Safety and Immunogenicity of a 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) in Chinese Children, Adults and the Elderly: A Phase 4, Randomized, Double-Blind, Active-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Randomization and Masking
2.3. Procedures
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Population
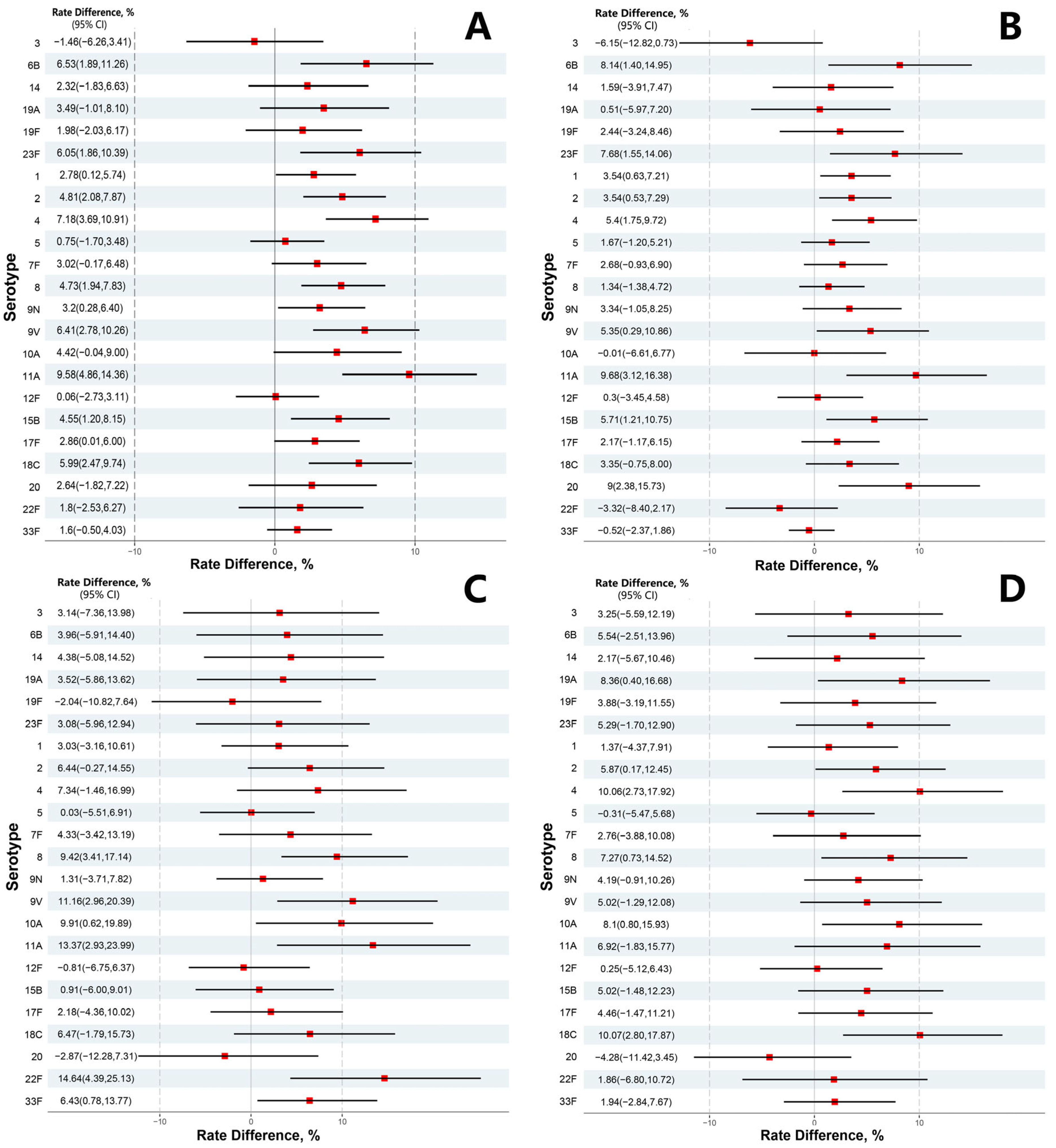
3.2. Immunogenicity Post-Vaccination
3.3. Subgroup Analysis
3.4. Adverse Reactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| CDC | Center for Disease Control and Prevention |
| ELISA | Enzyme-linked immune-sorbent assay |
| GMC | Geometric mean concentration |
| GMI | Geometric mean fold increase |
| IPD | Invasive pneumococcal disease |
| NMPA | National Medical Products Administration |
| PCV | Polysaccharide conjugate vaccine |
| PPSV | Pneumococcal polysaccharide vaccine |
| SAE | Serious adverse event |
| 95%CI | 95% confidence interval |
References
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [PubMed]
- Gierke, R.; Wodi, A.P.; Kobayashi, M. Pneumococcal Disease. In Epidemiology and Prevention of Vaccine Preventable Diseases, 14th ed.; Hall, E., Wodi, A.P., Hamborsky, J., Morelli, V., Schillie, S., Eds.; Center for Disease Control and Prevention: Washington, DC, USA, 2021; pp. 255–274. [Google Scholar]
- Torres, A.; Blasi, F.; Dartois, N.; Akova, M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax 2015, 70, 984–989. [Google Scholar] [CrossRef]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef]
- Bogaert, D.; de Groot, R.; Hermans, P.W.M. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, F.; Saad, J.S.; McGee, L.; van Tonder, A.J.; Bentley, S.D.; Lo, S.W.; Gladstone, R.A.; Turner, P.; Keenan, J.D.; Breiman, R.F.; et al. A New Pneumococcal Capsule Type, 10D, is the 100th Serotype and Has a Large cps Fragment from an Oral Streptococcus. mBio 2020, 11, e00937-20. [Google Scholar] [CrossRef]
- Kobayashi, M.; Pilishvili, T.; Farrar, J.L.; Leidner, A.J.; Gierke, R.; Prasad, N.; Moro, P.; Campos-Outcalt, D.; Morgan, R.L.; Long, S.S.; et al. Pneumococcal Vaccine for Adults Aged ≥19 Years: Recommendations of the Advisory Committee on Immunization Practices, United States, 2023. MMWR. Recomm. Rep. 2023, 72, 1–39. [Google Scholar] [CrossRef]
- Johnson, H.L.; Deloria-Knoll, M.; Levine, O.S.; Stoszek, S.K.; Freimanis Hance, L.; Reithinger, R.; Muenz, L.R.; O’Brien, K.L. Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children Under Five: The Pneumococcal Global Serotype Project. PLoS Med. 2010, 7, e1000348. [Google Scholar] [CrossRef]
- Lyu, S.; Hu, H.-L.; Yang, Y.-H.; Yao, K.-H. A systematic review about Streptococcus pneumoniae serotype distribution in children in mainland of China before the PCV13 was licensed. Expert Rev. Vaccines 2017, 16, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chung, D.R.; Song, J.-H.; Baek, J.Y.; Thamlikitkul, V.; Wang, H.; Carlos, C.; Ahmad, N.; Arushothy, R.; Tan, S.H.; et al. Changes in serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates from adult patients in Asia: Emergence of drug-resistant non-vaccine serotypes. Vaccine 2020, 38, 6065–6073. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-C.; Wang, Y.; Zhang, H.; Liu, Y.; Chen, X.-J.; Yang, H.-W.; Ma, P.; Wang, D.-C.; Zhang, B.-C.; Dong, A.-Y.; et al. Serotype distribution and clinical characteristics associated with Streptococcus pneumoniae among Chinese children and adults with invasive pneumococcal disease: A multicenter observational study. Hum. Vaccines Immunother. 2020, 17, 146–156. [Google Scholar] [CrossRef]
- World Health Organization. Considerations for pneumococcal vaccination in older adults. Wkly. Epidemiol. Rec. 2021, 96, 217–228. [Google Scholar]
- World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper–February 2019. Wkly. Epidemiol. Rec. 2019, 94, 85–104. [Google Scholar]
- Qin, Y.; Yu, H.-J.; Chinese Prevention Medicine Association. Technical guideline on application of pneumococcal vaccine in China (2012). Zhonghua Liu Xing Bing Xue Za Zhi 2012, 33, 1101–1110. [Google Scholar]
- Lai, X.; Lyu, Y.; Zhang, H.; Feng, H.; Fang, H. PPSV-23 recommendation and vaccination coverage in China: A cross-sectional survey among healthcare workers, older adults and chronic disease patients. Expert Rev. Vaccines 2022, 21, 1343–1353. [Google Scholar] [CrossRef]
- He, R.; Ren, X.; Huang, K.; Lei, J.; Niu, H.; Li, W.; Dong, F.; Li, B.; Wang, Y.; Yang, T.; et al. Influenza and pneumococcal vaccination coverage and associated factors in patients hospitalized with acute exacerbations of COPD in China: Findings from real-world data. Chin. Med. J. 2023, 137, 1179–1189. [Google Scholar] [CrossRef]
- Huang, L.; Wang, L.; Li, H.; Hu, Y.; Ru, W.; Han, W.; Shi, G.; Ye, Q.; Han, Z.; Xia, J.; et al. A phase III clinical trial to evaluate the safety and immunogenicity of 23-valent pneumococcal polysaccharide vaccine (PPV23) in healthy children, adults, and elderly. Hum. Vaccines Immunother. 2018, 15, 249–255. [Google Scholar] [CrossRef] [PubMed]
- National Medical Products Administration. Overseas Manufactured Drug: Pneumovax®, Basic Information for Approval Number SJ20140114. Available online: https://www.nmpa.gov.cn/datasearch/search-info.html?nmpa=aWQ9NDYyZjVlNjdhODcwMDRlYTVjMmIyMjJiMWM2MGE2ZDImaXRlbUlkPWZmODA4MDgxODNjYWQ3NTAwMTg0MDg4NjY1NzExODAw (accessed on 1 August 2025).
- National Medical Products Administration. Guidelines for Adverse Event Classification Standards for Clinical Trials of Pre-ventive Vaccines. 2019. Available online: https://www.cde.org.cn/zdyz/domesticinfopage?zdyzIdCODE=17d195260f53fff3cb3e4d8518a7a099 (accessed on 11 August 2025). (In Chinese).
- Konradsen, H.B.; Sørensen, U.B.S.; Henrichsen, J. A modified enzyme-linked immunosorbent assay for measuring type-specific anti-pneumococcal capsular polysaccharide antibodies. J. Immunol. Methods 1993, 164, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Smit, P.; Oberholzer, D.; Hayden-Smith, S.; Koornhof, H.J.; Hilleman, M.R. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA 1977, 238, 2613–2616. [Google Scholar] [CrossRef]
- Robbins, J.B.; Austrian, R.; Lee, C.J.; Rastogi, S.C.; Schiffman, G.; Henrichsen, J.; Makela, P.H.; Broome, C.V.; Facklam, R.R.; Tiesjema, R.H.; et al. Considerations for Formulating the Second-Generation Pneumococcal Capsular Polysaccharide Vaccine with Emphasis on the Cross-Reactive Types Within Groups. J. Infect. Dis. 1983, 148, 1136–1159. [Google Scholar] [CrossRef]
- World Health Organization. Immunization Agenda 2030: A Global Strategy to Leave No One Behind. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/ia2030 (accessed on 17 November 2024).
- Chen, Y.; Deng, W.; Wang, S.-M.; Mo, Q.-M.; Jia, H.; Wang, Q.; Li, S.-G.; Li, X.; Yao, B.-D.; Liu, C.-J.; et al. Burden of Pneumonia and Meningitis Caused by Streptococcus pneumoniae in China among Children under 5 Years of Age: A Systematic Literature Review. PLoS ONE 2011, 6, e27333. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lin, W.; Qian, C.; Zhang, Y.; Zhao, G.; Wang, W.; Zhang, T. Disease Burden of Meningitis Caused by Streptococcus pneumoniae Among Under-Fives in China: A Systematic Review and Meta-analysis. Infect. Dis. Ther. 2023, 12, 2567–2580. [Google Scholar] [CrossRef]
- Musher, D.M.; Manoff, S.B.; McFetridge, R.D.; Liss, C.L.; Marchese, R.D.; Raab, J.; Rueda, A.M.; Walker, M.L.; Hoover, P.A. Antibody persistence ten years after first and second doses of 23-valent pneumococcal polysaccharide vaccine, and immunogenicity and safety of second and third doses in older adults. Hum. Vaccines 2011, 7, 919–928. [Google Scholar] [CrossRef]
- Andrews, N.J.; A Waight, P.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: A postlicensure indirect cohort study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef]
- Babb, R.; Doyle, C.R.; Pirofski, L.-A.; Goldberg, J.B. Isolation and Characterization of Human Monoclonal Antibodies to Pneumococcal Capsular Polysaccharide 3. Microbiol. Spectr. 2021, 9, e0144621. [Google Scholar] [CrossRef] [PubMed]
- Linley, E.; Bell, A.; Gritzfeld, J.F.; Borrow, R. Should Pneumococcal Serotype 3 Be Included in Serotype-Specific Immunoassays? Vaccines 2019, 7, 4. [Google Scholar] [CrossRef]
- Yu, J.; Jing, H.; Lai, S.; Xu, W.; Li, M.; Wu, J.; Liu, W.; Yuan, Z.; Chen, Y.; Zhao, S.; et al. Etiology of diarrhea among children under the age five in China: Results from a five-year surveillance. J. Infect. 2015, 71, 19–27. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, W.; Jiang, Z.; Wang, L.; Li, C.; Li, Y.; Xia, J. Immunogenicity and safety of a 23-valent pneumococcal polysaccharide vaccine in Chinese healthy population aged >2 years: A randomized, double-blinded, active control, phase III trial. Hum. Vaccines Immunother. 2015, 11, 2425–2433. [Google Scholar] [CrossRef]
- World Health Organization. Global Manual on Surveillance of Adverse Events Following Immunization. 2016. Available online: https://www.who.int/publications/i/item/9789241507769 (accessed on 17 November 2024).
- Liu, H.; Yin, P.; Qi, J.; Zhou, M. Burden of non-communicable diseases in China and its provinces, 1990–2021: Results from the Global Burden of Disease Study 2021. Chin. Med. J. 2024, 137, 2325–2333. [Google Scholar] [CrossRef]
- Djennad, A.; Ramsay, M.E.; Pebody, R.; Fry, N.K.; Sheppard, C.; Ladhani, S.N.; Andrews, N.J. Effectiveness of 23-Valent Polysaccharide Pneumococcal Vaccine and Changes in Invasive Pneumococcal Disease Incidence from 2000 to 2017 in Those Aged 65 and Over in England and Wales. eClinicalMedicine 2018, 6, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.J.; Waight, P.A.; George, R.C.; Slack, M.P.; Miller, E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine 2012, 30, 6802–6808. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, X.; Li, H.; Xie, Z.; Zhu, T.; You, W.; Wang, Z.; Tan, J.; Feng, G.; Sun, Q.; et al. Immune persistence of a single dose of 23-valent pneumococcal polysaccharide vaccine: A 6-year follow-up. Hum. Vaccines Immunother. 2025, 21, 2517489. [Google Scholar] [CrossRef] [PubMed]
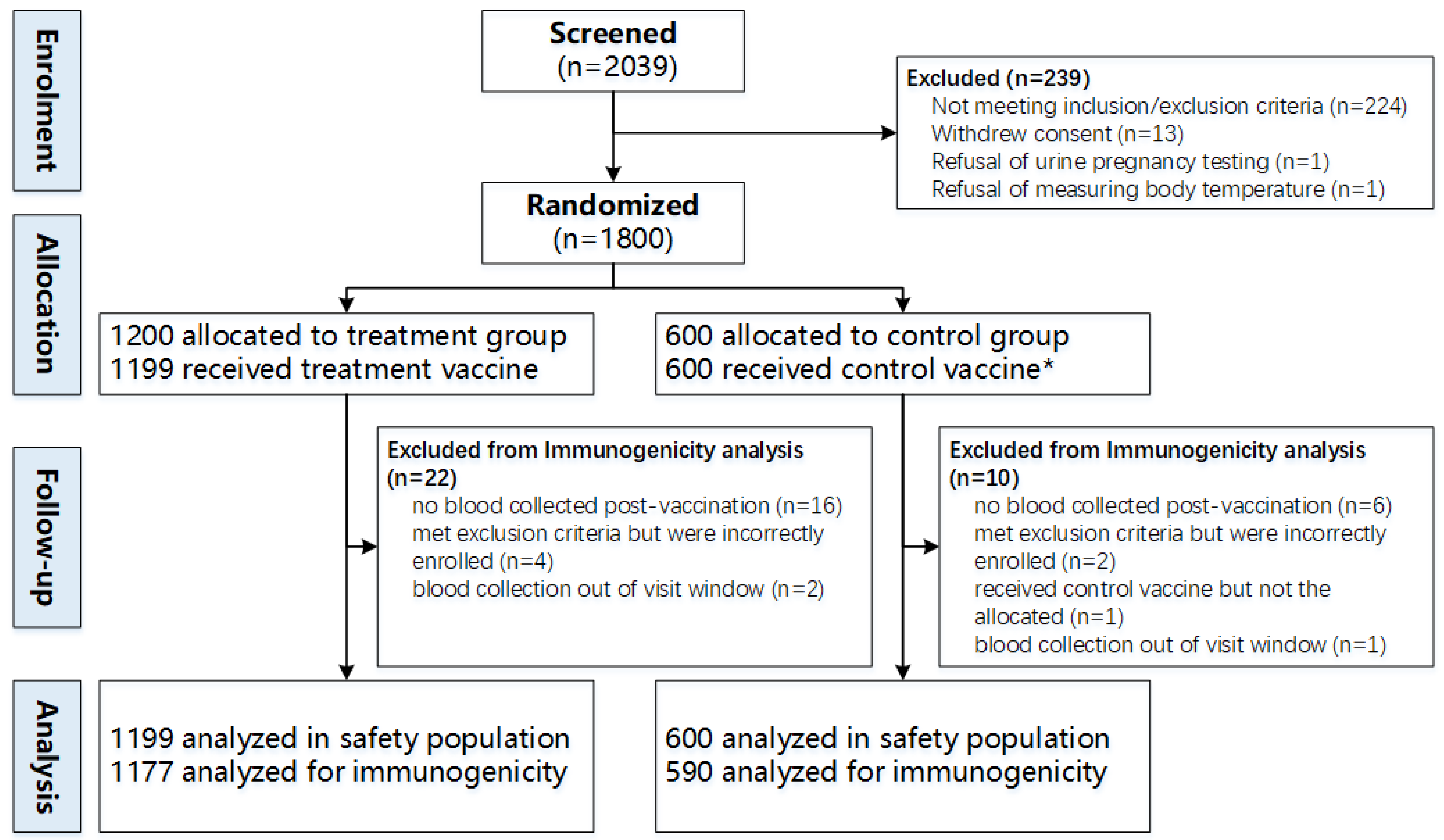
| Characteristics | Safety Population, No. (%) | |
|---|---|---|
| Treatment | Control | |
| No. of participants | 1199 | 600 |
| Mean age in years (SD) | 33.5 (25.56) | 33.7 (25.50) |
| Age group | ||
| 2–17 years | 599 (49.96) | 300 (50.00) |
| 18–59 years | 240 (20.02) | 121 (20.17) |
| 60+ years | 360 (30.03) | 179 (29.83) |
| Sex | ||
| Male | 582 (48.54) | 297 (49.50) |
| Female | 617 (51.46) | 303 (50.50) |
| Ethnicity | ||
| Han nationality | 1197 (99.83) | 599 (99.83) |
| Others | 2 (0.17) | 1 (0.17) |
| BMI, kg/m2 (SD) | 22.11 (4.86) | 22.08 (4.66) |
| Underlying medical conditions | 125 (10.42) | 67 (11.17) |
| Serotypes | GMC (95%CI) at Baseline | p-Value | GMC (95%CI) Post-Vaccination | GMC Ratio (95%CI) Post-Vaccination | p-Value | GMI (95%CI) Post-Vaccination | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (n = 1177) | Control (n = 590) | Treatment (n = 1177) | Control (n = 590) | Treatment (n = 1177) | Control (n = 590) | |||||
| 3 | 0.35 (0.32–0.38) | 0.37 (0.34–0.42) | 0.2858 | 1.06 (1.01–1.12) | 1.21 (1.13–1.29) | 0.88 (0.81–0.96) | 0.0035 | 3.04 (2.85–3.25) | 3.22 (2.93–3.54) | 0.3387 |
| 6B | 2.04 (1.94–2.14) | 2.04 (1.91–2.19) | 0.9505 | 6.92 (6.48–7.39) | 6.45 (5.88–7.08) | 1.07 (0.96–1.20) | 0.227 | 3.39 (3.22–3.58) | 3.15 (2.93–3.40) | 0.1182 |
| 14 | 6.95 (6.64–7.27) | 6.93 (6.50–7.38) | 0.9435 | 33.07 (31.01–35.28) | 30.64 (27.97–33.56) | 1.08 (0.97–1.21) | 0.1795 | 4.76 (4.47–5.07) | 4.42 (4.04–4.84) | 0.1873 |
| 19F | 3.36 (3.22–3.51) | 3.41 (3.21–3.63) | 0.6912 | 14.23 (13.43–15.09) | 12.95 (11.92–14.06) | 1.10 (0.99–1.22) | 0.0664 | 4.24 (4.02–4.46) | 3.80 (3.53–4.09) | 0.0171 |
| 19A | 3.71 (3.54–3.90) | 3.79 (3.54–4.06) | 0.624 | 12.98 (12.26–13.75) | 12.31 (11.34–13.35) | 1.06 (0.96–1.17) | 0.2909 | 3.50 (3.31–3.69) | 3.25 (3.01–3.51) | 0.1198 |
| 23F | 1.51 (1.44–1.58) | 1.46 (1.36–1.56) | 0.4271 | 6.43 (6.05–6.84) | 5.35 (4.91–5.84) | 1.2 (1.08–1.34) | 0.0008 | 4.27 (4.04–4.50) | 3.67 (3.40–3.97) | 0.0017 |
| 1 | 0.75 (0.70–0.80) | 0.79 (0.71–0.86) | 0.4281 | 6.13 (5.76–6.52) | 5.91 (5.41–6.45) | 1.04 (0.93–1.16) | 0.4993 | 8.17 (7.64–8.74) | 7.52 (6.84–8.26) | 0.1564 |
| 2 | 3.42 (3.27–3.58) | 3.54 (3.31–3.78) | 0.4186 | 25.06 (23.81–26.38) | 21.13 (19.65–22.71) | 1.19 (1.09–1.30) | 0.0002 | 7.32 (6.95–7.71) | 5.97 (5.55–6.43) | <0.0001 |
| 4 | 0.43 (0.40–0.47) | 0.42 (0.38–0.47) | 0.7733 | 3.65 (3.47–3.84) | 2.67 (2.48–2.87) | 1.37 (1.25–1.49) | <0.0001 | 8.46 (7.78–9.20) | 6.32 (5.61–7.11) | <0.0001 |
| 5 | 0.61 (0.58–0.65) | 0.63 (0.58–0.68) | 0.6485 | 4.46 (4.22–4.72) | 4.11 (3.79–4.45) | 1.09 (0.99–1.20) | 0.0962 | 7.29 (6.86–7.75) | 6.56 (6.02–7.15) | 0.0494 |
| 7F | 1.17 (1.11–1.25) | 1.21 (1.11–1.32) | 0.5463 | 7.38 (6.94–7.83) | 6.77 (6.22–7.38) | 1.09 (0.98–1.21) | 0.11 | 6.28 (5.91–6.67) | 5.59 (5.13–6.08) | 0.027 |
| 8 | 2.15 (2.04–2.27) | 2.16 (2.00–2.33) | 0.9238 | 18.61 (17.72–19.54) | 14.8 (13.81–15.86) | 1.26 (1.15–1.37) | <0.0001 | 8.66 (8.14–9.22) | 6.86 (6.28–7.49) | <0.0001 |
| 9N | 1.14 (1.05–1.25) | 1.19 (1.05–1.35) | 0.6068 | 12.02 (11.35–12.73) | 10.16 (9.37–11.02) | 1.18 (1.07–1.31) | 0.0009 | 10.5 (9.67–11.41) | 8.53 (7.59–9.58) | 0.0043 |
| 9V | 1.49 (1.40–1.60) | 1.54 (1.39–1.69) | 0.6526 | 7.26 (6.85–7.69) | 6.39 (5.88–6.94) | 1.14 (1.03–1.26) | 0.013 | 4.86 (4.59–5.14) | 4.16 (3.84–4.50) | 0.0018 |
| 10A | 2.89 (2.77–3.02) | 2.89 (2.72–3.07) | 0.9676 | 10.97 (10.27–11.71) | 9.86 (8.98–10.81) | 1.11 (0.99–1.25) | 0.0653 | 3.79 (3.60–3.99) | 3.41 (3.17–3.67) | 0.0197 |
| 11A | 1.90 (1.77–2.03) | 2.04 (1.85–2.25) | 0.2446 | 6.67 (6.36–6.99) | 5.66 (5.29–6.05) | 1.18 (1.09–1.28) | <0.0001 | 3.51 (3.32–3.72) | 2.78 (2.56–3.01) | <0.0001 |
| 12F | 0.32 (0.30–0.34) | 0.34 (0.31–0.37) | 0.2162 | 2.27 (2.14–2.41) | 2.25 (2.07–2.45) | 1.01 (0.91–1.12) | 0.8544 | 7.18 (6.73–7.67) | 6.63 (6.04–7.27) | 0.1647 |
| 15B | 3.15 (2.97–3.34) | 3.14 (2.89–3.41) | 0.9561 | 20.68 (19.35–22.09) | 17.06 (15.54–18.73) | 1.21 (1.08–1.36) | 0.001 | 6.56 (6.19–6.96) | 5.43 (5.00–5.90) | 0.0003 |
| 17F | 1.25 (1.17–1.33) | 1.31 (1.20–1.43) | 0.3613 | 8.45 (7.99–8.93) | 7.95 (7.35–8.60) | 1.06 (0.96–1.17) | 0.2192 | 6.77 (6.38–7.19) | 6.07 (5.58–6.60) | 0.0369 |
| 18C | 1.26 (1.17–1.35) | 1.37 (1.24–1.52) | 0.1741 | 7.49 (7.10–7.90) | 6.70 (6.20–7.23) | 1.12 (1.02–1.23) | 0.0189 | 5.96 (5.59–6.36) | 4.88 (4.45–5.35) | 0.0005 |
| 20 | 2.94 (2.78–3.11) | 2.99 (2.77–3.23) | 0.7141 | 10.00 (9.45–10.58) | 10.43 (9.63–11.29) | 0.96 (0.87–1.06) | 0.404 | 3.40 (3.23–3.58) | 3.48 (3.24–3.74) | 0.5965 |
| 22F | 2.23 (2.15–2.32) | 2.24 (2.12–2.37) | 0.9242 | 8.48 (8.08–8.90) | 8.23 (7.68–8.81) | 1.03 (0.95–1.12) | 0.4751 | 3.80 (3.61–4.00) | 3.67 (3.42–3.94) | 0.4484 |
| 33F | 1.11 (1.01–1.22) | 1.20 (1.06–1.37) | 0.3058 | 14.08 (13.18–15.03) | 12.69 (11.57–13.92) | 1.11 (0.99–1.24) | 0.073 | 12.72 (11.72–13.80) | 10.53 (9.38–11.82) | 0.0092 |
| Serotypes | Treatment Group (n = 1177) | Control Group (n = 590) | Rate Difference (95%CI) | p-Value | ||
|---|---|---|---|---|---|---|
| No. | Rate (95%CI) | No. | Rate (95%CI) | |||
| 3 | 697 | 59.22 (56.35–62.04) | 358 | 60.68 (56.61–64.64) | −1.46 (−6.26–3.41) | 0.5552 |
| 6B | 823 | 69.92 (67.21–72.53) | 374 | 63.39 (59.36–67.29) | 6.53 (1.89–11.26) | 0.0056 |
| 14 | 911 | 77.40 (74.90–79.76) | 443 | 75.08 (71.39–78.53) | 2.32 (−1.83–6.63) | 0.2781 |
| 19F | 933 | 79.27 (76.84–81.55) | 456 | 77.29 (73.69–80.61) | 1.98 (−2.03–6.17) | 0.3382 |
| 19A | 843 | 71.62 (68.95–74.18) | 402 | 68.14 (64.21–71.88) | 3.49 (−1.01–8.10) | 0.1297 |
| 23F | 931 | 79.10 (76.66–81.39) | 431 | 73.05 (69.28–76.59) | 6.05 (1.86–10.39) | 0.0043 |
| 1 | 1096 | 93.12 (91.52–94.50) | 533 | 90.34 (87.66–92.60) | 2.78 (0.12–5.74) | 0.0400 |
| 2 | 1104 | 93.80 (92.26–95.11) | 525 | 88.98 (86.17–91.39) | 4.81 (2.08–7.87) | 0.0004 |
| 4 | 1046 | 88.87 (86.93–90.61) | 482 | 81.69 (78.33–84.73) | 7.18 (3.69–10.91) | <0.0001 |
| 5 | 1098 | 93.29 (91.70–94.65) | 546 | 92.54 (90.12–94.53) | 0.75 (−1.70–3.48) | 0.5613 |
| 7F | 1049 | 89.12 (87.21–90.85) | 508 | 86.10 (83.04–88.79) | 3.02 (−0.17–6.48) | 0.0640 |
| 8 | 1099 | 93.37 (91.80–94.73) | 523 | 88.64 (85.80–91.09) | 4.73 (1.94–7.83) | 0.0006 |
| 9N | 1077 | 91.50 (89.76–93.03) | 521 | 88.31 (85.43–90.79) | 3.20 (0.28–6.40) | 0.0311 |
| 9V | 1023 | 86.92 (84.85–88.79) | 475 | 80.51 (77.08–83.63) | 6.41 (2.78–10.26) | 0.0004 |
| 10A | 860 | 73.07 (70.43–75.58) | 405 | 68.64 (64.73–72.37) | 4.42 (−0.04–9.00) | 0.0519 |
| 11A | 815 | 69.24 (66.52–71.87) | 352 | 59.66 (55.58–63.65) | 9.58 (4.86–14.36) | <0.0001 |
| 12F | 1066 | 90.57 (88.75–92.18) | 534 | 90.51 (87.85–92.75) | 0.06 (−2.73–3.11) | 0.9672 |
| 15B | 1043 | 88.62 (86.66–90.37) | 496 | 84.07 (80.86–86.93) | 4.55 (1.20–8.15) | 0.0072 |
| 17F | 1081 | 91.84 (90.13–93.34) | 525 | 88.98 (86.17–91.39) | 2.86 (0.01–6.00) | 0.0488 |
| 18C | 1034 | 87.85 (85.85–89.66) | 483 | 81.86 (78.51–84.89) | 5.99 (2.47–9.74) | 0.0007 |
| 20 | 847 | 71.96 (69.30–74.51) | 409 | 69.32 (65.43–73.02) | 2.64 (−1.82–7.22) | 0.2483 |
| 22F | 871 | 74.00 (71.40–76.49) | 426 | 72.20 (68.40–75.78) | 1.80 (−2.53–6.27) | 0.4198 |
| 33F | 1126 | 95.67 (94.34–96.76) | 555 | 94.07 (91.85–95.83) | 1.60 (−0.50–4.03) | 0.1407 |
| Events | Any Severity | p-Value | Grade 3 | p-Value | ||
|---|---|---|---|---|---|---|
| Treatment Group (n = 1199) | Control Group (n = 600) | Treatment Group (n = 1199) | Control Group (n = 600) | |||
| Overall adverse reactions within 30 days | 236 (19.68) | 118 (19.67) | 1.0000 | 8 (0.67) | 5 (0.83) | 0.7697 |
| Injection site adverse reactions | ||||||
| Pain | 191 (15.93) | 98 (16.33) | 0.8383 | 0 (0.00) | 2 (0.33) | 0.1111 |
| Induration | 21 (1.75) | 4 (0.67) | 0.0855 | 1 (0.08) | 0 (0.00) | 1.0000 |
| Swelling | 32 (2.67) | 10 (1.67) | 0.2458 | 1 (0.08) | 1 (0.17) | 1.0000 |
| Erythema | 20 (1.67) | 6 (1.00) | 0.3019 | 2 (0.17) | 1 (0.17) | 1.0000 |
| Rash | 1 (0.08) | 0 (0.00) | 1.0000 | 0 (0.00) | 0 (0.00) | 1.0000 |
| Pruritus | 18 (1.50) | 5 (0.83) | 0.2732 | 0 (0.00) | 0 (0.00) | 1.0000 |
| Systemic adverse reactions | ||||||
| Fever | 32 (2.67) | 18 (3.00) | 0.7611 | 5 (0.42) | 3 (0.50) | 1.0000 |
| Acute allergic reaction | 2 (0.17) | 1 (0.17) | 1.0000 | 0 (0.00) | 0 (0.00) | 1.0000 |
| Cough | 15 (1.25) | 3 (0.50) | 0.2070 | 0 (0.00) | 0 (0.00) | 1.0000 |
| Myalgia | 6 (0.50) | 7 (1.17) | 0.1409 | 0 (0.00) | 1 (0.17) | 0.3335 |
| Arthralgia | 2 (0.17) | 2 (0.33) | 0.6047 | 0 (0.00) | 0 (0.00) | 1.0000 |
| Headache | 7 (0.58) | 1 (0.17) | 0.2818 | 0 (0.00) | 0 (0.00) | 1.0000 |
| Fatigue | 9 (0.75) | 5 (0.83) | 1.0000 | 0 (0.00) | 0 (0.00) | 1.0000 |
| Dizziness | 1 (0.08) | 0 (0.00) | 1.0000 | 0 (0.00) | 0 (0.00) | 1.0000 |
| Pruritus | 0 (0.00) | 2 (0.33) | 0.1111 | 0 (0.00) | 0 (0.00) | 1.0000 |
| Urticaria | 1 (0.08) | 0 (0.00) | 1.0000 | 1 (0.08) | 0 (0.00) | 1.0000 |
| Limb discomfort | 1 (0.08) | 0 (0.00) | 1.0000 | 0 (0.00) | 0 (0.00) | 1.0000 |
| Serious adverse events (SAEs) during the study | 6 (0.50) | 0 (0.00) | 0.1872 | 6 (0.50) | 0 (0.00) | 0.1872 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Shi, G.; Dong, Y.; Yang, W.; Wang, Y.; Ye, X.; Zhang, J.; Yang, X.; Yu, D.; Song, D.; et al. Safety and Immunogenicity of a 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) in Chinese Children, Adults and the Elderly: A Phase 4, Randomized, Double-Blind, Active-Controlled Clinical Trial. Vaccines 2025, 13, 866. https://doi.org/10.3390/vaccines13080866
Liu X, Shi G, Dong Y, Yang W, Wang Y, Ye X, Zhang J, Yang X, Yu D, Song D, et al. Safety and Immunogenicity of a 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) in Chinese Children, Adults and the Elderly: A Phase 4, Randomized, Double-Blind, Active-Controlled Clinical Trial. Vaccines. 2025; 13(8):866. https://doi.org/10.3390/vaccines13080866
Chicago/Turabian StyleLiu, Xiaoyu, Gang Shi, Yuanyuan Dong, Wanqi Yang, Yinan Wang, Xianying Ye, Juxiang Zhang, Xinyi Yang, Dan Yu, Dan Song, and et al. 2025. "Safety and Immunogenicity of a 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) in Chinese Children, Adults and the Elderly: A Phase 4, Randomized, Double-Blind, Active-Controlled Clinical Trial" Vaccines 13, no. 8: 866. https://doi.org/10.3390/vaccines13080866
APA StyleLiu, X., Shi, G., Dong, Y., Yang, W., Wang, Y., Ye, X., Zhang, J., Yang, X., Yu, D., Song, D., Ma, Y., Wang, Z., Li, H., & Hu, W. (2025). Safety and Immunogenicity of a 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) in Chinese Children, Adults and the Elderly: A Phase 4, Randomized, Double-Blind, Active-Controlled Clinical Trial. Vaccines, 13(8), 866. https://doi.org/10.3390/vaccines13080866






