Humoral and Cellular Immune Responses Against SARS-CoV-2 Following COVID-19 Vaccination in Older Adults: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Objectives
2.3. Eligibility Criteria
2.4. Information Sources
2.5. Search Strategy
2.6. Study Selection Process
2.7. Data Extraction Process
2.8. Risk of Bias and Quality Assessment
2.9. Statistical Analysis
3. Results
3.1. Selection of Studies
3.2. Characteristics of the Included Studies
3.3. Humoral and Cellular Immune Response
3.3.1. Humoral Immune Response
3.3.2. Cellular Immune Response
3.4. Sensitivity Analyses
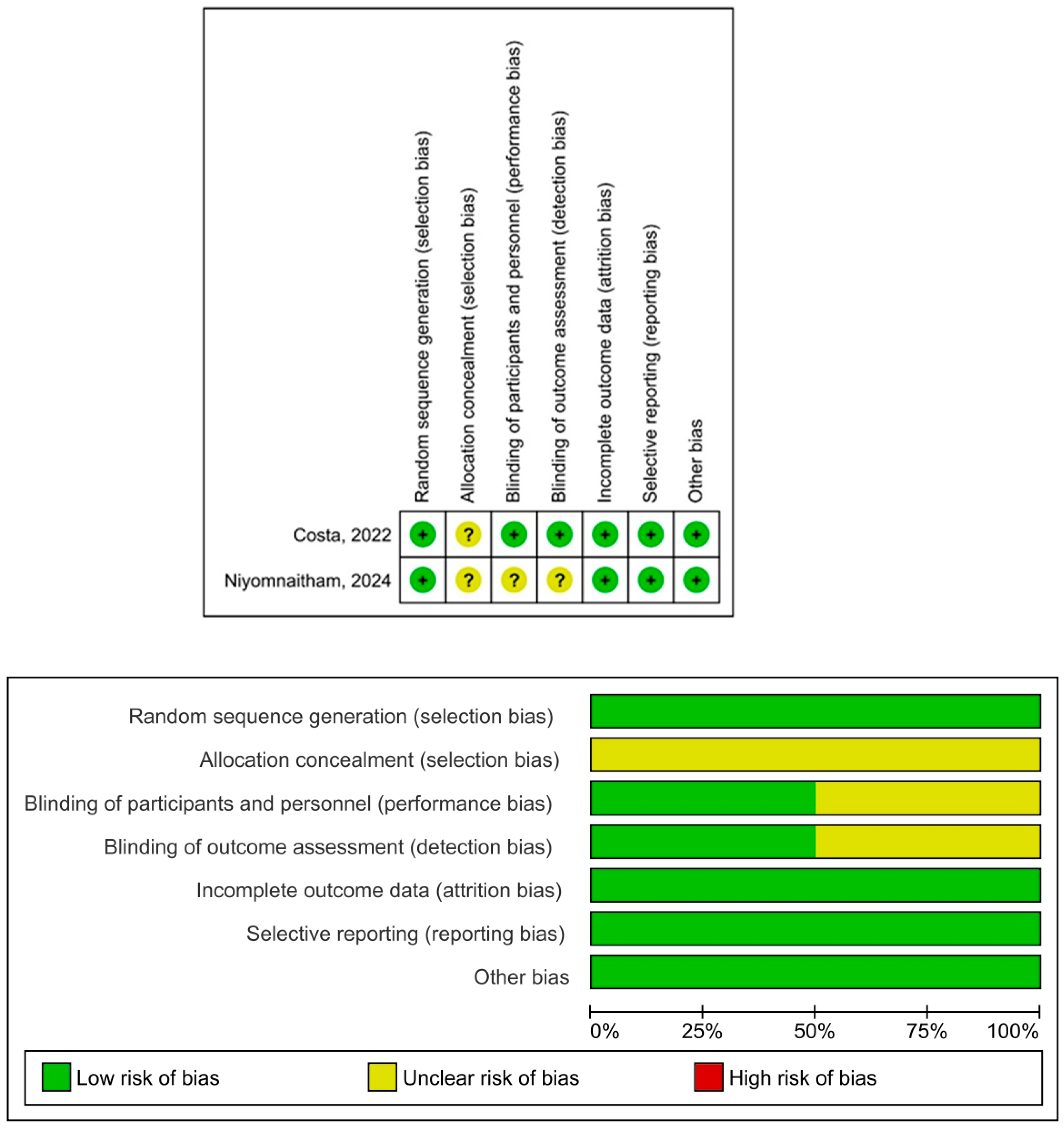
3.5. Risk of Bias and Quality of the Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Stasi, C.; Fallani, S.; Voller, F.; Silvestri, C. Treatment for COVID-19: An overview. Eur. J. Pharmacol. 2020, 889, 173644. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef]
- Abul, Y.; Leeder, C.; Gravenstein, S. Epidemiology and clinical presentation of COVID-19 in older adults. Infect. Dis. Clin. N. Am. 2023, 37, 1–26. [Google Scholar] [CrossRef]
- Chary, M.; Barbuto, A.F.; Izadmehr, S.; Tarsillo, M.; Fleischer, E.; Burns, M.M. COVID-19 therapeutics: Use, mechanism of action, and toxicity (vaccines, monoclonal antibodies, and immunotherapeutics). J. Med. Toxicol. 2023, 19, 205–218. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Tian, L.; Pang, Z.; Yang, Q.; Huang, T.; Fan, J.; Song, L.; Tong, Y.; Fan, H. COVID-19 vaccine development: Milestones, lessons and prospects. Signal Transduct. Target. Ther. 2022, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Older Adults and COVID-19 Vaccines—Social Networking Collection. PAHO/WHO. 2021. Available online: https://www.paho.org/es/documentos/adultos-mayores-vacunas-contra-covid-19-coleccion-redes-sociales (accessed on 1 May 2025).
- Xu, K.; Wang, Z.; Qin, M.; Gao, Y.; Luo, N.; Xie, W.; Zou, Y.; Wang, J.; Ma, X. A systematic review and meta-analysis of the effectiveness and safety of COVID-19 vaccination in older adults. Front. Immunol. 2023, 14, 1113156. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Flores, R.R.; Jang, I.H.; Saathoff, A.; Robbins, P.D. Immune senescence, immunosenescence and aging. Front. Aging 2022, 3, 900028. [Google Scholar] [CrossRef]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Ouslander, J.G.; Saliba, D. Early success of COVID-19 vaccines in nursing homes: Will it stick? J. Am. Geriatr. Soc. 2021, 69, 2060–2062. [Google Scholar] [CrossRef]
- Fukushima, K.; Kubo, T.; Ito, Y.; Oda, Y.; Nagayoshi, Y.; Fukuda, M.; Takazono, T.; Sakamoto, N.; Mukae, H. Humoral and cellular immune responses to mRNA COVID-19 vaccinations in the elderly: A longitudinal study in Japan. J. Infect. Chemother. 2025, 31, 102695. [Google Scholar] [CrossRef]
- Bredholt, G.; Sævik, M.; Søyland, H.; Ueland, T.; Zhou, F.; Pathirana, R.; Madsen, A.; Vahokoski, J.; Lartey, S.; Halvorsen, B.E.; et al. Three doses of SARS-CoV-2 mRNA vaccine in older adults result in similar antibody responses but reduced cellular cytokine responses relative to younger adults. Vaccine X 2024, 20, 100564. [Google Scholar] [CrossRef]
- Dalla Gasperina, D.; Veronesi, G.; Castelletti, C.M.; Varchetta, S.; Ottolini, S.; Mele, D.; Ferrari, G.; Shaik, A.K.B.; Celesti, F.; Dentali, F.; et al. Humoral and cellular immune response elicited by the BNT162b2 COVID-19 vaccine booster in elderly. Int. J. Mol. Sci. 2023, 24, 13728. [Google Scholar] [CrossRef]
- Saiag, E.; Alcalay, Y.; Marudi, O.; Orr-Urtreger, A.; Hagin, D. Cellular and humoral immune response to the fourth Pfizer-BioNTech COVID-19 vaccine dose in individuals aged 60 years and older. Vaccine 2023, 41, 914–921. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.K.L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Tut, G.; Lancaster, T.; Krutikov, M.; Sylla, P.; Bone, D.; Kaur, N.; Spalkova, E.; Bentley, C.; Amin, U.; Jadir, A.T.; et al. Profile of humoral and cellular immune responses to single doses of BNT162b2 or ChAdOx1 nCoV-19 vaccines in residents and staff within residential care homes (VIVALDI): An observational study. Lancet Healthy Longev. 2021, 2, e544–e553. [Google Scholar] [CrossRef]
- Costa, P.R.; Correia, C.A.; Marmorato, M.P.; Dias, J.Z.C.; Thomazella, M.V.; Cabral da Silva, A.; de Oliveira, A.C.S.; Gusmão, A.F.; Ferrari, L.; Freitas, A.C.; et al. Humoral and cellular immune responses to CoronaVac up to one year after vaccination. Front. Immunol. 2022, 13, 1032411. [Google Scholar] [CrossRef]
- Rouers, A.; Wong, N.; Goh, Y.S.; Torres-Ruesta, A.; Tay, M.Z.; Chang, Z.W.; Fong, S.W.; Neo, V.; Kam, I.K.J.; Yeo, N.K.; et al. Efficient recall of SARS-CoV-2 variant-reactive B cells and T responses in the elderly upon heterologous mRNA vaccines as boosters. J. Med. Virol. 2023, 95, e28258. [Google Scholar] [CrossRef]
- Chaiwong, W.; Takheaw, N.; Pata, S.; Laopajon, W.; Duangjit, P.; Inchai, J.; Pothirat, C.; Bumroongkit, C.; Deesomchok, A.; Theerakittikul, T.; et al. Neutralizing antibody and T-cell responses against SARS-CoV-2 variants by heterologous CoronaVac/ChAdOx-1 vaccination in elderly subjects with chronic obstructive pulmonary disease. Vaccine 2023, 41, 5901–5909. [Google Scholar] [CrossRef]
- Dudley, H.M.; O’Mara, M.; Auma, A.; Gong, J.; Ross, Y.; Gurevich, N.; Carbone, S.; Reihs, A.; Nguyen, Y.; McComsey, G.A.; et al. Rheumatoid arthritis and older age are associated with lower humoral and cellular immune response to primary series COVID-19 mRNA vaccine. Vaccine 2023, 41, 6112–6119. [Google Scholar] [CrossRef]
- Kometani, K.; Yorimitsu, T.; Jo, N.; Yamaguchi, E.; Kikuchi, O.; Fukahori, M.; Sawada, T.; Tsujimoto, Y.; Sunami, A.; Li, M.; et al. Booster COVID-19 mRNA vaccination ameliorates impaired B-cell but not T-cell responses in older adults. Front. Immunol. 2024, 15, 1455334. [Google Scholar] [CrossRef]
- Niyomnaitham, S.; Chokephaibulkit, K.; Pheerapanyawaranun, C.; Toh, Z.Q.; Licciardi, P.V.; Satayasanskul, A.; Jansarikit, L.; Assantachai, P. Immunogenicity of BNT162b2 as a first booster after a ChAdOx1 primary series in a Thai geriatric population living with frailty. J. Nutr. Health Aging 2024, 28, 100315. [Google Scholar] [CrossRef]
- Segato, I.; Mele, D.; Forlani, G.; Dalla Gasperina, D.; Mondelli, M.U.; Varchetta, S. T cell responses to BA.2.86 and JN.1 SARS-CoV-2 variants in elderly subjects. Vaccines 2024, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.W.T.; Boon, L.H.; Cui, J.; Juequn, Z.; Shunmuganathan, B.; Gupta, R.; Tan, N.Y.J.; Qian, X.; Purushotorman, K.; SCOPE Cohort Study Group; et al. Relative deficiency in interferon-γ-secreting CD4+ T cells is strongly associated with poorer COVID-19 vaccination responses in older adults. Aging Cell 2024, 23, e14099. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.K.; Lee, W.J.; Peng, L.N.; Meng, L.C.; Hsiao, F.Y.; Chen, L.K. COVID-19 vaccines in older adults. Clin. Geriatr. Med. 2022, 38, 605–620. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Li, F.; Li, Y.; Li, Y.; Peng, P.; Li, S.; He, L.; Liu, T. Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 965971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, L.; Tian, T.; Li, W.; Pan, Y.; Wang, Y. Efficacy and safety of COVID-19 vaccination in older adults: A systematic review and meta-analysis. Vaccines 2022, 11, 33. [Google Scholar] [CrossRef]
- Berber, E.; Ross, T.M. Factors predicting COVID-19 vaccine effectiveness and longevity of humoral immune responses. Vaccines 2024, 12, 1284. [Google Scholar] [CrossRef]
- Scourfield, D.O.; Reed, S.G.; Quastel, M.; Alderson, J.; Bart, V.M.T.; Teijeira Crespo, A.; Jones, R.; Pring, E.; Richter, F.C.; Oxford-Cardiff COVID-19 Literature Consortium; et al. The role and uses of antibodies in COVID-19 infections: A living review. Oxf. Open Immunol. 2021, 2, iqab003. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, K.; Li, Y.; Lu, C.; Ling, K.; Cai, C.; Wang, W.; Ye, D. Targeting TNF-α for COVID-19: Recent Advanced and Controversies. Front. Public. Health 2022, 10, 833967. [Google Scholar] [CrossRef]
- Mohd Zawawi, Z.; Kalyanasundram, J.; Mohd Zain, R.; Thayan, R.; Basri, D.F.; Yap, W.B. Prospective Roles of Tumor Necrosis Factor-Alpha (TNF-α) in COVID-19: Prognosis, Therapeutic and Management. Int. J. Mol. Sci. 2023, 24, 6142. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Han, Y.; Gu, Z.; Sun, C. Immunosenescence, aging and successful aging. Front. Immunol. 2022, 13, 942796. [Google Scholar] [CrossRef]
- de Mol, J.; Kuiper, J.; Tsiantoulas, D.; Foks, A.C. The Dynamics of B Cell Aging in Health and Disease. Front. Immunol. 2021, 12, 733566. [Google Scholar] [CrossRef]
- Martinez, F.; Novarino, J.; Mejía, J.E.; Fazilleau, N.; Aloulou, M. Ageing of T-dependent B cell responses. Immunol. Lett. 2021, 233, 97–103. [Google Scholar] [CrossRef]
- Sturmlechner, I.; Jain, A.; Mu, Y.; Weyand, C.M.; Goronzy, J.J. T cell fate decisions during memory cell generation with aging. Semin. Immunol. 2023, 69, 101800. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Lee, W.W.; Cui, D.; Hiruma, Y.; Lamar, D.L.; Yang, Z.Z.; Ouslander, J.G.; Weyand, C.M.; Goronzy, J.J. T cell subset-specific susceptibility to aging. Clin. Immunol. 2008, 127, 107–118. [Google Scholar] [CrossRef]
- Rodriguez, I.J.; Lalinde Ruiz, N.; Llano León, M.; Martínez Enríquez, L.; Montilla Velásquez, M.D.P.; Ortiz Aguirre, J.P.; Rodríguez Bohórquez, O.M.; Velandia Vargas, E.A.; Hernández, E.D.; Parra López, C.A. Immunosenescence Study of T Cells: A Systematic Review. Front. Immunol. 2021, 11, 604591. [Google Scholar] [CrossRef]
- Coperchini, F.; Greco, A.; Teliti, M.; Croce, L.; Chytiris, S.; Magri, F.; Gaetano, C.; Rotondi, M. Inflamm-ageing: How cytokines and nutrition shape the trajectory of ageing. Cytokine Growth Factor Rev. 2025, 82, 31–42. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Immunosenescence: The potential role of myeloid-derived suppressor cells (MDSC) in age-related immune deficiency. Cell Mol. Life Sci. 2019, 76, 1901–1918. [Google Scholar] [CrossRef]
- Collins, C.P.; Longo, D.L.; Murphy, W.J. The immunobiology of SARS-CoV-2 infection and vaccine responses: Potential influences of cross-reactive memory responses and aging on efficacy and off-target effects. Front. Immunol. 2024, 15, 1345499. [Google Scholar] [CrossRef]
- Silva-Cayetano, A.; Fra-Bido, S.; Robert, P.A.; Innocentin, S.; Burton, A.R.; Watson, E.M.; Lee, J.L.; Webb, L.M.C.; Foster, W.S.; McKenzie, R.C.J.; et al. Spatial dysregulation of T follicular helper cells impairs vaccine responses in aging. Nat. Immunol. 2023, 24, 1124–1137. [Google Scholar] [CrossRef]
- Gustafson, C.E.; Kim, C.; Weyand, C.M.; Goronzy, J.J. Influence of immune aging on vaccine responses. J. Allergy Clin. Immunol. 2020, 145, 1309–1321. [Google Scholar] [CrossRef]
- Joosten, S.A. Individual- and population-associated heterogeneity in vaccine-induced immune responses. The impact of inflammatory status and diabetic comorbidity. Semin. Immunol. 2025, 78, 101964. [Google Scholar] [CrossRef]
- Buja, A.; Grotto, G.; Taha, M.; Cocchio, S.; Baldo, V. Use of information and communication technology strategies to increase vaccination coverage in older adults: A systematic review. Vaccines 2023, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Pinho, C.; Oliveira, A.; Santos, R.; Felgueiras, M.; Martins, J.P. Vaccination Promotion Strategies in the Elderly: Systematic Review and Meta-Analysis. Vaccines 2024, 12, 1395. [Google Scholar] [CrossRef]
- Meng, H.; Mao, J.; Ye, Q. Strategies and safety considerations of booster vaccination in COVID-19. Bosn. J. Basic Med. Sci. 2022, 22, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kani, R.; Iwagami, M.; Takagi, H.; Yasuhara, J.; Kuno, T. Assessment of efficacy and safety of mRNA COVID-19 vaccines in children aged 5 to 11 years. JAMA Pediatr. 2023, 177, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Luo, X.; Shen, Q.; Lei, R.; Liu, X.; Liu, E.; Li, Q.; Chen, Y. Safety, immunogenicity, and efficacy of COVID-19 vaccines in children and adolescents: A systematic review. Vaccines 2021, 9, 1102. [Google Scholar] [CrossRef]
- Wang, H.; Gan, M.; Wu, B.; Zeng, R.; Wang, Z.; Xu, J.; Li, J.; Zhang, Y.; Cao, J.; Chen, L.; et al. Humoral and cellular immunity of two-dose inactivated COVID-19 vaccination in Chinese children: A prospective cohort study. J. Med. Virol. 2023, 95, e28380. [Google Scholar] [CrossRef]
- Wang, J.; Tong, Y.; Li, D.; Li, J.; Li, Y. The impact of age difference on the efficacy and safety of COVID-19 vaccines: A systematic review and meta-analysis. Front. Immunol. 2021, 12, 758294. [Google Scholar] [CrossRef]
- Wagner, A.; Garner-Spitzer, E.; Jasinska, J.; Kollaritsch, H.; Stiasny, K.; Kundi, M.; Wiedermann, U. Age-related differences in humoral and cellular immune responses after primary immunisation: Indications for stratified vaccination schedules. Sci. Rep. 2018, 8, 9825. [Google Scholar] [CrossRef]
- Hayati, T.; Kartinah, N.T.; Wibowo, H.; Purwoko, R.Y. Comparison of inflammatory mediator cytokine responses to inactivated virus platform COVID-19 vaccines between elderly and young adult populations. Narra J. 2024, 4, e1380. [Google Scholar] [CrossRef] [PubMed]
- Montmaneix-Engels, F.; Dimeglio, C.; Staes, L.; Da Silva, I.; Porcheron, M.; Jougla, I.; Hérin, F.; Izopet, J. Study of the cellular and humoral immune responses to SARS-CoV-2 vaccination. Heliyon 2024, 10, e29116. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Dallan, B.; Proietto, D.; De Laurentis, M.; Gallerani, E.; Martino, M.; Ghisellini, S.; Zurlo, A.; Volpato, S.; Govoni, B.; Borghesi, M.; et al. Age differentially impacts adaptive immune responses induced by adenoviral versus mRNA vaccines against COVID-19. Nat. Aging 2024, 4, 1121–1136. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Cohen, A.A.; Provost, G.; Khalil, A.; Lacombe, G.; Rodrigues, S.; Desroches, M.; Hirokawa, K.; et al. Immunosenescence and Altered Vaccine Efficiency in Older Subjects: A Myth Difficult to Change. Vaccines 2022, 10, 607. [Google Scholar] [CrossRef]
| First Author Name/Year of Publication | Country | Design | Period | Total, Sample n | Age—Older Adults | Antecedents—Comorbidities | Sample Immune Response | Masculine n (%) | Vaccine Type (Technology) | Dose | Test or Assay to Evaluate CIR | Test or Assay to Evaluate HIR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tut et al., 2021 [19] | UK | Cohort | 11 December 2020–16 February 2021 | 35 | ≥65 | None | 35 | NR | Pfizer-BioNTech (mRNA) Oxford-AstraZeneca (viral vector) | 1st | Human IFN-γ ELISpotPRO | V-PLEX SARS-CoV-2 Panel 2 |
| Costa et al., 2022 [20] | Brazil | Controlled clinical trial | July 2020—NR | 24 | ≥60 | None | 24 | 16 (66.7) | CoronaVac (Inactivated) | 2nd | AIM/flow cytometry | Electrochemiluminescence multiplex serology assay |
| Rouers et al., 2022 [21] | Singapore | Cohort | NR | 41 | ≥60 | None | 41 | 20 (48.7) | Pfizer-BioNTech (mRNA) Moderna (mRNA) | 2nd | ICS Multicolored flow cytometry | ELISpot Flow Cytometry Pseudovirus neutralization |
| Chaiwong et al., 2023 [22] | Thailand | Cohort | July 2021 and January 2022 | 30 | ≥65 | COPD | 30 | 25 (83.3) | CoronaVac (Inactivated) Oxford—AstraZeneca (viral vector) | 2nd | BD FACSCelestaTM flow cytometer | cPass SARS-CoV-2 |
| Dalla et al., 2023 [13] | Italy | Cohort | October 2021–January 2022 | 49 | ≥70 | Dementia CVD Diabetes COPD Autoimmune disease | 49 | 12 (24.4) | Pfizer-BioNTech (mRNA) | 3rd | ELISpot | ELISA Competitive ELISA for Nab |
| Dudley et al., 2023 [23] | USA | Cohort | February 2021–January 2022 | 46 | ≥60 | RA HBP Diabetes CAD | 46 | 37 (80) | Pfizer-BioNTech (mRNA) Moderna (mRNA) | 3rd | ELISPOT | Bead multiplex immunoassay Pseudovirus neutralization assay |
| Saiag et al., 2023 [14] | Israel | Cohort | August 2021–January 2022 | 133 | ≥60 | None | 133 | 50 (37.5) | Pfizer-BioNTech (mRNA) | 4th | SARS-CoV-2 T-Cell Analysis Kits for human PBMCs | ADVIA Centaur SARS-CoV-2 IgG CMIA |
| Bredholt et al., 2024 [12] | Norway | Cohort | NR | 68 | ≥70 | CHD CLD RD Diabetes Cancer Immunosuppression CKD Neurological disease | 68 | 29 (43) | Pfizer–BioNTech (mRNA) Moderna (mRNA) | 3rd | FluoroSpot ELISpot | ELISA Microneutralization |
| Kometani et al., 2024 [24] | Japan | Cohort | NR | 105 | ≥65 | None | 105 | 55 (50.4) | Pfizer-BioNTech (mRNA) | 3rd | AIM | Surrogate Virus Neutralization Assay Flow cytometry |
| Niyomnaitham et al., 2024 [25] | Thailand | Randomized clinical trial | 9 January–8 August 2022 | 139 | ≥65 | HBP Dyslipidemia DM | 139 | 45 (32.37) | Pfizer-BioNTech (mRNA) Oxford-AstraZeneca (viral vector) | 2nd | ELISpot | CMIA Neutralization assay |
| Segato et al., 2024 [26] | Italy | Cohort | January 2024–15 March 2024 | 18 | ≥65 | Diabetes COPD CVD CRF | 18 | 12 (66.6) | Pfizer-BioNTech (mRNA) | ≥3rd | ELISpot AIM | ELISA |
| Vanda et al., 2024 [27] | Singapore | Cohort | NR | 14 | ≥70 | None | 14 | NR | Pfizer-BioNTech (mRNA) | 2nd | ICS Flow cytometry | High-dimensional flow cytometry |
| Fukushima et al., 2025 [11] | Japan | Cohort | September 2021–March 2022 | 80 | ≥70 | None | 80 | 9 (11.2) | mRNA | 2nd | QuantiFERON | SARS-CoV-2 IgG II Quant |
| First Author Name/Year of Publication | Humoral Immune Response | Findings HIR | Cellular Immune Response | Findings CIR | Results | Overall Conclusions |
|---|---|---|---|---|---|---|
| Tut et al., 2021 [19] | ↑ IgG levels | Moderate inhibition against B.1.1.7, B.1.351, and P.1 variants after the first dose of Pfizer-BioNTech or AstraZeneca; reduced humoral response. | ↑ IFN-γ | Cellular response comparable to young adults after first dose of Pfizer or AstraZeneca. | No correlation was observed between IgG and IFN-γ values. | HIR decreases in older adults without prior infection. CIR is maintained under pre-infection conditions. |
| Costa et al., 2022 [20] | IgG levels are maintained | Stable IgG levels over one year with CoronaVac; no significant increase against N protein. | ↓ IFN-γ levels | Increase in CD40L+ T cells during first 180 days with CoronaVac; decline thereafter. | HIR is maintained and the CIR decreases; no correlation between IgG and IFN-γ. | The HIR is maintained and the CIR decreases in older adults. |
| Rouers et al., 2022 [21] | ↑ IgG levels | Higher levels of neutralizing antibodies and IgG memory B cells after heterologous mRNA booster. | ↑ IFN-γ levels | Increase in Th1, Th2, Th17, and follicular helper T cells after heterologous mRNA booster. | HIR and CIR are enhanced by heterologous boosters in the elderly. | CIR and HIR increase in older adults after the heterologous mRNA booster vaccine. |
| Chaiwong et al., 2023 [22] | ↑ IgG anti-S1 levels | Neutralizing antibodies detected against Wuhan, Alpha, Beta, and Delta variants; weak response to Omicron. | Limited IFN-γ and TNF-α response | Induction of CD4+ TNF-α, IFN-γ, IL-4, IL-17, IL-10, and FasL at 4 weeks after CoronaVac/ChAdOx1 schedule. | Humoral response against the Alpha, Beta, and Delta variants, and a limited cellular response. | CIR limited and HIR increases in older adults. |
| Dalla et al., 2023 [13] | ↑ IgG levels | Significant IgG increase after the second dose and Pfizer-BioNTech booster. | ↑ IFN-γ levels | Significant increase in T-cell response after second dose and Pfizer booster. | HIR and CIR increase after booster vaccine; no correlation between IgG and IFN-γ values. | CIR and HIR increase in older adult patients after BNT162b2 booster. |
| Dudley et al., 2023 [23] | ↓ IgG levels | Low IgG and neutralizing antibody levels after second dose; negative correlation with age. | ↓ IFN-γ levels | Reduced IL-2, IFN-γ, and T-cell response in older adults. | A correlation was observed between IgG and IFN-γ levels. | CIR and HIR decreases in older adult patients. |
| Saiag et al., 2023 [14] | ↑ IgG levels | Transient increase in anti-Spike antibodies after the fourth dose; progressive IgG decline. | ↑ IFN-γ and TNF-α | Increase in CD4+ IFN-γ and TNF-α after the fourth Pfizer dose. | The fourth dose of vaccine significantly enhanced both humoral and cell reactivity. | CIR and HIR increase in older adult patients. |
| Bredholt et al., 2024 [12] | ↑ IgG levels | Increased neutralizing antibodies after Pfizer-BioNTech booster; no improvement in cellular immunity. | There is no significant improvement in T-cell immunity. | No robust cellular response after third dose; possible T-cell exhaustion. | Lower humoral and cell responses in older adults. | Booster dose improves HIR and CIR decreases in older adult patients. |
| Kometani et al., 2024 [24] | ↑ IgG levels | Pfizer booster stimulated memory B cells; low CD8+ T-cell response. | ↓ Cells T CD8+ and cTfh1 | Reduced production of follicular helper T cells; improved after booster. | HIR increase after mRNA booster; no correlation between IgG levels and T cells. | HIR increase in older adults, while the CIR decreases with age. |
| Niyomnaitham et al., 2024 [25] | ↑ IgG levels | Lower antibody titers against Wuhan strain in frail older adults; comparable Omicron response. | ↑ IFN-γ | Spike-specific T-cell response similar between frail and non-frail participants. | Reduced IgG antibody levels in the frail IM group; similar IFN-γ secretion in both groups. | CIR and HIR increase in older adult patients. |
| Segato et al., 2024 [26] | IgG levels are maintained | Reduced IgG against JN.1 after Pfizer booster. | ↓ IFN-γ levels | Lower CD4+ responses against JN.1 and BA.2.86 after Pfizer booster. | IgG antibody levels were uniform and levels of IFN-γ were reduced. | CIR decreases in older adult patients. |
| Vanda et al., 2024 [27] | ↓ IgG levels | It focused mainly on T cells. | ↓ IFN-γ and TNFα levels | Reduced IFN-γ levels and lower proportion of IFN-γ–secreting CD4+ T cells. | HIR and CIR are reduced in older adults; correlation between IgG and IFN-γ. | HIR and CIR are reduced in older adults. |
| Fukushima et al., 2025 [11] | ↓ IgG levels | Progressive decline in IgG levels with age and time after mRNA vaccination. | ↓ IFN-γ levels | Progressive IFN-γ decline with age in individuals over 70. | No correlation was observed between IgG antibody levels and secreted IFN-γ values. | CIR and HIR decreases in older adult patients. |
| First Author/Year of Publication | Selection | Comparability | Exposure/Outcome | Score |
|---|---|---|---|---|
| Tut et al., 2021 [19] | **** | * | *** | 8 |
| Rouers et al., 2022 [21] | *** | * | *** | 7 |
| Chaiwong et al., 2023 [22] | *** | * | *** | 7 |
| Dalla et al., 2023 [13] | **** | * | ** | 7 |
| Dudley et al., 2023 [23] | **** | * | ** | 7 |
| Bredholt et al., 2024 [12] | *** | * | *** | 7 |
| Saiag et al., 2023 [14] | *** | * | *** | 7 |
| Kometani et al., 2024 [24] | **** | * | ** | 7 |
| Segato et al., 2024 [26] | **** | * | *** | 8 |
| Vanda et al., 2024 [27] | **** | * | ** | 7 |
| Fukushima et al., 2025 [11] | *** | * | *** | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
la Cruz, R.A.R.-D.; Flores-Córdova, J.M.; Calderon-Hernandez, C.C.; Cahuapaza-Gutierrez, N.L.; Ccallalli-Ruiz, N.A.; Runzer-Colmenares, F.M. Humoral and Cellular Immune Responses Against SARS-CoV-2 Following COVID-19 Vaccination in Older Adults: A Systematic Review. Vaccines 2025, 13, 852. https://doi.org/10.3390/vaccines13080852
la Cruz RAR-D, Flores-Córdova JM, Calderon-Hernandez CC, Cahuapaza-Gutierrez NL, Ccallalli-Ruiz NA, Runzer-Colmenares FM. Humoral and Cellular Immune Responses Against SARS-CoV-2 Following COVID-19 Vaccination in Older Adults: A Systematic Review. Vaccines. 2025; 13(8):852. https://doi.org/10.3390/vaccines13080852
Chicago/Turabian Stylela Cruz, Ruth Angélica Rojas-De, Janeth M. Flores-Córdova, Cielo Cinthya Calderon-Hernandez, Nelson Luis Cahuapaza-Gutierrez, Nino Arturo Ccallalli-Ruiz, and Fernando M. Runzer-Colmenares. 2025. "Humoral and Cellular Immune Responses Against SARS-CoV-2 Following COVID-19 Vaccination in Older Adults: A Systematic Review" Vaccines 13, no. 8: 852. https://doi.org/10.3390/vaccines13080852
APA Stylela Cruz, R. A. R.-D., Flores-Córdova, J. M., Calderon-Hernandez, C. C., Cahuapaza-Gutierrez, N. L., Ccallalli-Ruiz, N. A., & Runzer-Colmenares, F. M. (2025). Humoral and Cellular Immune Responses Against SARS-CoV-2 Following COVID-19 Vaccination in Older Adults: A Systematic Review. Vaccines, 13(8), 852. https://doi.org/10.3390/vaccines13080852