFU | PyMT-TS1, 4T1, E0771 | i.v. | Reduced tumor growth, prolonged animal survival. | |||
| 2023 | Bortezomib; 1 × 106 PFU | JJN-3, 5TGM1 | i.v. | Lower tumor burden rates, prevented myeloma cell regrowth. | |||
| 2022 | BRAFi; 5 × 105 PFU | 4434, Mel888 | i.t. | Enhanced survival, but cannot fully control tumors. | |||
| G207 [104,105] (HSV-1) | 2012 | 1 × 107 PFU | HT29, PLC5 | i.t. | Provided potential targets to overcome resistance. | ||
| 2016 | 1 × 107 PFU | D425, D341 | i.t. | Pediatric medulloblastoma may be an excellent target. | |||
| ICP34.5, ICP47 (US12); GALV-GP-R− | Enhances viral oncolytic effect, increases immunogenic cell death | Virus 16 [106] (HSV-1) | 2019 | CTLA-4 antibody; 5 × 106 PFU | A20, A549, MDA-MB-231 | i.t. | Reduced tumor growth. |
| ICP34.5, ICP47 (US12) | VT09X [117] (HSV-1) | 2022 | Pembrolizumab; 1 × 107 PFU | B16-F10 | i.t. | Antitumor immune response, prolonged animal survival. | |
| ICP6 (UL39), ICP47, US11 | Promotes viral DNA synthesis; affects MHC-I molecule expression | G47Δ [90,91,92,93,94,95,96] (HSV-1) | 2014 | 3 × 106 PFU | MPNST, S462 | i.t. | Reduced tumor growth, prolonged animal survival. |
| 2017 | PD-1 and CTLA-4 antibody; 5 × 105 PFU | 005 GSCs, CT-2A | i.t. | Prolonged animal survival. | |||
| 2020 | O6-BG, TMZ; 5 × 105 PFU | 005 GSCs | i.t. | Prolonged animal survival. | |||
| 2020 | 2 × 106 PFU | 4T1 | i.t. | Reduced tumor burden and metastasis. | |||
| 2018 | PD-1 and CTLA-4 antibody; 5 × 105 PFU | 005 GSCs | i.t. | Antitumor immune response. | |||
| 2018 | Axitinib; 2.5 × 105 PFU | 005 GSCs, MGG123 | i.t. | Prolonged animal survival. | |||
| 2024 | 5 × 105 PFU | 005 GSCs, CT-2A, GL261 | i.t. | Stimulated antitumor immunity, prolonged median survival. | |||
| VP16 (UL48) | Initiates immediate-early gene transcription | KM100 [118] (HSV-1) | 2016 | 2 × 107 PFU | TUBO | i.t. | Prolonged animal survival. |
| gK (UL53), ICP27 (UL54) | Promotes membrane fusion; affects mRNA splicing | HF10 [109,110,111,112,113,114] (HSV-1) | 2014 | Erlotinib; 1 × 105 PFU | BxPC3, PANC-1 | i.t. | Combination therapy is more effective. |
| 2021 | 1 × 107 PFU | NMOC1 | i.t. | Prolonged animal survival. | |||
| 2017 | 1 × 107 PFU | MC26 | i.t. | Inhibited tumor metastasis. | |||
| 2017 | DTIC; 1 × 107 PFU | clone M3 | i.t. | Induced anti-tumor immune response and prolonged survival. | |||
| 2019 | Cetuximab; 5 × 106 PFU | HT-29 | i.t. | Antitumor immune response, suppressed angiogenesis. | |||
| 2020 | 1.5 × 106 PFU | FaDu, SCC-VII | i.t. | Reduced tumor growth, prolonged animal survival. |
| Name | Target | Indications | Combination Therapy | Phase/Status | ROA | Year | Clinical Trial No. | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|
| HSV1716 | ICP34.5 | Malignant Glioma | / | Phase I/Terminated | i.t. | 2013 | NCT02031965 | Data not reported. |
| C134 | ICP34.5; HCMV-TRS1 | Recurrent Glioblastoma | / | Phase Ib/Active, not recruiting | i.t. | 2024 | NCT06193174 | Clinical studies are ongoing. |
| / | Phase I/Active, not recruiting | i.t. | 2019 | NCT03657576 | ||||
| Malignant Glioma | / | Phase II/Active, not recruiting | i.t. | 2024 | NCT06614855 | |||
| G47Δ | ICP34.5, ICP47; LacZ | Malignant Glioma | / | Phase I-II/Completed | i.t. | 2019 | UMIN000002661 | Median OS 23.3 months, 1-year survival rate 92.3%. |
| / | Phase II/Completed | i.t. | 2020 | UMIN000015995 | ||||
| rQNestin34.5v.2 | ICP34.5, UL39 | Malignant Glioma | / | Phase I/Recruiting | i.t. | 2017 | NCT03152318 | Clinical studies are ongoing. |
| G207 | ICP34.5; LacZ | Malignant Glioma | 5Gy radiotherapy | Phase II/Recruiting | i.t. | 2024 | NCT04482933 | |
| Recurrent Brain Tumor | 5Gy radiotherapy | Phase I/Recruiting | i.t. | 2019 | NCT03911388 | |||
| Phase I/Completed | i.t. | 2020 | NCT02457845 | Median OS 23.3 months. | ||||
| ON-01 | TK, RR, UNG | Malignant Glioma | / | Phase I-II/Completed | i.t. | 2022 | NCT06562621 | Data not reported. |
| MVR-C5252 | ICP34.5; IL-12, PD-1 | Malignant Glioma | / | Phase I/Recruiting | i.t. | 2024 | NCT06126744 | Clinical studies are ongoing. |
| M032 | ICP34.5; IL-12 | Recurrent Malignant Glioma | / | Phase I/Active, not recruiting | i.t. | 2022 | NCT02062827 | Data not reported. |
| Pembrolizumab | Phase I-II/Recruiting | i.t. | 2022 | NCT05084430 | Clinical studies are ongoing. | |||
| OH2 | GM-CSF | Recurrent Glioblastoma | / | Phase I-II/Recruiting | i.t. | 2021 | NCT05235074 |
| Name | Target | Indications | Combination Therapy | Phase/Status | ROA | Year | Clinical Trial No. | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|
| T-VEC | GM-CSF | Melanoma | / | Phase III/Completed | i.t. | 2014 | NCT00769704 | ORR 31.5%, median OS 23.3 months, DRR 19%. |
| EBRT | Phase II/Completed | i.t. | 2016 | NCT02819843 | 0% grade 3 AEs. | |||
| Ipilimumab | Phase II/Completed | i.t. | 2021 | NCT01740297 | ORR 35.7%, median PFS 13.5 months. | |||
| Nivolumab | Phase II/Completed | i.t. | 2020 | NCT04330430 | Pathologic CR 45%. | |||
| Pembrolizumab | Phase II/Recruiting | i.t. | 2019 | NCT03842943 | Clinical studies are ongoing. | |||
| Advanced Soft Tissue Sarcoma | EBRT | Phase I/Recruiting | i.t. | 2024 | NCT06660810 | |||
| Nivolumab | Phase II/Recruiting | i.t. | 2019 | NCT03886311 | ||||
| Non-melanoma Skin Cancer | / | Phase I/Completed | i.t. | 2022 | NCT03458117 | Data not reported. | ||
| ONCR-177 | IL-12, CCL4, FLT3LG, PD-1 and CTLA-4 antibodies | Skin/Subcutaneous Malignancies | Pembrolizumab | Phase I/Terminated | i.t. | 2020 | NCT04348916 | Data not reported. |
| OrienX010 | GM-CSF | Malignant Melanoma | / | Phase I/Unknown | i.t. | 2016 | NCT03048253 | Data not reported. |
| Melanoma | / | Phase I/Completed | i.t. | 2012 | NCT01935453 | |||
| T3011 | IL-12, PD-1 antibody | Non-melanoma Skin Cancer, Sarcoma | / | Phase I-II/Unknown | i.t. | 2020 | NCT05602792 | Results unknown. |
| RP1 | GM-CSF, GALV-GP R- | Melanoma | / | Phase I/Recruiting | i.t. | 2024 | NCT06216938 | Clinical studies are ongoing. |
| Squamous Cell Carcinoma | / | Phase I-II/Recruiting | i.t. | 2023 | NCT05858229 | |||
| Advanced Skin Malignancies | / | Phase I-II/Recruiting | i.t. | 2020 | NCT04349436 | |||
| Non-melanoma Skin Cancer | Nivolumab | Phase II/Recruiting | i.t. | 2017 | NCT03767348 | |||
| RP2 | GM-CSF, GALV-GP R-, CTLA-4 antibody | Metastatic Uveal Melanoma | Nivolumab | Phase II-III/Recruiting | i.t. | 2024 | NCT06581406 | Clinical studies are ongoing. |
| HF10 | UL43, UL49.5, UL55, UL56, LAT | Melanoma | Nivolumab | Phase II/Completed | i.t. | 2018 | NCT03259425 | ORR 83.3%, 14.3% grade 3 AEs. |
| Ipilimumab | Phase II/Completed | i.t. | 2018 | NCT03153085 | Data not reported. | |||
| 2016 | NCT02272855 | |||||||
| / | Phase I/Completed | i.t. | 2015 | NCT01017185 | ||||
| OH2 | GM-CSF | Melanoma | Pembrolizumab | Phase I-II/Recruiting | i.t. | 2018 | NCT04386967 | Clinical studies are ongoing. |
| PD-1 antibody HX008 | Phase I-II/Recruiting | i.t. | 2020 | NCT04616443 | ||||
| / | Phase III/Recruiting | i.t. | 2023 | NCT05868707 | ||||
| Soft Tissue Sarcoma | PD-1 antibody HX008 | Phase I-II/Recruiting | i.t. | 2019 | NCT03866525 | |||
| R130 | CD3 scFv, CD86, PD-1, HSV2-US11 | Melanoma | / | Phase I/Recruiting | i.t. | 2023 | NCT05961111 NCT06171282 | Clinical studies are ongoing. |
| Advanced Bone/Soft Tissue Tumors | / | Phase I/Recruiting | i.t. | 2023 | ||||
| NCT05851456 | ||||||||
| Sarcoma | / | Phase I/Recruiting | i.t. | 2023 | NCT05860374 | |||
| KB707 | IL-2, IL-12 | Melanoma | / | Phase I-II/Recruiting | i.t. | 2023 | NCT05970497 | |
| HSV1716 | ICP34.5 | Sarcoma | / | Phase I/Completed | i.t. | 2018 | NCT00931931 | Data not reported. |
| Indications | Name | Target | Combination Therapy | Phase/Status | ROA | Year | Clinical Trial No. | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|
| Respiratory System | ||||||||
| Head and Neck Cancer | T3011 | IL-12, PD-1 antibody | / | Phase I–II/Unknown | i.t. | 2020 | NCT05602792 | Results unknown. |
| T-VEC | GM-CSF | Pembrolizumab | Phase I/Completed | i.t. | 2017 | NCT02626000 | Median PFS 3.0 months, OS 5.8 months. | |
| HF10 | UL43, UL49.5, UL55, UL56, LAT | / | Phase I/Completed | i.t. | 2015 | NCT01017185 | Data not reported. | |
| OH2 | GM-CSF | PD-1 antibody HX008 | Phase I–II/Recruiting | i.t. | 2019 | NCT03866525 | Clinical studies are ongoing. | |
| R130 | CD3 scFv, CD86, PD-1, HSV2-US11 | / | Phase I/Recruiting | i.t. | 2023 | NCT05961111 | ||
| / | Phase I/Unknown | i.t. | 2023 | NCT05886075 | Results unknown. | |||
| / | Phase I/Recruiting | i.t. | 2023 | NCT05830240 | Clinical studies are ongoing. | |||
| Lung Cancer | OrienX010 | GM-CSF | / | Phase I/Completed | i.t. | 2012 | NCT01935453 | Data not reported. |
| T3011 | IL-12, PD-1 antibody | / | Phase I–II/Unknown | i.v. | 2022 | NCT05598268 | Results unknown. | |
| RP2 | GM-CSF, GALV-GP R-, CTLA-4 antibody | Nivolumab | Phase I/Recruiting | i.t. | 2019 | NCT04336241 | Clinical studies are ongoing. | |
| R130 | CD3 scFv, CD86, PD-1, HSV2-US11 | / | Phase I/Recruiting | i.t. | 2023 | NCT05961111 | ||
| / | Phase I/Unknown | i.t. | 2023 | NCT05886075 | Results unknown. | |||
| KB707 | IL-2, IL-12 | / | Phase I–II/Recruiting | nebulization | 2024 | NCT06228326 | Clinical studies are ongoing. | |
| Pleural Mesothelioma | HSV1716 | ICP34.5 | / | Phase I–II/Completed | i.p. | 2016 | NCT01721018 | Data not reported. |
| Digestive System | ||||||||
| Gastric Cancer | VG161 | IL-12, IL-15, PD-L1B | Nivolumab | Phase I–II/Recruiting | i.t. | 2022 | NCT06008925 | Clinical studies are ongoing. |
| OH2 | GM-CSF | PD-1 antibody HX008 | Phase I–II/Recruiting | i.t. | 2019 | NCT03866525 | ||
| Liver Cancer | OrienX010 | GM-CSF | / | Phase I/Completed | i.t. | 2012 | NCT01935453 | Data not reported. |
| T3011 | IL-12 and PD-1 antibody | / | Phase I–II/Unknown | i.v. | 2022 | NCT05598268 | Results unknown. | |
| T-VEC | GM-CSF | / | Phase I/Completed | i.t. | 2018 | NCT03256344 | ORR 10%, PFS 5.4 months, OS 19.2 months. | |
| RP2 | GM-CSF, GALV-GP R-, CTLA-4 antibody | Nivolumab | Phase I/Recruiting | i.t. | 2019 | NCT04336241 | Clinical studies are ongoing. | |
| Atezolizumab and Bevacizumab | Phase II/Recruiting | i.t. | 2024 | NCT05733598 | ||||
| VG161 | IL-12, IL-15, PD-L1B | Camrelizumab | Phase I-II/Not yet recruiting | i.t. | 2023 | NCT06124001 | ||
| / | Phase I/Recruiting | i.t. | 2021 | NCT04806464 | ||||
| R130 | CD3 scFv, CD86, PD-1, HSV2-US11 | / | Phase I/Recruiting | i.t. | 2023 | NCT05860374 | ||
| Pancreatic Cancer | OrienX010 | GM-CSF | / | Phase I/Completed | i.t. | 2012 | NCT01935453 | Data not reported. |
| HF10 | UL43, UL49.5, UL55, UL56, LAT | Gemcitabine + Paclitaxel | Phase I/Active, not recruiting | i.t. | 2020 | NCT03252808 | Clinical studies are ongoing. | |
| Erlotinib + Gemcitabine | Phase I/Completed | i.t. | 2018 | UMIN000010150 | Median PFS 6.3 months, median OS 15.5 months. | |||
| T-VEC | GM-CSF | / | Phase I/Completed | i.t. | 2017 | NCT03086642 | Median OS 7.8 months. | |
| VG161 | IL-12, IL-15, PD-L1B | Nivolumab | Phase I-II/Recruiting | i.t. | 2022 | NCT05162118 | Clinical studies are ongoing. | |
| OH2 | GM-CSF | / | Phase I-II/Terminated | i.t. | 2021 | NCT04637698 | Results unknown. | |
| Colorectal Cancer | ONCR-177 | IL-12, CCL4, FLT3LG, PD-1 and CTLA-4 antibody | Pembrolizumab | Phase I/Terminated | i.t. | 2020 | NCT04348916 | Results unknown. |
| T3011 | IL-12 and PD-1 antibody | Toripalimab + Regorafenib | Phase I/Recruiting | i.v. | 2024 | NCT06283303 | Clinical studies are ongoing. | |
| Regorafenib | Phase I/Recruiting | i.v. | 2023 | NCT06200363 | ||||
| R130 | CD3 scFv, CD86, PD-1, HSV2-US11 | / | Phase I/Recruiting | i.t. | 2023 | NCT05860374 | ||
| Urogenital System | ||||||||
| Bladder Cancer | T3011 | IL-12 and PD-1 antibody | / | Phase I/Recruiting | bid. | 2023 | NCT06427291 | Clinical studies are ongoing. |
| OH2 | GM-CSF | / | Phase I-II/Recruiting | bid. | 2022 | NCT05232136 | ||
| / | Phase II/Recruiting | i.t. | 2022 | NCT05248789 | ||||
| Ovarian Cancer | R130 | CD3 scFv, CD86, PD-1, HSV2-US11 | / | Phase I/Recruiting | i.t. or i.v. | 2022 | NCT05801783 | |
| Cervical Cancer | / | Phase I/Recruiting | i.t. or i.v. | 2023 | NCT05812677 | |||
| BS-006 | CD3 and PD-L1 antibod | / | Phase I/Recruiting | i.t. | 2022 | NCT05393440 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Pei, Y.; Dong, C.; Liang, J.; Cai, T.; Zhang, Y.; Tan, D.; Wang, J.; He, Q. Oncolytic Herpes Simplex Virus Therapy: Latest Advances, Core Challenges, and Future Outlook. Vaccines 2025, 13, 880. https://doi.org/10.3390/vaccines13080880
Zheng Y, Pei Y, Dong C, Liang J, Cai T, Zhang Y, Tan D, Wang J, He Q. Oncolytic Herpes Simplex Virus Therapy: Latest Advances, Core Challenges, and Future Outlook. Vaccines. 2025; 13(8):880. https://doi.org/10.3390/vaccines13080880
Chicago/Turabian StyleZheng, Yiyang, Yusheng Pei, Chunyan Dong, Jinghui Liang, Tong Cai, Yuan Zhang, Dejiang Tan, Junzhi Wang, and Qing He. 2025. "Oncolytic Herpes Simplex Virus Therapy: Latest Advances, Core Challenges, and Future Outlook" Vaccines 13, no. 8: 880. https://doi.org/10.3390/vaccines13080880
APA StyleZheng, Y., Pei, Y., Dong, C., Liang, J., Cai, T., Zhang, Y., Tan, D., Wang, J., & He, Q. (2025). Oncolytic Herpes Simplex Virus Therapy: Latest Advances, Core Challenges, and Future Outlook. Vaccines, 13(8), 880. https://doi.org/10.3390/vaccines13080880






