Multisystem Endothelial Inflammation: A Key Driver of Adverse Events Following mRNA-Containing COVID-19 Vaccines
Abstract
1. Introduction
2. Spectrum of mRNA-LNP-Induced Inflammatory Complications
3. The Root Causes of mRNA-LNP-Induced Inflammatory Complications
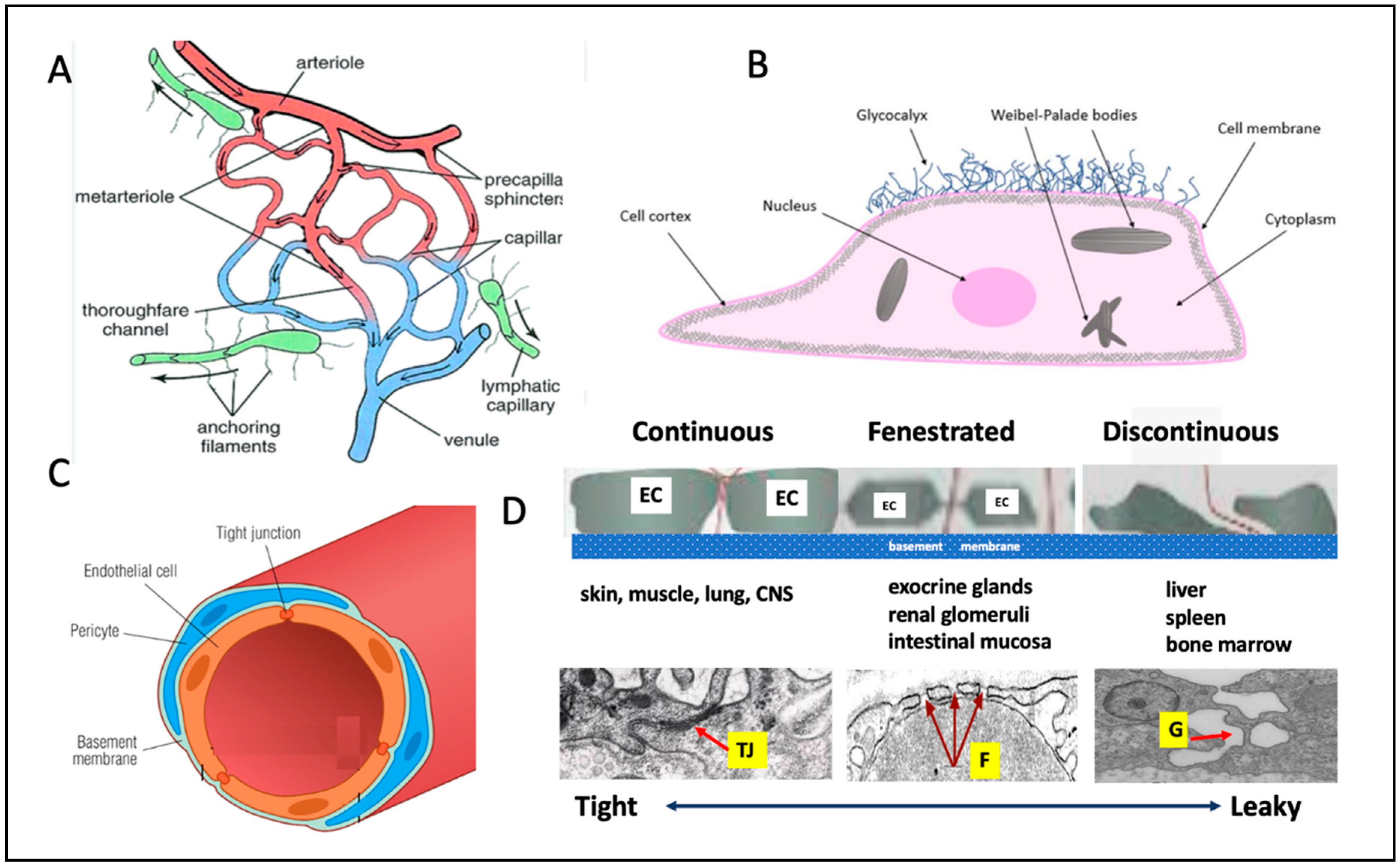
4. Components and Organization of Microcirculation Across Tissues
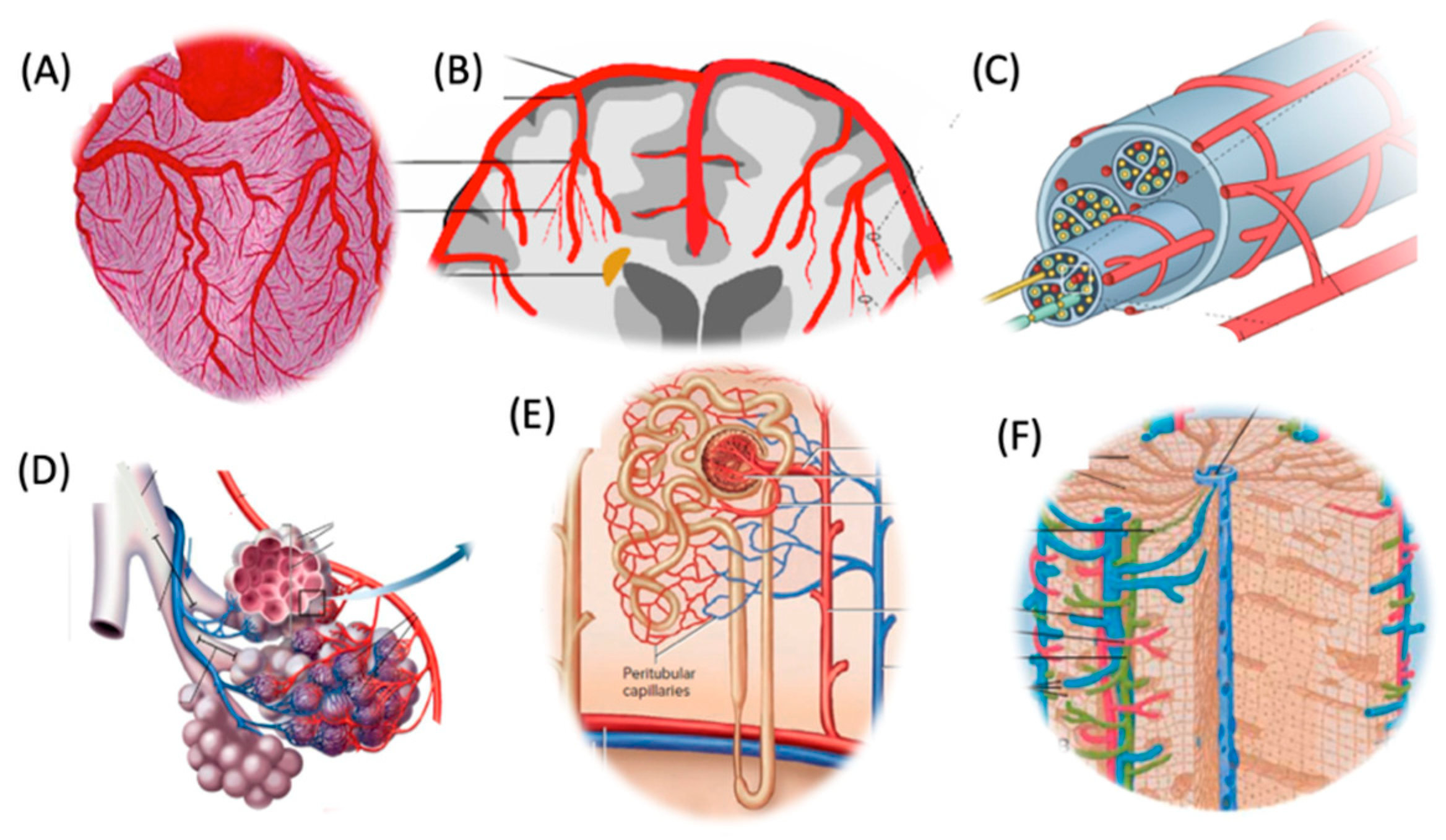
5. Distinctive Microcirculatory Architectures Across Organs and Their Impacts on Vaccine-Induced Inflammations
Organ-Specific Microcirculatory Structures with Impact on Vaccine AEs
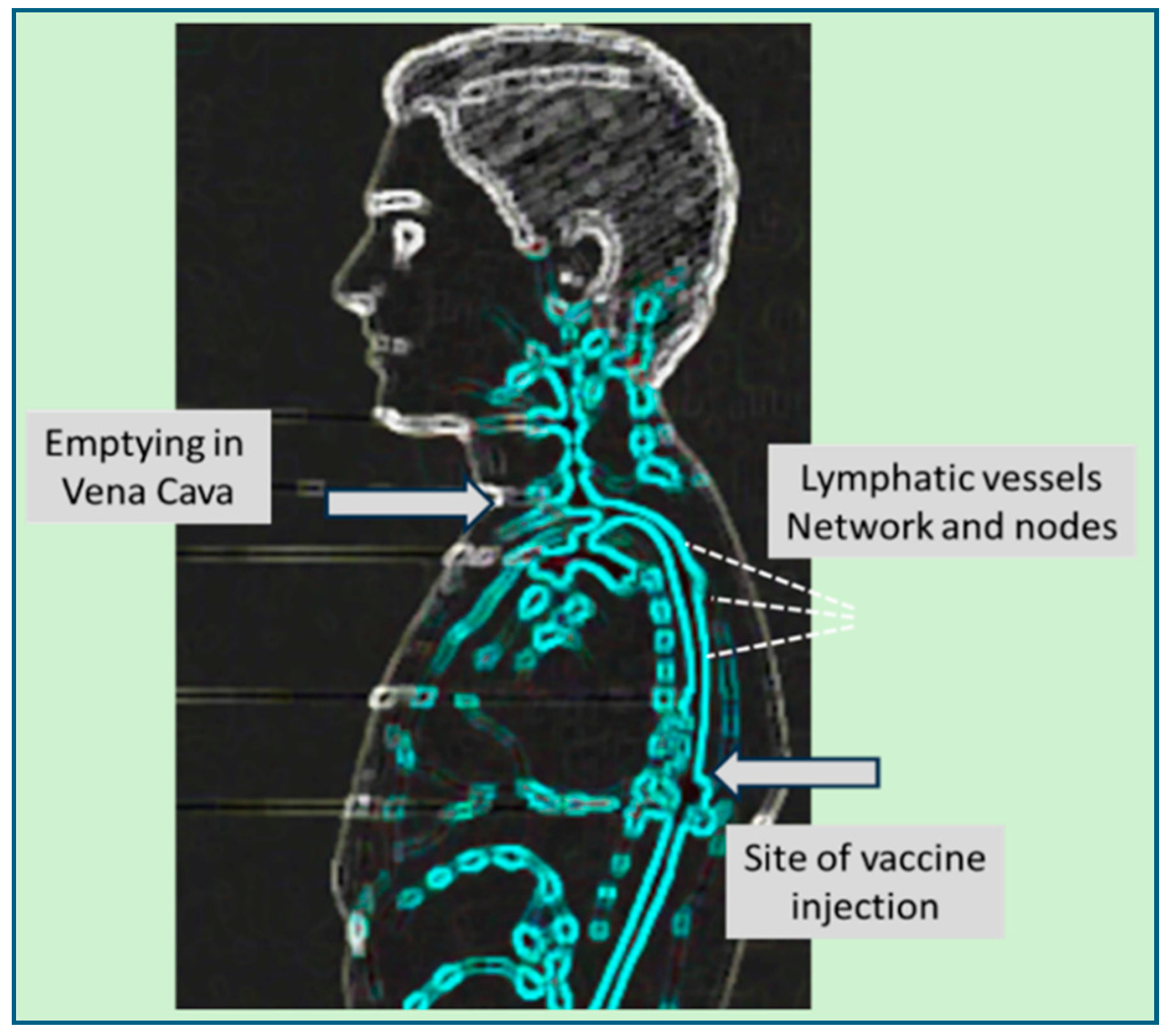
6. The Journey of mRNA-LNPs from the Deltoid Muscle to the Sites of Inflammations
7. Stoichiometry of Vaccine-Endothelium Interactions
8. The Pathophysiology, Diagnosis and Example of Vaccine-Induced Vasculitis
8.1. Pathophysiology
8.2. Diagnosis
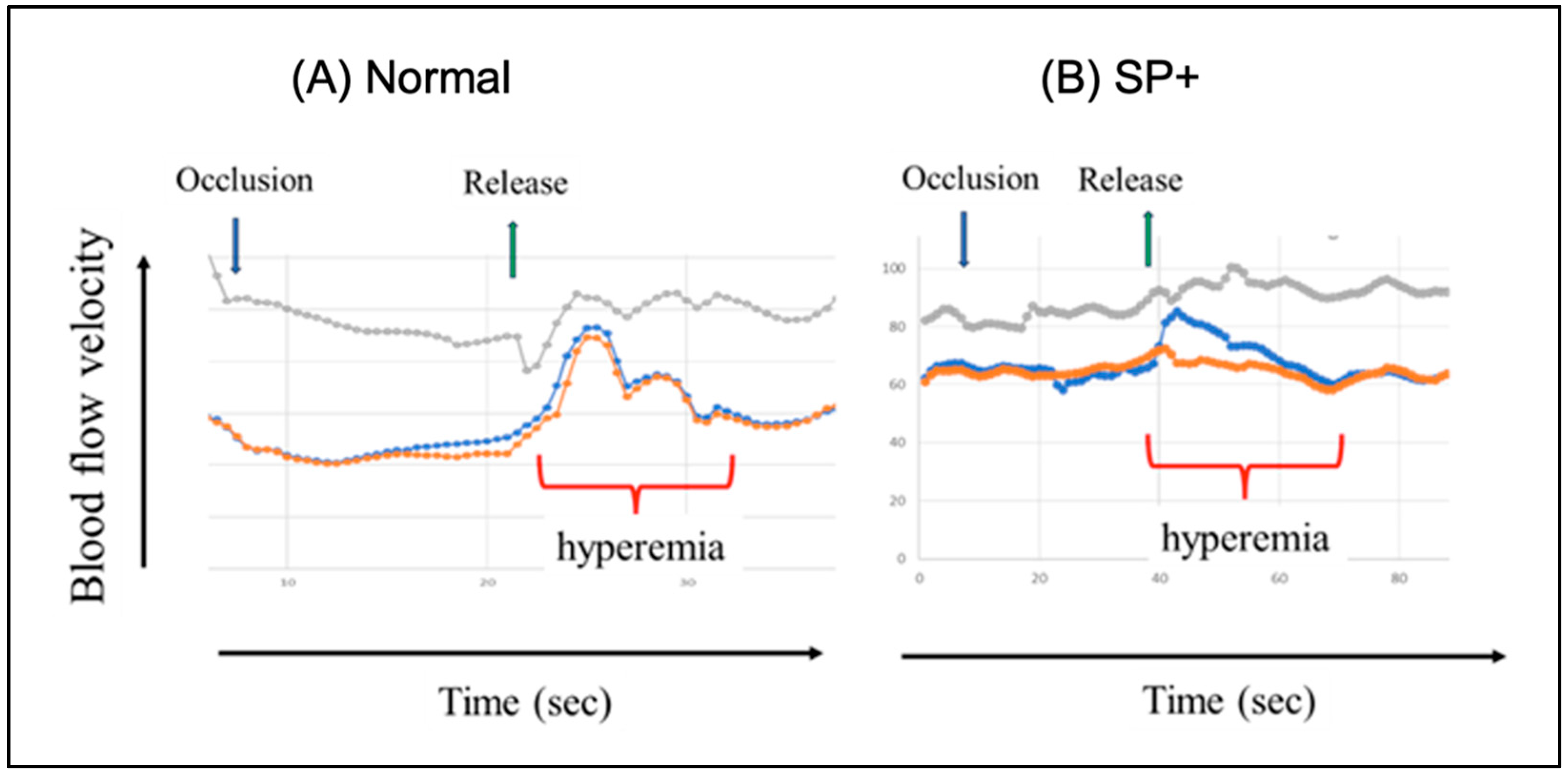
8.3. Experimental Evidence of Spike Protein-Induced Cerebral Abnormality
9. Non-Target Organ Uptake of mRNA-LNPs
9.1. The Role of Ionizable Lipid in Multiorgan Transfection
9.2. Systemic Transfection vs. in Loco Immunogenicity of mRNA-LNP-Derived Spike Protein: The Achilles’ Heel of mRNA Vaccines
9.3. mRNA-LNP Internalization by Endothelial Cells
10. Endothelial Cells in the Frontline of Systemic Inflammation
10.1. Spike Protein Toxicity on Endothelial Cells
10.2. Vaccine-Induced Autoimmune Damage on Endothelial Cells
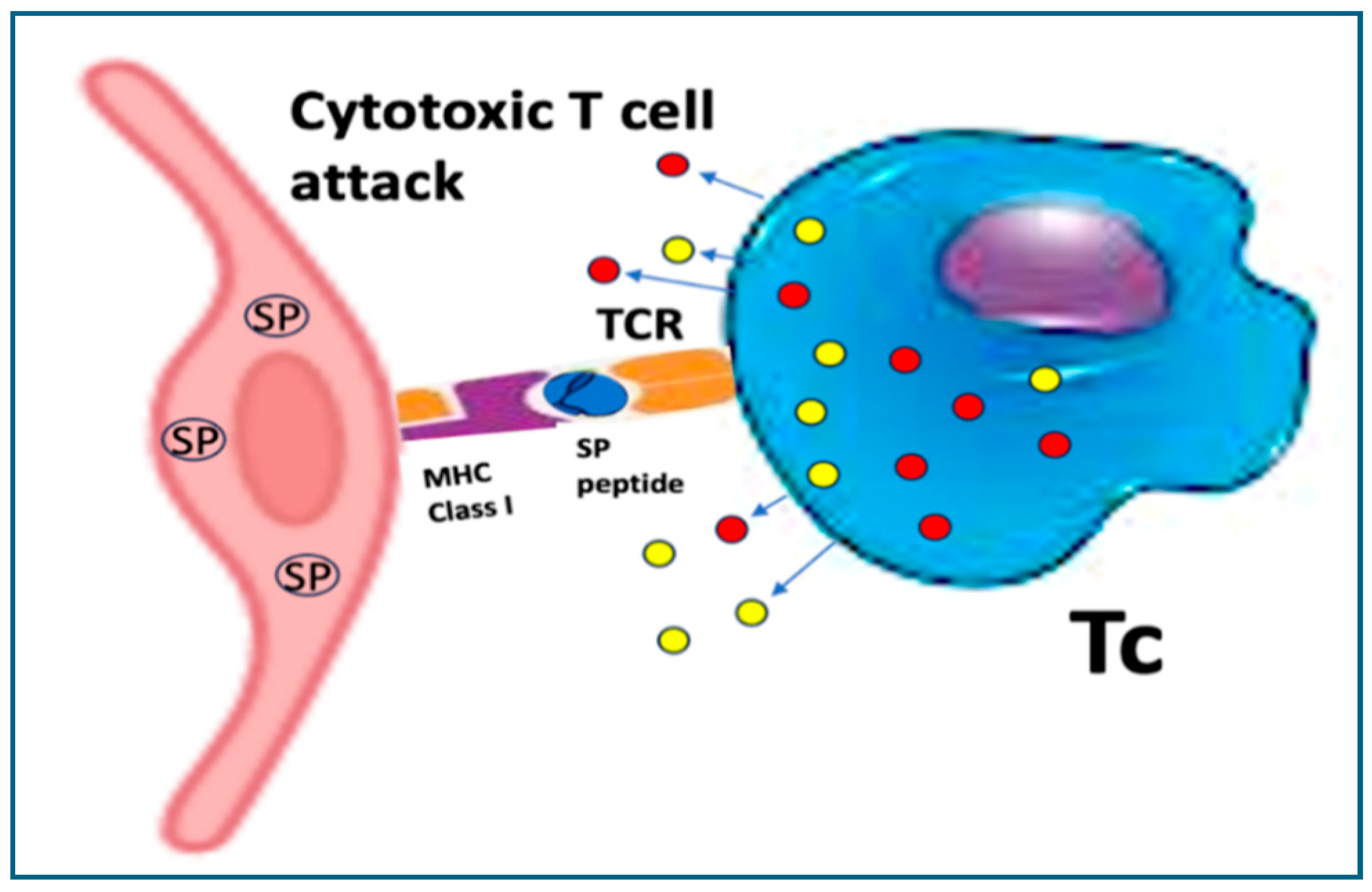
10.2.1. Cross-Presentation Behind Cytotoxic T Cell Attack
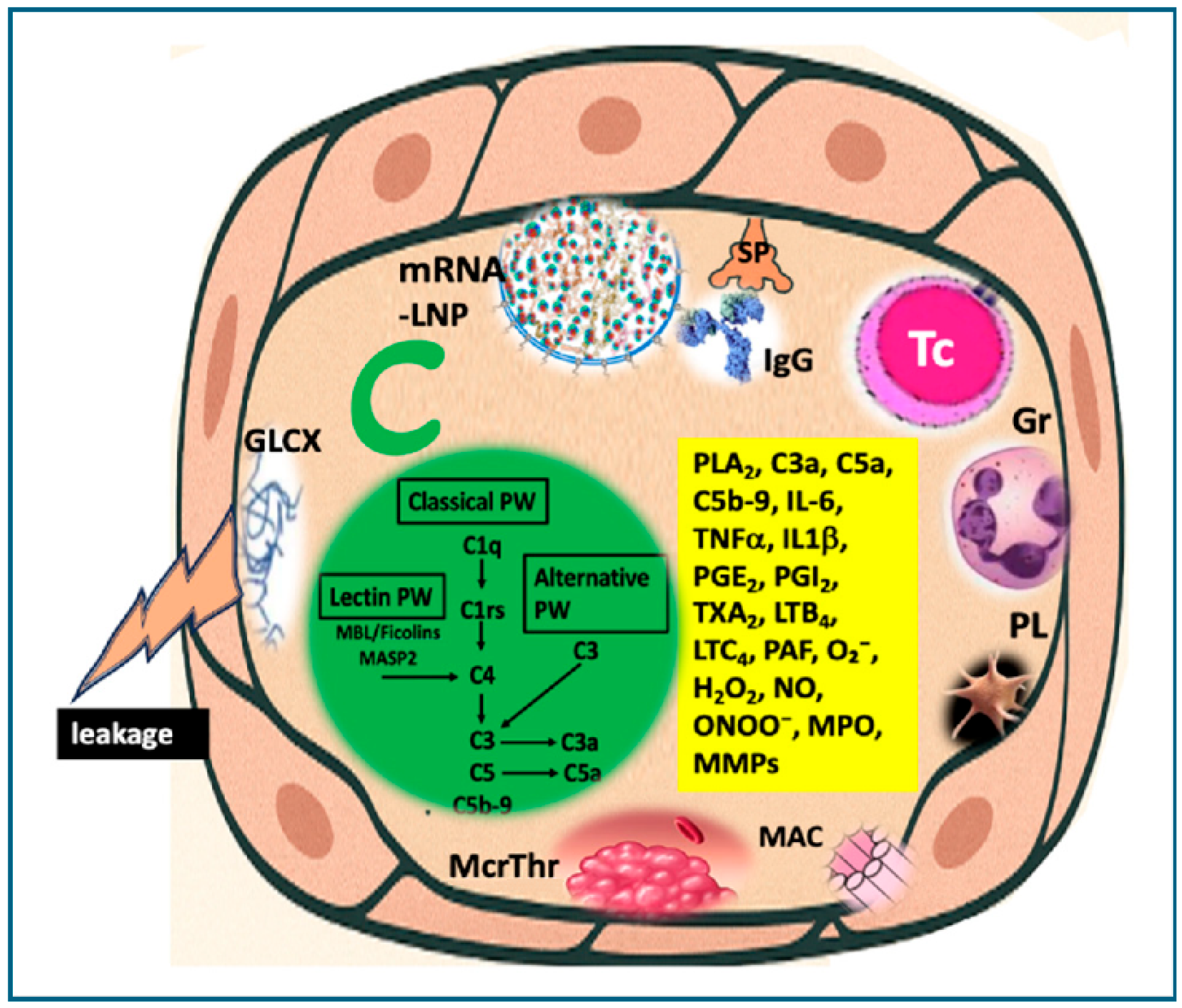
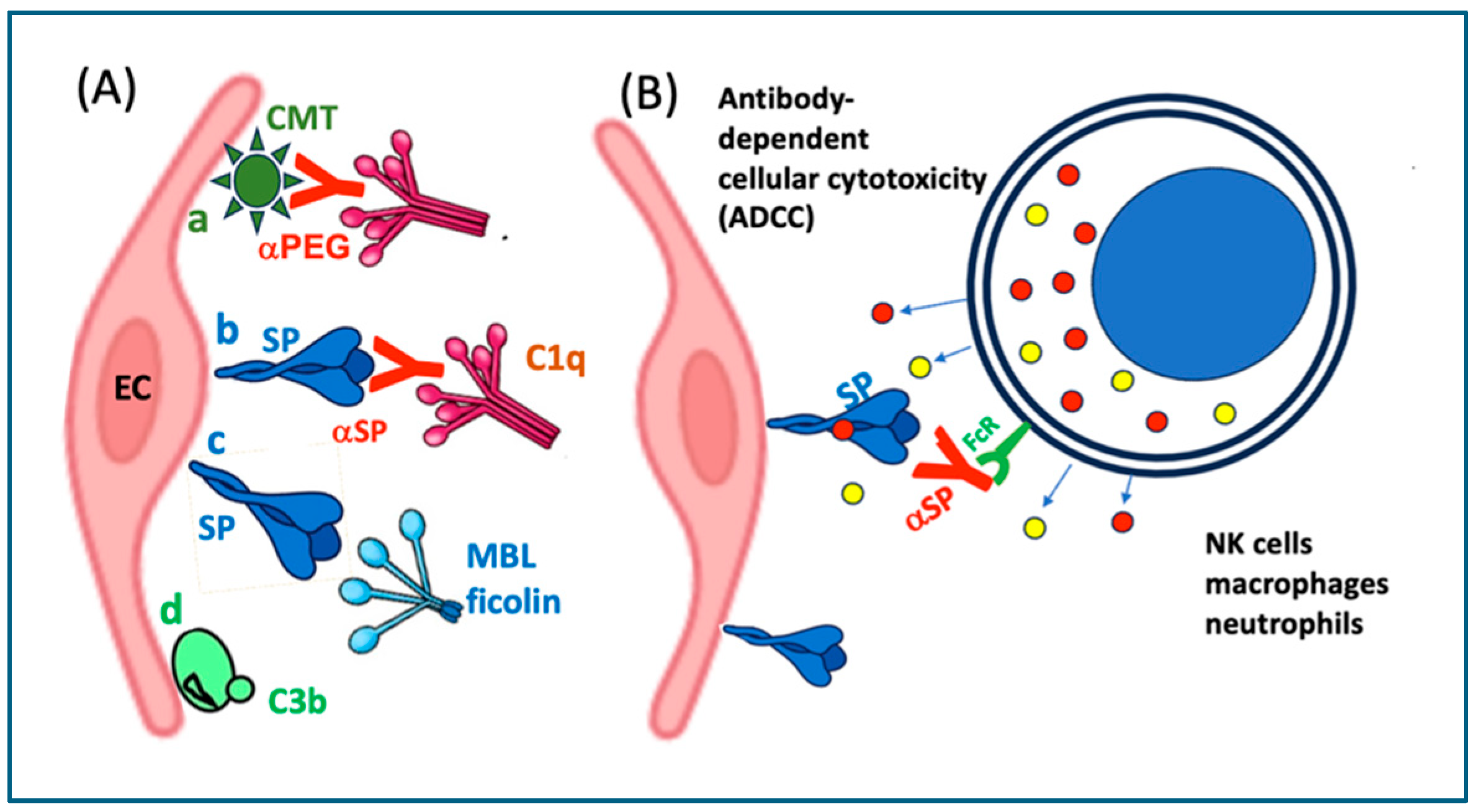
10.2.2. Complement-Mediated Cytotoxicity
10.2.3. Antibody-Dependent Cellular Cytotoxicity (ADCC)
10.2.4. LNP-Induced Inflammatory Signaling in Endothelial Cells
11. Outlook
11.1. Vaccine-Induced Pseudo-Infection: Functional Mimicry of Pathogenicity in a Few?
11.2. Scope and Justification
11.3. Limitations of the Review
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novak, N.; Tordesillas, L.; Cabanillas, B. Adverse rare events to vaccines for COVID-19: From hypersensitivity reactions to thrombosis and thrombocytopenia. Int. Rev. Immunol. 2022, 41, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Oueijan, R.I.; Hill, O.R.; Ahiawodzi, P.D.; Fasinu, P.S.; Thompson, D.K. Rare Heterogeneous Adverse Events Associated with mRNA-Based COVID-19 Vaccines: A Systematic Review. Medicines 2022, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M.; Wu, Y.; Sawano, M.; Shah, R.; Zhou, T.; Arun, A.S.; Khosla, P.; Kaleem, S.; Vashist, A.; Bhattacharjee, B.; et al. Post-Vaccination Syndrome: A Descriptive Analysis of Reported Symptoms and Patient Experiences After Covid-19 Immunization. medRxiv 2023. [Google Scholar] [CrossRef]
- Palmer, M.; Bhakdi, S.; Hooker, B.; Holland, M.; DesBois, M.; Rasnick, D.; Fitts, C.A. mRNA Vaccine Toxicity. 2023. Available online: https://doctors4covidethics.org/mRNA-vaccine-toxicity (accessed on 30 July 2025).
- Costa, C.; Moniati, F. The Epidemiology of COVID-19 Vaccine-Induced Myocarditis. Adv. Med. 2024, 2024, 4470326. [Google Scholar] [CrossRef]
- Padilla-Flores, T.; Sampieri, A.; Vaca, L. Incidence and management of the main serious adverse events reported after COVID-19 vaccination. Pharmacol. Res. Perspect. 2024, 12, e1224. [Google Scholar] [CrossRef]
- Kang, H.; Sun, Y.; Fang, Z.; Ding, W.; Bai, T.; Yang, K.; Jiang, D. Illuminating the shadows—perspectives on mRNA vaccine adverse events—mechanisms, risks and management: A review. Int. J. Biol. Macromol. 2025, 318, 145010. [Google Scholar] [CrossRef]
- Szebeni, J. Expanded Spectrum and Increased Incidence of Adverse Events Linked to COVID-19 Genetic Vaccines: New Concepts on Prophylactic Immuno-Gene Therapy, Iatrogenic Orphan Disease, and Platform-Inherent Challenges. Pharmaceutics 2025, 17, 45. [Google Scholar] [CrossRef]
- FDA. FDA Approves Required Updated Warning in Labeling of mRNA COVID-19 Vaccines Regarding Myocarditis and Pericarditis Following Vaccination. Available online: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-approves-required-updated-warning-labeling-mrna-covid-19-vaccines-regarding-myocarditis-and (accessed on 30 July 2025).
- Gardner, S.; David Lim, D. The CDC Is No Longer Recommending Covid Vaccines for Healthy Children, Pregnant Women. 2025. Available online: https://www.politico.com/news/2025/05/27/the-cdc-is-no-longer-recommending-covid-vaccines-for-healthy-children-pregnant-women-00370864?utm (accessed on 30 July 2025).
- Perrone, M.; Neergaard, L. New Trump Vaccine Policy Limits Access to COVID Shots. 2025. Available online: https://apnews.com/article/vaccines-fda-kennedy-covid-shots-rfk-trump-bb4de15b6ff955d6cd0b406aaec3cdc5 (accessed on 30 July 2025).
- Prasad, V.; Makary, M.A. An Evidence-Based Approach to Covid-19 Vaccination. N. Engl. J. Med. 2025, 392, 2484–2486. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M. New COVID Booster Rules Could Limit Shots for People Under 65. 2025. Available online: https://www.axios.com/2025/05/20/fda-limits-covid-boosters?utm (accessed on 30 July 2025).
- Nathan-Kazis, J. Kennedy’s Edict Results in New, Narrowed Vaccine Guidance from CDC. Barron’s Online, 30 May 2025. [Google Scholar]
- Worldwide Safety. Cumulative Analysis of Post-Authorization Adverse Event Reports of PF-07302048 (BNT162b2) Received Through 28-Feb-2021. 2021. Available online: https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf?fbclid=IwAR2tWI7DKw0cc2lj8 (accessed on 30 July 2025).
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine 2022, 40, 5798–5805. [Google Scholar] [CrossRef]
- Pual-Ehrlich-Institut. Safety Report. 2022. Available online: https://www.pei.de/SharedDocs/Downloads/EN/newsroom-en/dossiers/safety-reports/safety-report-27-december-2020-31-march-2022.pdf?__blob=publicationFile&v=8 (accessed on 30 July 2025).
- Yousaf, A.R.; Miller, A.D.; Lindsey, K.; Shah, A.B.; Wu, M.J.; Melgar, M.; Zambrano, L.D.; Campbell, A.P. Multisystem Inflammatory Syndrome in Children Among Persons Who Completed a Two-dose COVID-19 Vaccine Primary Series Compared with Those Reporting No COVID-19 Vaccination, US National MIS-C Surveillance. Pediatr. Infect. Dis. J. 2023, 42, e476–e478. [Google Scholar] [CrossRef]
- Salzman, M.B.; Huang, C.W.; O’Brien, C.M.; Castillo, R.D. Multisystem Inflammatory Syndrome after SARS-CoV-2 Infection and COVID-19 Vaccination. Emerg. Infect. Dis. 2021, 27, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Chang, Y. Cytomegalovirus Proctitis Developed after COVID-19 Vaccine: A Case Report and Literature Review. Vaccines 2022, 10, 1417. [Google Scholar] [CrossRef]
- Chakravorty, S.; Cochrane, A.B.; Psotka, M.A.; Regmi, A.; Marinak, L.; Thatcher, A.; Shlobin, O.A.; Brown, A.W.; King, C.S.; Ahmad, K.; et al. CMV Infection Following mRNA SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients. Transpl. Direct 2022, 8, e1344. [Google Scholar] [CrossRef] [PubMed]
- Herzum, A.; Trave, I.; D’Agostino, F.; Burlando, M.; Cozzani, E.; Parodi, A. Epstein-Barr virus reactivation after COVID-19 vaccination in a young immunocompetent man: A case report. Clin. Exp. Vaccine Res. 2022, 11, 222–225. [Google Scholar] [CrossRef]
- Navarro-Bielsa, A.; Gracia-Cazana, T.; Aldea-Manrique, B.; Abadias-Granado, I.; Ballano, A.; Bernad, I.; Gilaberte, Y. COVID-19 infection and vaccines: Potential triggers of Herpesviridae reactivation. An. Bras. Dermatol. 2023, 98, 347–354. [Google Scholar] [CrossRef]
- Shafiee, A.; Amini, M.J.; Arabzadeh Bahri, R.; Jafarabady, K.; Salehi, S.A.; Hajishah, H.; Mozhgani, S.H. Herpesviruses reactivation following COVID-19 vaccination: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 278. [Google Scholar] [CrossRef]
- Maple, P.A.C. COVID-19, SARS-CoV-2 Vaccination, and Human Herpesviruses Infections. Vaccines 2023, 11, 232. [Google Scholar] [CrossRef]
- Martinez-Reviejo, R.; Tejada, S.; Adebanjo, G.A.R.; Chello, C.; Machado, M.C.; Parisella, F.R.; Campins, M.; Tammaro, A.; Rello, J. Varicella-Zoster virus reactivation following severe acute respiratory syndrome coronavirus 2 vaccination or infection: New insights. Eur. J. Intern. Med. 2022, 104, 73–79. [Google Scholar] [CrossRef]
- Elbaz, M.; Hoffman, T.; Yahav, D.; Dovrat, S.; Ghanem-Zoubi, N.; Atamna, A.; Grupel, D.; Reisfeld, S.; Hershman-Sarafov, M.; Ciobotaro, P.; et al. Varicella-Zoster Virus-Induced Neurologic Disease After COVID-19 Vaccination: A Multicenter Observational Cohort Study. Open Forum Infect. Dis. 2024, 11, ofae287. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J. The Unique Features and Collateral Immune Effects of mRNA-Based COVID-19 Vaccines: Potential Plausible Causes of Adverse Events and Complications. Preprints 2025. [Google Scholar] [CrossRef]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Kariko, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.R.; Muramatsu, H.; Nallagatla, S.R.; Bevilacqua, P.C.; Sansing, L.H.; Weissman, D.; Kariko, K. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010, 38, 5884–5892. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Jha, B.K.; Silverman, R.H.; Weissman, D.; Kariko, K. Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase, L. Nucleic Acids Res. 2011, 39, 9329–9338. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef] [PubMed]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef]
- Gerritsen, M.E.; Bloor, C.M. Endothelial cell gene expression in response to injury. FASEB J. 1993, 7, 523–532. [Google Scholar] [CrossRef]
- Allport, J.R.; Ding, H.; Collins, T.; Gerritsen, M.E.; Luscinskas, F.W. Endothelial-dependent mechanisms regulate leukocyte transmigration: A process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J. Exp. Med. 1997, 186, 517–527. [Google Scholar] [CrossRef]
- Takacs, J.; Deak, D.; Koller, A. Higher level of physical activity reduces mental and neurological symptoms during and two years after COVID-19 infection in young women. Sci. Rep. 2024, 14, 6927. [Google Scholar] [CrossRef]
- Stoker, M.E.; Gerdes, A.M.; May, J.F. Regional differences in capillary density and myocyte size in the normal human heart. Anat. Rec. 1982, 202, 187–191. [Google Scholar] [CrossRef]
- Hudlicka, O.; Brown, M.; Egginton, S. Angiogenesis in skeletal and cardiac muscle. Physiol. Rev. 1992, 72, 369–417. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis Following Immunization with mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021, 6, 1202–1206. [Google Scholar] [CrossRef]
- Verma, A.K.; Lavine, K.J.; Lin, C.Y. Myocarditis after Covid-19 mRNA Vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef]
- Shay, D.K.; Shimabukuro, T.T.; DeStefano, F. Myocarditis Occurring After Immunization with mRNA-Based COVID-19 Vaccines. JAMA Cardiol. 2021, 6, 1115–1117. [Google Scholar] [CrossRef]
- Meyer-Szary, J.; Bazgier, M.; Lubocka, P.; Dorniak, K.; Sabiniewicz, R. Cardiac magnetic resonance characteristics of acute myocarditis occurring after mRNA-based COVID-19 vaccines immunization. Cardiol. J. 2022, 29, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.R.; Tashjian, R.; Duncanson, E. Autopsy Histopathologic Cardiac Findings in 2 Adolescents Following the Second COVID-19 Vaccine Dose. Arch. Pathol. Lab. Med. 2022, 146, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef]
- Nemeth, Z.; Cziraki, A.; Szabados, S.; Biri, B.; Keki, S.; Koller, A. Elevated Levels of Asymmetric Dimethylarginine (ADMA) in the Pericardial Fluid of Cardiac Patients Correlate with Cardiac Hypertrophy. PLoS ONE 2015, 10, e0135498. [Google Scholar] [CrossRef]
- Nemeth, Z.; Cziraki, A.; Szabados, S.; Horvath, I.; Koller, A. Pericardial fluid of cardiac patients elicits arterial constriction: Role of endothelin. Can. J. Physiol. Pharmacol. 2015, 93, 779–785. [Google Scholar] [CrossRef]
- Rinaldi, V.; Bellucci, G.; Buscarinu, M.C.; Renie, R.; Marrone, A.; Nasello, M.; Zancan, V.; Nistri, R.; Palumbo, R.; Salerno, A.; et al. CNS inflammatory demyelinating events after COVID-19 vaccines: A case series and systematic review. Front. Neurol. 2022, 13, 1018785. [Google Scholar] [CrossRef]
- Patone, M.; Handunnetthi, L.; Saatci, D.; Pan, J.; Katikireddi, S.V.; Razvi, S.; Hunt, D.; Mei, X.W.; Dixon, S.; Zaccardi, F.; et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021, 27, 2144–2153. [Google Scholar] [CrossRef]
- Khayat-Khoei, M.; Bhattacharyya, S.; Katz, J.; Harrison, D.; Tauhid, S.; Bruso, P.; Houtchens, M.K.; Edwards, K.R.; Bakshi, R. COVID-19 mRNA vaccination leading to CNS inflammation: A case series. J. Neurol. 2022, 269, 1093–1106. [Google Scholar] [CrossRef]
- Ota, N.; Itani, M.; Aoki, T.; Sakurai, A.; Fujisawa, T.; Okada, Y.; Noda, K.; Arakawa, Y.; Tokuda, S.; Tanikawa, R. Expression of SARS-CoV-2 spike protein in cerebral Arteries: Implications for hemorrhagic stroke Post-mRNA vaccination. J. Clin. Neurosci. 2025, 136, 111223. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Tomomatsu, K.; Okazaki, E.; Takeuchi, T.; Horio, Y.; Kondo, Y.; Oguma, T.; Asano, K. COVID-19 vaccine-associated organizing pneumonia. Respirol. Case Rep. 2022, 10, e0944. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, J.H.; Park, S.; Hwang, Y.I.; Kim, H.I.; Jang, S.H.; Jung, K.S.; Kim, Y.K.; Kim, H.A.; Lee, I.J. Clinical characteristics of patients with COVID-19 vaccine-related pneumonitis: A case series and literature review. Korean J. Intern. Med. 2022, 37, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Kervella, D.; Jacquemont, L.; Chapelet-Debout, A.; Deltombe, C.; Ville, S. Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney Int. 2021, 100, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, J.; Ye, Q. Renal Side Effects of COVID-19 Vaccination. Vaccines 2022, 10, 1783. [Google Scholar] [CrossRef]
- Rocco, A.; Sgamato, C.; Compare, D.; Nardone, G. Autoimmune hepatitis following SARS-CoV-2 vaccine: May not be a casuality. J. Hepatol. 2021, 75, 728–729. [Google Scholar] [CrossRef]
- Schinas, G.; Polyzou, E.; Dimakopoulou, V.; Tsoupra, S.; Gogos, C.; Akinosoglou, K. Immune-mediated liver injury following COVID-19 vaccination. World J. Virol. 2023, 12, 100–108. [Google Scholar] [CrossRef]
- Sergi, C.M. COVID-19 vaccination-related autoimmune hepatitis-a perspective. Front. Pharmacol. 2023, 14, 1190367. [Google Scholar] [CrossRef]
- Chen, C.; Xie, D.; Xiao, J. Real-world evidence of autoimmune hepatitis following COVID-19 vaccination: A population-based pharmacovigilance analysis. Front. Pharmacol. 2023, 14, 1100617. [Google Scholar] [CrossRef]
- Kim, J.H.; Chae, H.B.; Woo, S.; Song, M.S.; Kim, H.J.; Woo, C.G. Clinicopathological Characteristics of Autoimmune-Like Hepatitis Induced by COVID-19 mRNA Vaccine (Pfizer-BioNTech, BNT162b2): A Case Report and Literature Review. Int. J. Surg. Pathol. 2023, 31, 1156–1162. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Rohss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo After Modified mRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- Szabo, G.T.; Mahiny, A.J.; Vlatkovic, I. COVID-19 mRNA vaccines: Platforms and current developments. Mol. Ther. 2022, 30, 1850–1868. [Google Scholar] [CrossRef]
- Buckley, M.; Arainga, M.; Maiorino, L.; Pires, I.S.; Kim, B.J.; Michaels, K.K.; Dye, J.; Qureshi, K.; Zhang, Y.J.; Mak, H.; et al. Visualizing lipid nanoparticle trafficking for mRNA vaccine delivery in non-human primates. Mol. Ther. 2025, 33, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyarto, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, R.; Koller, A.; Kaley, G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am. J. Physiol. 1998, 274, R790–R796. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Mizuno, R.; Kaley, G. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: Role of endothelial prostanoids. Am. J. Physiol. 1999, 277, R1683–R1689. [Google Scholar] [CrossRef]
- Breslin, J.W. Mechanical forces and lymphatic transport. Microvasc. Res. 2014, 96, 46–54. [Google Scholar] [CrossRef] [PubMed]
- von der Weid, P.Y. Lymphatic Vessel Pumping. Adv. Exp. Med. Biol. 2019, 1124, 357–377. [Google Scholar] [CrossRef]
- Alazraki, N.; Glass, E.C.; Castronovo, F.; Olmos, R.A.; Podoloff, D.; Society of Nuclear, M. Procedure guideline for lymphoscintigraphy and the use of intraoperative gamma probe for sentinel lymph node localization in melanoma of intermediate thickness. J. Nucl. Med. 2002, 43, 1414–1418. [Google Scholar] [PubMed]
- Dixon, J.B.; Greiner, S.T.; Gashev, A.A.; Cote, G.L.; Moore, J.E.; Zawieja, D.C. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 2006, 13, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Borresen, B.; Hansen, A.E.; Fliedner, F.P.; Henriksen, J.R.; Elema, D.R.; Brandt-Larsen, M.; Kristensen, L.K.; Kristensen, A.T.; Andresen, T.L.; Kjaer, A. Noninvasive Molecular Imaging of the Enhanced Permeability and Retention Effect by (64)Cu-Liposomes: In vivo Correlations with (68)Ga-RGD, Fluid Pressure, Diffusivity and (18)F-FDG. Int. J. Nanomed. 2020, 15, 8571–8581. [Google Scholar] [CrossRef]
- Pfizer Australia Pty Ltd. Nonclinical Evaluation Report: BNT162b2 [mRNA] COVID-19 Vaccine (COMIRNATYTM). 2021. Available online: https://www.tga.gov.au/sites/default/files/foi-2389-06.pdf (accessed on 30 July 2025).
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Li, S.; Hu, Y.; Li, A.; Lin, J.; Hsieh, K.; Schneiderman, Z.; Zhang, P.; Zhu, Y.; Qiu, C.; Kokkoli, E.; et al. Payload distribution and capacity of mRNA lipid nanoparticles. Nat. Commun. 2022, 13, 5561. [Google Scholar] [CrossRef]
- Szebeni, J.; Storm, G.; Ljubimova, J.Y.; Castells, M.; Phillips, E.J.; Turjeman, K.; Barenholz, Y.; Crommelin, D.J.A.; Dobrovolskaia, M.A. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat. Nanotechnol. 2022, 17, 337–346. [Google Scholar] [CrossRef]
- Jaffe, E.A. Cell biology of endothelial cells. Hum. Pathol. 1987, 18, 234–239. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef]
- Gilio, M.; De Stefano, G. Large-vessel vasculitis following the Pfizer-BioNTech COVID-19 vaccine. Intern. Emerg. Med. 2022, 17, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Bostan, E.; Zaid, F.; Akdogan, N.; Gokoz, O. Possible case of mRNA COVID-19 vaccine-induced small-vessel vasculitis. J. Cosmet. Dermatol. 2022, 21, 51–53. [Google Scholar] [CrossRef]
- Abuhammad, A.; Albandak, M.; Ayyad, M.; Refayeh, E.; Qawasma, B.; Hour, S.; Abu Thraiee, Y.; Sowaity, Z.A.; Dukmak, O.; Jobran, A.W.M.; et al. COVID-19 vaccine-associated vasculitis: A systematic review. SAGE Open Med. 2024, 12, 20503121241261165. [Google Scholar] [CrossRef]
- Abdelmaksoud, A.; Wollina, U.; Temiz, S.A.; Hasan, A. SARS-CoV-2 vaccination-induced cutaneous vasculitis: Report of two new cases and literature review. Dermatol. Ther. 2022, 35, e15458. [Google Scholar] [CrossRef]
- Rice, C.M.; Scolding, N.J. The diagnosis of primary central nervous system vasculitis. Pract. Neurol. 2020, 20, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Hiremath, S.B.; Aviv, R.I.; Wilson, N. Childhood Cerebral Vasculitis : A Multidisciplinary Approach. Clin. Neuroradiol. 2023, 33, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Takacs, J.; Deak, D.; Seregely, B.; Koller, A. Cognitive Slowing, Dysfunction in Verbal Working Memory, Divided Attention and Response Inhibition in Post COVID-19 Condition in Young Adults. Life 2025, 15, 821. [Google Scholar] [CrossRef]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef]
- Horejs, C. From lipids to lipid nanoparticles to mRNA vaccines. Nat. Rev. Mater. 2021, 6, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.W.; Madden, T.D.; Cullis, P.R. Bilayer Stabilizing Components and Their Use in Forming Programmable Fusogenic Liposomes. U.S. Patent 5,885,613, 23 March 1999. [Google Scholar]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Darjuan, M.M.; Mercer, J.E.; Chen, S.; van der Meel, R.; Thewalt, J.L.; Tam, Y.Y.C.; Cullis, P.R. On the Formation and Morphology of Lipid Nanoparticles Containing Ionizable Cationic Lipids and siRNA. ACS Nano 2018, 12, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Pateev, I.; Seregina, K.; Ivanov, R.; Reshetnikov, V. Biodistribution of RNA Vaccines and of Their Products: Evidence from Human and Animal Studies. Biomedicines 2023, 12, 59. [Google Scholar] [CrossRef]
- Dalby, B.; Cates, S.; Harris, A.; Ohki, E.C.; Tilkins, M.L.; Price, P.J.; Ciccarone, V.C. Advanced transfection with Lipofectamine 2000 reagent: Primary neurons, siRNA, and high-throughput applications. Methods 2004, 33, 95–103. [Google Scholar] [CrossRef]
- Ferraresso, F.; Badior, K.; Seadler, M.; Zhang, Y.; Wietrzny, A.; Cau, M.F.; Haugen, A.; Rodriguez, G.G.; Dyer, M.R.; Cullis, P.R.; et al. Protein is expressed in all major organs after intravenous infusion of mRNA-lipid nanoparticles in swine. Mol. Ther. Methods Clin. Dev. 2024, 32, 101314. [Google Scholar] [CrossRef]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef]
- Dézsi, L.; Kökény, G.; Szénási, G.; Révész, C.; Mészáros, T.; Barta, A.B.; Facsko, R.; Szilasi, A.; Bakos, T.; Kozma, G.T.; et al. Acute anaphylactic and multiorgan inflammatory effects of Comirnaty in pigs: Evidence of spike protein mRNA transfection and paralleling inflammatory cytokine upregulation. BioRxiv 2025, 658379. Available online: https://www.biorxiv.org/content/biorxiv/early/2025/06/10/2025.06.07.658379.full.pdf (accessed on 30 July 2025).
- Sago, C.D.; Lokugamage, M.P.; Paunovska, K.; Vanover, D.A.; Monaco, C.M.; Shah, N.N.; Gamboa Castro, M.; Anderson, S.E.; Rudoltz, T.G.; Lando, G.N.; et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc. Natl. Acad. Sci. USA 2018, 115, E9944–E9952. [Google Scholar] [CrossRef]
- Francia, V.; Schiffelers, R.M.; Cullis, P.R.; Witzigmann, D. The Biomolecular Corona of Lipid Nanoparticles for Gene Therapy. Bioconjug. Chem. 2020, 31, 2046–2059. [Google Scholar] [CrossRef]
- Cheng, M.H.Y.; Leung, J.; Zhang, Y.; Strong, C.; Basha, G.; Momeni, A.; Chen, Y.; Jan, E.; Abdolahzadeh, A.; Wang, X.; et al. Induction of Bleb Structures in Lipid Nanoparticle Formulations of mRNA Leads to Improved Transfection Potency. Adv. Mater. 2023, 35, e2303370. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.W.; Guzman, E.B.; Menon, N.; Langer, R.S. Lipid Nanoparticles for Nucleic Acid Delivery to Endothelial Cells. Pharm. Res. 2023, 40, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; Felgner, P.L. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat. Rev. Drug. Discov. 2024, 23, 709–722. [Google Scholar] [CrossRef]
- Yazdi, M.; Pohmerer, J.; Hasanzadeh Kafshgari, M.; Seidl, J.; Grau, M.; Hohn, M.; Vetter, V.; Hoch, C.C.; Wollenberg, B.; Multhoff, G.; et al. In Vivo Endothelial Cell Gene Silencing by siRNA-LNPs Tuned with Lipoamino Bundle Chemical and Ligand Targeting. Small 2024, 20, e2400643. [Google Scholar] [CrossRef] [PubMed]
- Petersen, D.M.S.; Weiss, R.M.; Hajj, K.A.; Yerneni, S.S.; Chaudhary, N.; Newby, A.N.; Arral, M.L.; Whitehead, K.A. Branched-Tail Lipid Nanoparticles for Intravenous mRNA Delivery to Lung Immune, Endothelial, and Alveolar Cells in Mice. Adv. Healthc. Mater. 2024, 13, e2400225. [Google Scholar] [CrossRef]
- Papp, T.E.; Zeng, J.; Shahnawaz, H.; Akyianu, A.; Breda, L.; Yadegari, A.; Steward, J.; Shi, R.; Li, Q.; Mui, B.L.; et al. CD47 peptide-cloaked lipid nanoparticles promote cell-specific mRNA delivery. Mol. Ther. 2025, 33, 3195–3208. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Yu, J.; Yuan, X.; Chen, H.; Chaturvedi, S.; Braunstein, E.M.; Brodsky, R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood 2020, 136, 2080–2089. [Google Scholar] [CrossRef]
- Ali, Y.M.; Ferrari, M.; Lynch, N.J.; Yaseen, S.; Dudler, T.; Gragerov, S.; Demopulos, G.; Heeney, J.L.; Schwaeble, W.J. Lectin Pathway Mediates Complement Activation by SARS-CoV-2 Proteins. Front. Immunol. 2021, 12, 714511. [Google Scholar] [CrossRef]
- Perico, L.; Morigi, M.; Galbusera, M.; Pezzotta, A.; Gastoldi, S.; Imberti, B.; Perna, A.; Ruggenenti, P.; Donadelli, R.; Benigni, A.; et al. SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation. Front. Immunol. 2022, 13, 827146. [Google Scholar] [CrossRef]
- Aird, W.C. Endothelial cell heterogeneity. Crit. Care Med. 2003, 31, S221–S230. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflüg. Arch.-Eur. J. Physiol. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Weinbaum, S.; Tarbell, J.M.; Damiano, E.R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007, 9, 121–167. [Google Scholar] [CrossRef] [PubMed]
- Robles, J.P.; Zamora, M.; Adan-Castro, E.; Siqueiros-Marquez, L.; Martinez de la Escalera, G.; Clapp, C. The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin alpha5beta1 and NF-kappaB signaling. J. Biol. Chem. 2022, 298, 101695. [Google Scholar] [CrossRef] [PubMed]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef]
- Avolio, E.; Carrabba, M.; Milligan, R.; Kavanagh Williamson, M.; Beltrami, A.P.; Gupta, K.; Elvers, K.T.; Gamez, M.; Foster, R.R.; Gillespie, K.; et al. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: A potential non-infective mechanism of COVID-19 microvascular disease. Clin. Sci. 2021, 135, 2667–2689. [Google Scholar] [CrossRef]
- Perico, L.; Morigi, M.; Pezzotta, A.; Locatelli, M.; Imberti, B.; Corna, D.; Cerullo, D.; Benigni, A.; Remuzzi, G. SARS-CoV-2 spike protein induces lung endothelial cell dysfunction and thrombo-inflammation depending on the C3a/C3a receptor signalling. Sci. Rep. 2023, 13, 11392. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Zhang, L.; Bhushan, A.; Swanson, B.; Zhang, L.; Mamede, J.I.; Voigt, R.M.; Shaikh, M.; Engen, P.A.; Keshavarzian, A. The SARS-CoV-2 S1 Spike Protein Promotes MAPK and NF-kB Activation in Human Lung Cells and Inflammatory Cytokine Production in Human Lung and Intestinal Epithelial Cells. Microorganisms 2022, 10, 1996. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Liang, T.; Chen, Y.; Zhu, S.; Zhou, L.; Chen, N.; Qian, L.; Wang, Y.; Li, M.; Zhou, X.; et al. SARS-CoV-2 spike protein induces the cytokine release syndrome by stimulating T cells to produce more IL-2. Front. Immunol. 2024, 15, 1444643. [Google Scholar] [CrossRef]
- Shouman, S.; El-Kholy, N.; Hussien, A.E.; El-Derby, A.M.; Magdy, S.; Abou-Shanab, A.M.; Elmehrath, A.O.; Abdelwaly, A.; Helal, M.; El-Badri, N. SARS-CoV-2-associated lymphopenia: Possible mechanisms and the role of CD147. Cell Commun. Signal. 2024, 22, 349. [Google Scholar] [CrossRef]
- Vettori, M.; Dima, F.; Henry, B.M.; Carpene, G.; Gelati, M.; Celegon, G.; Salvagno, G.L.; Lippi, G. Effects of Different Types of Recombinant SARS-CoV-2 Spike Protein on Circulating Monocytes’ Structure. Int. J. Mol. Sci. 2023, 24, 9373. [Google Scholar] [CrossRef]
- Vettori, M.; Carpene, G.; Salvagno, G.L.; Gelati, M.; Dima, F.; Celegon, G.; Favaloro, E.J.; Lippi, G. Effects of Recombinant SARS-CoV-2 Spike Protein Variants on Platelet Morphology and Activation. Semin. Thromb. Hemost. 2023, 50, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Terentes-Printzios, D.; Gardikioti, V.; Solomou, E.; Emmanouil, E.; Gourgouli, I.; Xydis, P.; Christopoulou, G.; Georgakopoulos, C.; Dima, I.; Miliou, A.; et al. The effect of an mRNA vaccine against COVID-19 on endothelial function and arterial stiffness. Hypertens. Res. 2022, 45, 846–855. [Google Scholar] [CrossRef]
- Castro-Robles, B.; Cimas, F.J.; Arias-Salazar, L.; Ontanon, J.; Lozano, J.; Lopez-Lopez, S.; Andres-Pretel, F.; Requena-Calleja, M.A.; Mas, A.; Serrano-Heras, G.; et al. Distinct response patterns of endothelial markers to the BNT162b2 mRNA COVID-19 booster vaccine are associated with the spike-specific IgG antibody production. Front. Immunol. 2024, 15, 1471401. [Google Scholar] [CrossRef] [PubMed]
- Tukaj, S.; Sitna, M.; Sitko, K. The impact of the mRNA COVID-19 vaccine on the Th-like cytokine profile in individuals with no history of COVID-19: Insights into autoimmunity targeting heat shock proteins. Front. Immunol. 2025, 16, 1549739. [Google Scholar] [CrossRef]
- Frasca, L.; Ocone, G.; Palazzo, R. Safety of COVID-19 Vaccines in Patients with Autoimmune Diseases, in Patients with Cardiac Issues, and in the Healthy Population. Pathogens 2023, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Kariko, K.; Tureci, O. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Huynh, T.V.; Rethi, L.; Lee, T.W.; Higa, S.; Kao, Y.H.; Chen, Y.J. Spike Protein Impairs Mitochondrial Function in Human Cardiomyocytes: Mechanisms Underlying Cardiac Injury in COVID-19. Cells 2023, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; Aparicio-Alonso, M.; Henry, M.; Radman, M.; Attal, R.; Bakkar, A. Toxicity of the spike protein of COVID-19 is a redox shift phenomenon: A novel therapeutic approach. Free Radic. Biol. Med. 2023, 206, 106–110. [Google Scholar] [CrossRef]
- Mogi, M. Is COVID-19 vaccination beneficial or harmful to endothelial cells? Hypertens. Res. 2022, 45, 920–921. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Rao, E.; Grover, P.; Zhang, H. Thrombosis after SARS-CoV2 infection or COVID-19 vaccination: Will a nonpathologic anti-PF4 antibody be a solution?-A narrative review. J. Bio-X Res. 2022, 5, 97–103. [Google Scholar] [CrossRef]
- Fujimura, Y.; Holland, L.Z. COVID-19 microthrombosis: Unusually large VWF multimers are a platform for activation of the alternative complement pathway under cytokine storm. Int. J. Hematol. 2022, 115, 457–469. [Google Scholar] [CrossRef]
- Indes, J.E.; Koleilat, I.; Hatch, A.N.; Choinski, K.; Jones, D.B.; Aldailami, H.; Billett, H.; Denesopolis, J.M.; Lipsitz, E. Early experience with arterial thromboembolic complications in patients with COVID-19. J. Vasc. Surg. 2021, 73, 381–389. [Google Scholar] [CrossRef]
- Zhou, Y.; Nishikawa, M.; Kanno, H.; Yang, R.; Ibayashi, Y.; Xiao, T.H.; Peterson, W.; Herbig, M.; Nitta, N.; Miyata, S.; et al. Long-term effects of Pfizer-BioNTech COVID-19 vaccinations on platelets. Cytom. A 2023, 103, 162–167. [Google Scholar] [CrossRef]
- Thachil, J.; Favaloro, E.J.; Lippi, G. D-dimers-“Normal” Levels versus Elevated Levels Due to a Range of Conditions, Including “D-dimeritis,” Inflammation, Thromboembolism, Disseminated Intravascular Coagulation, and COVID-19. Semin. Thromb. Hemost. 2022, 48, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Grobbelaar, L.M.; Venter, C.; Vlok, M.; Ngoepe, M.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: Implications for microclot formation in COVID-19. Biosci. Rep. 2021, 41, BSR20210611. [Google Scholar] [CrossRef]
- Jabagi, M.J.; Botton, J.; Bertrand, M.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA 2022, 327, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, Y.; Rojas, M.; Beltran, S.; Polo, F.; Camacho-Dominguez, L.; Morales, S.D.; Gershwin, M.E.; Anaya, J.M. Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J. Autoimmun. 2022, 132, 102898. [Google Scholar] [CrossRef] [PubMed]
- Fraenza, F.; Cagnotta, C.; Gaio, M.; Sportiello, L.; Scavone, C.; Capuano, A.; Trama, U. Disproportionality analysis of European safety reports on autoimmune and rheumatic diseases following COVID-19 vaccination. Sci. Rep. 2025, 15, 14740. [Google Scholar] [CrossRef] [PubMed]
- Jaycox, J.R.; Lucas, C.; Yildirim, I.; Dai, Y.; Wang, E.Y.; Monteiro, V.; Lord, S.; Carlin, J.; Kita, M.; Buckner, J.H.; et al. SARS-CoV-2 mRNA vaccines decouple anti-viral immunity from humoral autoimmunity. Nat. Commun. 2023, 14, 1299. [Google Scholar] [CrossRef]
- Freiberg, C.; Dotan, A.; Arnheim, D.; Aviel, Y.B. Investigating the association between SARS-CoV-2 infection, COVID-19 vaccination, and autoimmune diseases in a pediatric population: A comprehensive analysis. Pediatr. Rheumatol. 2025, 23, 52. [Google Scholar] [CrossRef]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer-BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021, 207, 2405–2410. [Google Scholar] [CrossRef]
- Montezano, A.C.; Camargo, L.L.; Mary, S.; Neves, K.B.; Rios, F.J.; Stein, R.; Lopes, R.A.; Beattie, W.; Thomson, J.; Herder, V.; et al. SARS-CoV-2 spike protein induces endothelial inflammation via ACE2 independently of viral replication. Sci. Rep. 2023, 13, 14086. [Google Scholar] [CrossRef]
- Embgenbroich, M.; Burgdorf, S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018, 9, 1643. [Google Scholar] [CrossRef]
- Morgan, B.P.; Harris, C.L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 2015, 14, 857–877. [Google Scholar] [CrossRef]
- Serna, M.; Giles, J.L.; Morgan, B.P.; Bubeck, D. Structural basis of complement membrane attack complex formation. Nat. Commun. 2016, 7, 10587. [Google Scholar] [CrossRef]
- Wiedmer, T.; Hall, S.E.; Ortel, T.L.; Kane, W.H.; Rosse, W.F.; Sims, P.J. Complement-induced vesiculation and exposure of membrane prothrombinase sites in platelets of paroxysmal nocturnal hemoglobinuria. Blood 1993, 82, 1192–1196. [Google Scholar] [CrossRef]
- Noris, M.; Remuzzi, G. Overview of complement activation and regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef]
- Gibson, B.G.; Cox, T.E.; Marchbank, K.J. Contribution of animal models to the mechanistic understanding of Alternative Pathway and Amplification Loop (AP/AL)-driven Complement-mediated Diseases. Immunol. Rev. 2023, 313, 194–216. [Google Scholar] [CrossRef]
- Brouwer, N.; Dolman, K.M.; van Zwieten, R.; Nieuwenhuys, E.; Hart, M.; Aarden, L.A.; Roos, D.; Kuijpers, T.W. Mannan-binding lectin (MBL)-mediated opsonization is enhanced by the alternative pathway amplification loop. Mol. Immunol. 2006, 43, 2051–2060. [Google Scholar] [CrossRef]
- De Vrieze, J. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science 2020, 10, 1126. [Google Scholar] [CrossRef]
- Kozma, G.T.; Meszaros, T.; Berenyi, P.; Facsko, R.; Patko, Z.; Olah, C.Z.; Nagy, A.; Fulop, T.G.; Glatter, K.A.; Radovits, T.; et al. Role of anti-polyethylene glycol (PEG) antibodies in the allergic reactions to PEG-containing Covid-19 vaccines: Evidence for immunogenicity of PEG. Vaccine 2023, 41, 4561–4570. [Google Scholar] [CrossRef] [PubMed]
- Dézsi, L.; Mészáros, T.; Kozma, G.; H-Velkei, M.; Oláh, C.Z.; Szabó, M.; Patkó, Z.; Fülöp, T.; Hennies, M.; Szebeni, M.; et al. A naturally hypersensitive porcine model may help understand the mechanism of COVID-19 mRNA vaccine-induced rare (pseudo) allergic reactions: Complement activation as a possible contributing factor. Geroscience 2022, 44, 597–618. [Google Scholar] [CrossRef]
- Bakos, T.; Meszaros, T.; Kozma, G.T.; Berenyi, P.; Facsko, R.; Farkas, H.; Dezsi, L.; Heirman, C.; de Koker, S.; Schiffelers, R.; et al. mRNA-LNP COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions. Int. J. Mol. Sci. 2024, 25, 3595. [Google Scholar] [CrossRef]
- Barta, B.A.; Radovits, T.; Dobos, A.B.; Kozma, G.T.; Mészáros, T.; Berényi, P.; Facskó, R.; Fülöp, T.; Merkely, B.; Szebeni, J. Comirnaty-induced cardiopulmonary distress and other symptoms of complement-mediated pseudo-anaphylaxis in a hyperimmune pig model: Causal role of anti-PEG antibodies. Vaccine X 2024, 19, 100497. [Google Scholar] [CrossRef]
- Warren, C.M.; Snow, T.T.; Lee, A.S.; Shah, M.M.; Heider, A.; Blomkalns, A.; Betts, B.; Buzzanco, A.S.; Gonzalez, J.; Chinthrajah, R.S.; et al. Assessment of Allergic and Anaphylactic Reactions to mRNA COVID-19 Vaccines with Confirmatory Testing in a US Regional Health System. JAMA Netw. Open 2021, 4, e2125524. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Sharma, S.; Kumar, G.; Thakur, S. Unravelling the complexity of CARPA: A review of emerging advancements in therapeutic strategies. Arch. Dermatol. Res. 2025, 317, 439. [Google Scholar] [CrossRef]
- Risso, V.; Lafont, E.; Le Gallo, M. Therapeutic approaches targeting CD95L/CD95 signaling in cancer and autoimmune diseases. Cell Death Dis. 2022, 13, 248. [Google Scholar] [CrossRef]
- Ramirez-Labrada, A.; Pesini, C.; Santiago, L.; Hidalgo, S.; Calvo-Perez, A.; Onate, C.; Andres-Tovar, A.; Garzon-Tituana, M.; Uranga-Murillo, I.; Arias, M.A.; et al. All About (NK Cell-Mediated) Death in Two Acts and an Unexpected Encore: Initiation, Execution and Activation of Adaptive Immunity. Front. Immunol. 2022, 13, 896228. [Google Scholar] [CrossRef]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A.; et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Vlatkovic, I. Non-Immunotherapy Application of LNP-mRNA: Maximizing Efficacy and Safety. Biomedicines 2021, 9, 530. [Google Scholar] [CrossRef]
- Igyarto, B.; Qin, Z. The mRNA-LNP vaccines—The good, the bad and the ugly? Front. Immunol. 2024, 15, 1336906. [Google Scholar] [CrossRef] [PubMed]
- Igyarto, B.Z.; Jacobsen, S.; Ndeupen, S. Future considerations for the mRNA-lipid nanoparticle vaccine platform. Curr. Opin. Virol. 2021, 48, 65–72. [Google Scholar] [CrossRef]
- Kew, O.M.; Sutter, R.W.; de Gourville, E.M.; Dowdle, W.R.; Pallansch, M.A. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 2005, 59, 587–635. [Google Scholar] [CrossRef]
- Barrett, A.D.; Teuwen, D.E. Yellow fever vaccine—How does it work and why do rare cases of serious adverse events take place? Curr. Opin. Immunol. 2009, 21, 308–313. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Different Types of COVID-19 Vaccines. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained (accessed on 30 July 2025).
- Castruita, J.A.S.; Schneider, U.V.; Mollerup, S.; Leineweber, T.D.; Weis, N.; Bukh, J.; Pedersen, M.S.; Westh, H. SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. APMIS 2023, 131, 128–132. [Google Scholar] [CrossRef]
- Hanna, N.; Heffes-Doon, A.; Lin, X.; Manzano De Mejia, C.; Botros, B.; Gurzenda, E.; Nayak, A. Detection of Messenger RNA COVID-19 Vaccines in Human Breast Milk. JAMA Pediatr. 2022, 176, 1268–1270. [Google Scholar] [CrossRef]
- Hanna, N.; De Mejia, C.M.; Heffes-Doon, A.; Lin, X.; Botros, B.; Gurzenda, E.; Clauss-Pascarelli, C.; Nayak, A. Biodistribution of mRNA COVID-19 vaccines in human breast milk. EBioMedicine 2023, 96, 104800. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; Kyriakopoulos, A.M.; Brogna, C.; Piscopo, M.; McCullough, P.A.; Seneff, S. Long-lasting, biochemically modified mRNA, and its frameshifted recombinant spike proteins in human tissues and circulation after COVID-19 vaccination. Pharmacol. Res. Perspect. 2024, 12, e1218. [Google Scholar] [CrossRef] [PubMed]

|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| |
| Strains | References |
|---|---|
| Cytomegalovirus (CMV) | [21,22] |
| Epstein–Barr virus (EBV) | [23] |
| Herpes simplex virus (HSV) | [24,25,26] |
| Varicella Zoster virus (VZV) | [27,28] |
| Vessel Size | Types of Vasculitis | Typical Symptoms |
|---|---|---|
| Small |
|
|
| Medium |
|
|
| Large |
|
|
| Abnormality | Contributing Mechanisms | Potential Consequences | References |
|---|---|---|---|
| Endothelial cell activation |
|
| [109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133] |
| Vascular injury |
| ||
| Thrombogenesis |
|
| [47,119,134,135,136,137,138,139,140,141] |
| Autoimmune damages |
|
| [127,128,142,143,144,145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szebeni, J.; Koller, A. Multisystem Endothelial Inflammation: A Key Driver of Adverse Events Following mRNA-Containing COVID-19 Vaccines. Vaccines 2025, 13, 855. https://doi.org/10.3390/vaccines13080855
Szebeni J, Koller A. Multisystem Endothelial Inflammation: A Key Driver of Adverse Events Following mRNA-Containing COVID-19 Vaccines. Vaccines. 2025; 13(8):855. https://doi.org/10.3390/vaccines13080855
Chicago/Turabian StyleSzebeni, János, and Akos Koller. 2025. "Multisystem Endothelial Inflammation: A Key Driver of Adverse Events Following mRNA-Containing COVID-19 Vaccines" Vaccines 13, no. 8: 855. https://doi.org/10.3390/vaccines13080855
APA StyleSzebeni, J., & Koller, A. (2025). Multisystem Endothelial Inflammation: A Key Driver of Adverse Events Following mRNA-Containing COVID-19 Vaccines. Vaccines, 13(8), 855. https://doi.org/10.3390/vaccines13080855







