Baseline Analysis of Serotype-Specific IgG Antibody Levels for 13-Valent Pneumococcal Conjugate Vaccine in Healthy Chinese Individuals: A Multicenter Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Pneumococcal Polysaccharide Antigen
2.3. Serotype-Specific IgG Antibody ELISA
2.4. Statistical Analysis
- (1)
- Descriptive Statistics: Overall distribution of IgG levels was summarized nationwide without stratification.
- (2)
- Two-Way ANOVA: Baseline antibody levels (log-transformed) were assessed for fixed effects of region, age, and gender.
- (3)
- Pairwise Comparisons: GMC and seropositivity by region; region-stratified comparisons by age group; GMC and seropositivity by age group; age-stratified comparisons across regions.
- (4)
- Gender-Based Comparisons: National-level GMC and seropositivity differences between males and females; region-stratified comparisons by gender; age group-stratified comparisons by gender.
2.5. Ethical Approval
3. Results
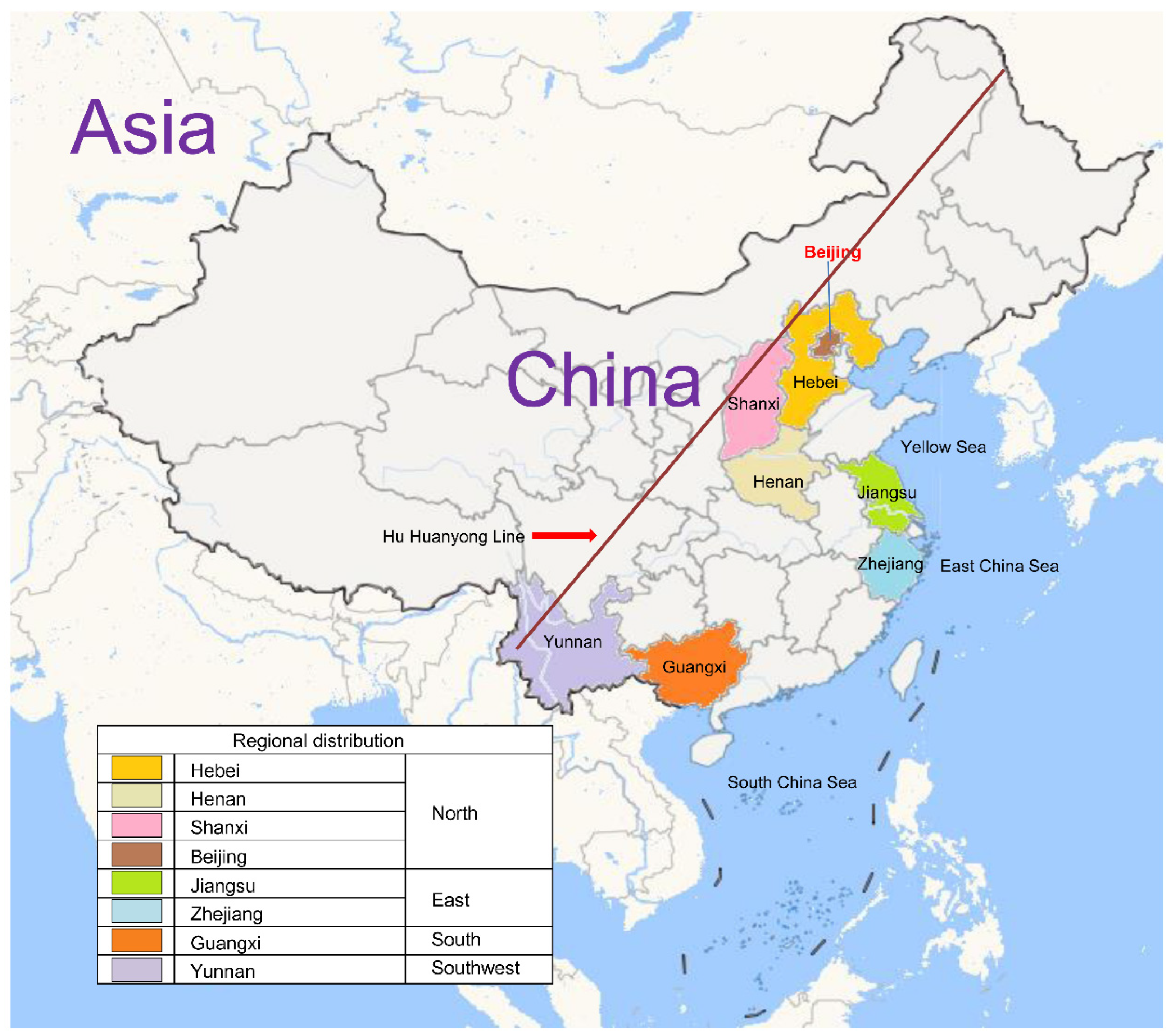
3.1. Demographic Characteristics and Geographical Distribution
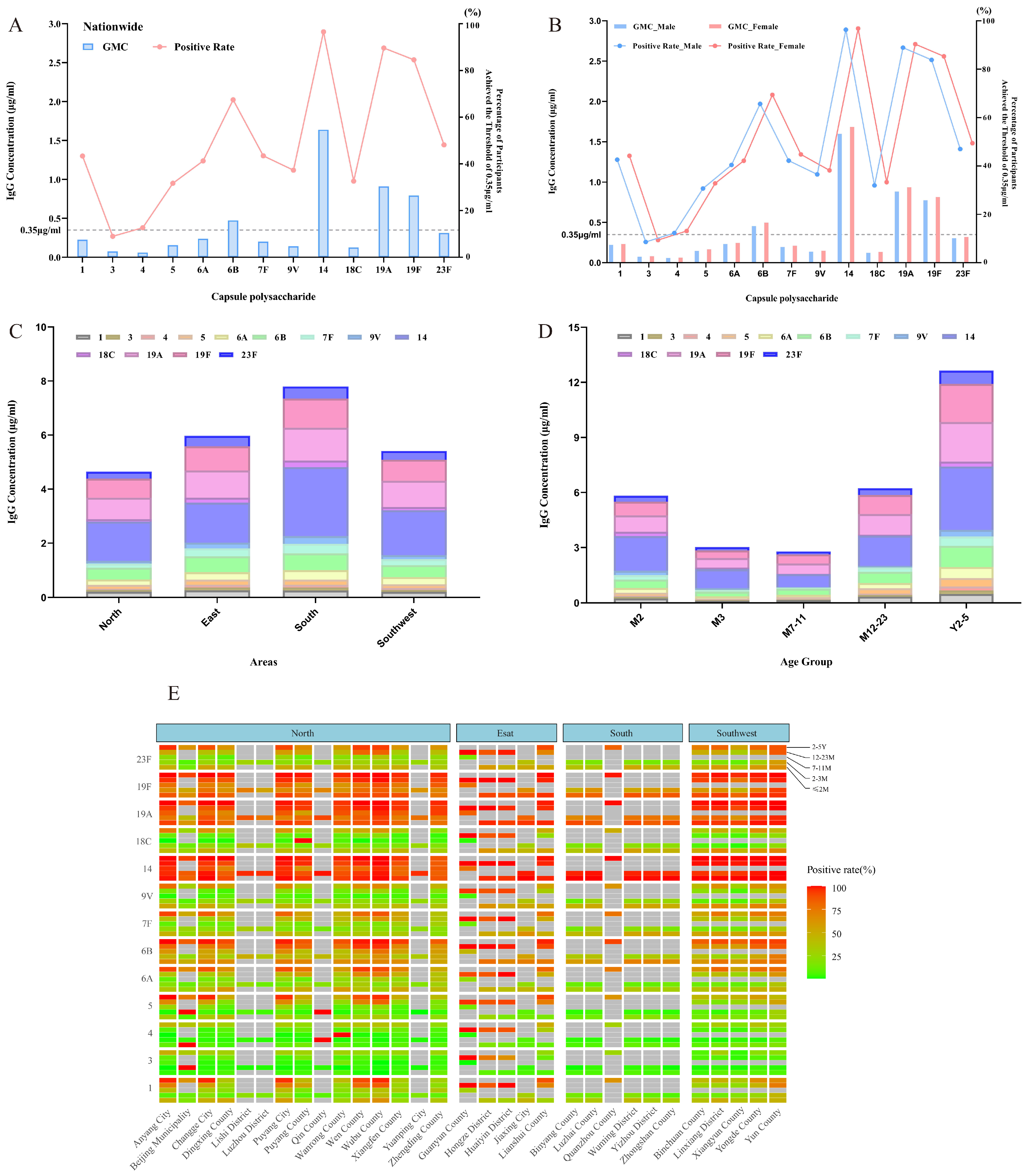
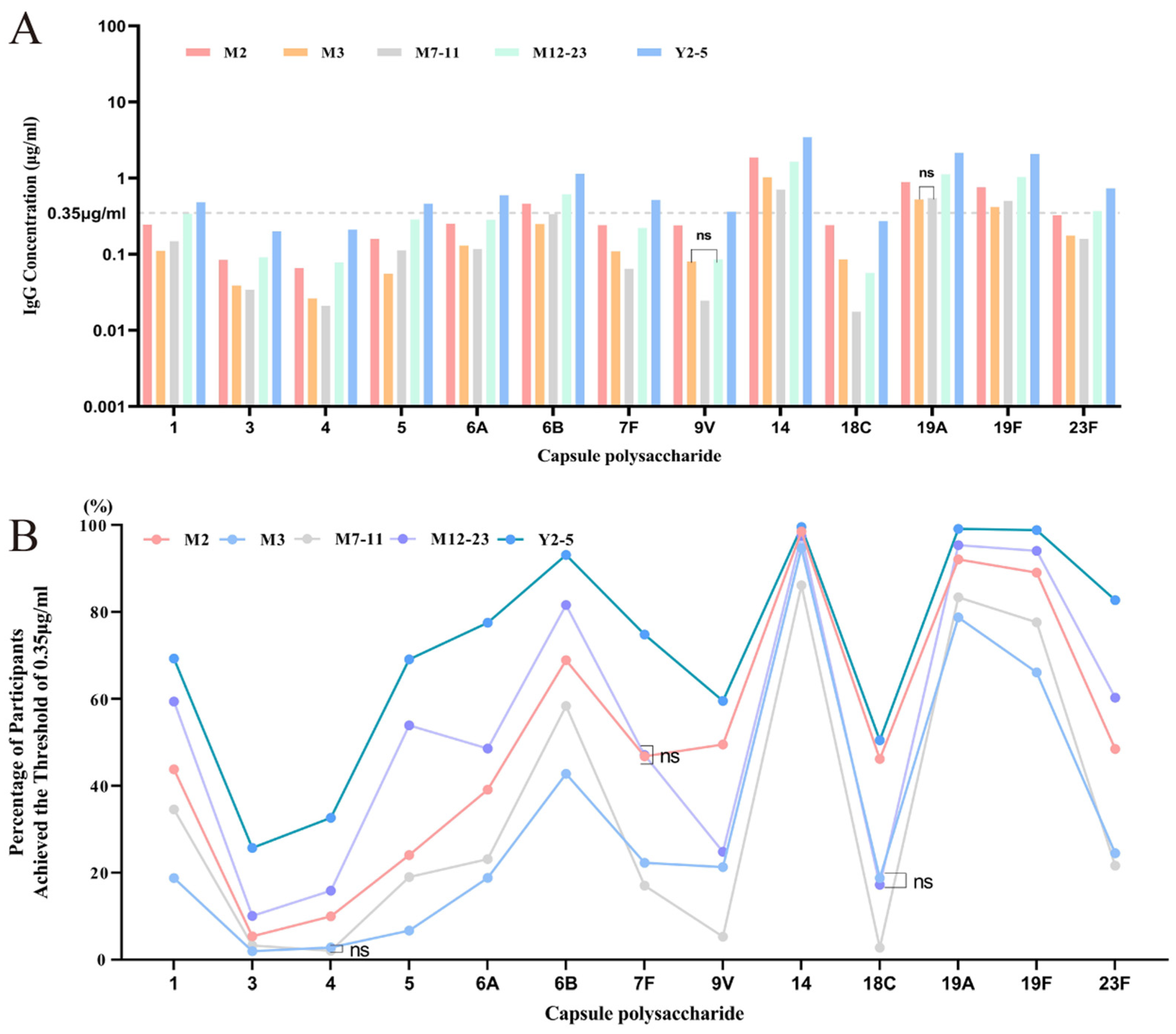
3.2. Nationwide Overview of Baseline Antibody Levels
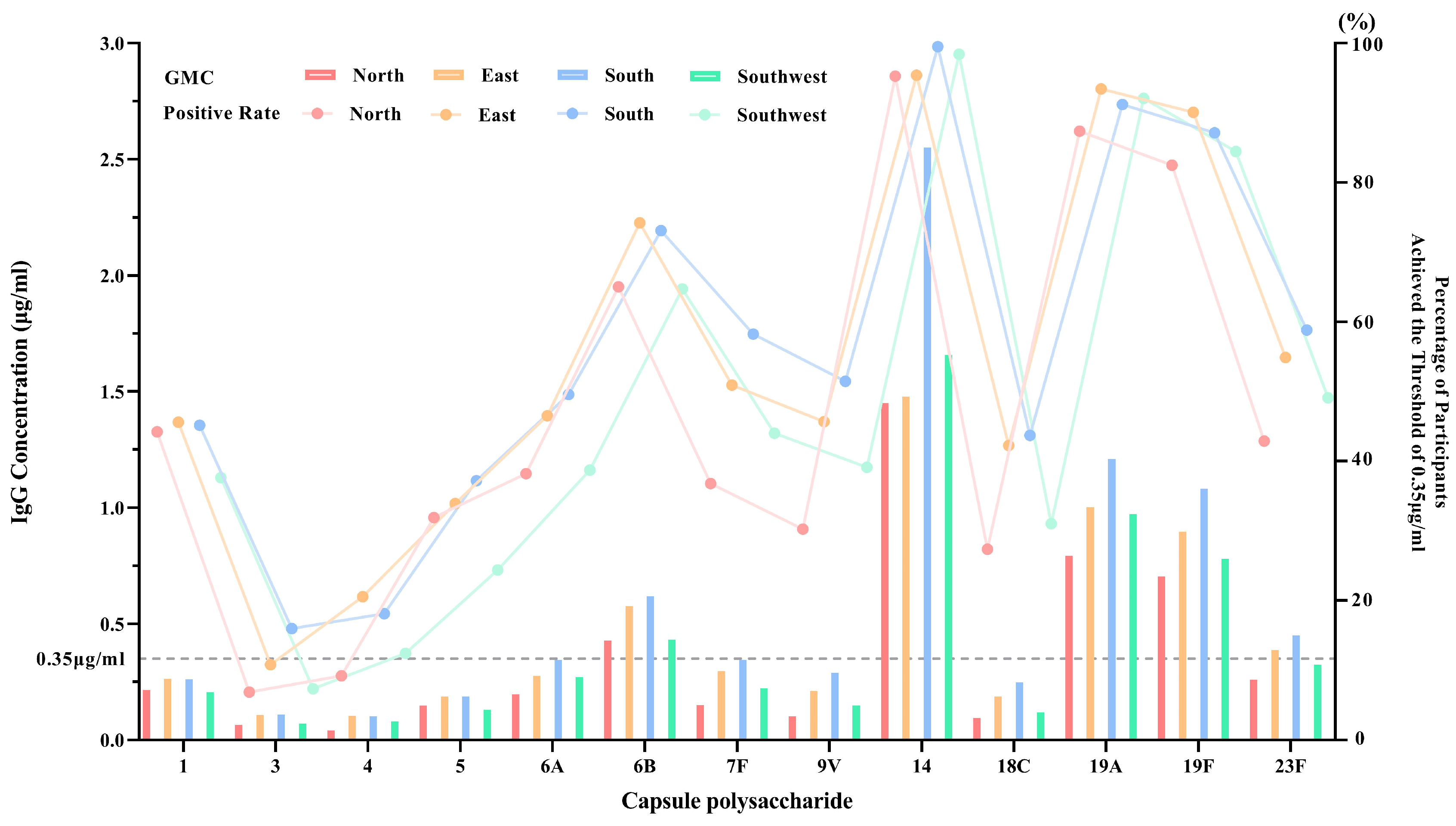
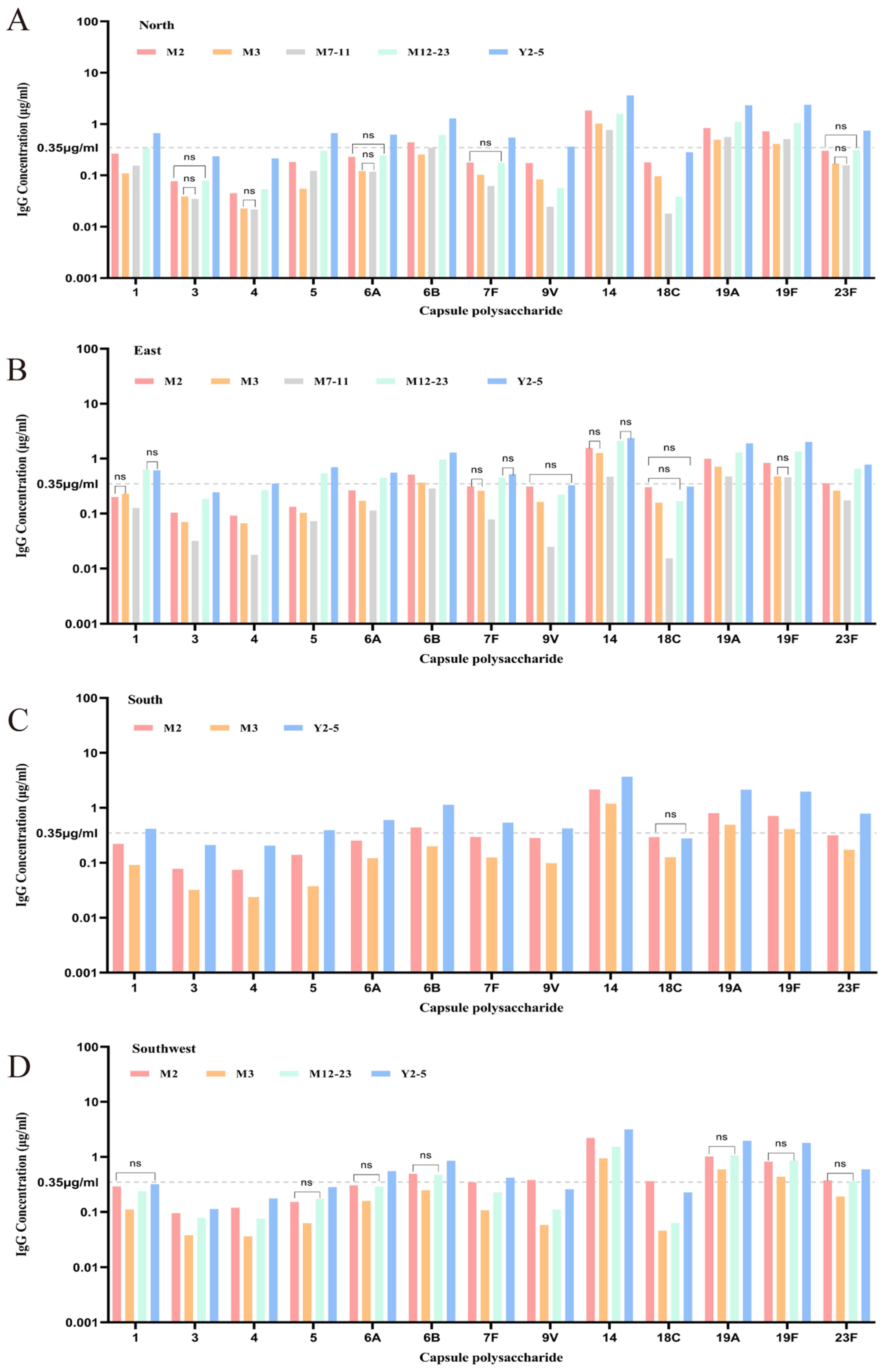
3.2.1. Regional Baseline GMC and Seropositivity Without Age Stratification
3.2.2. Regional Baseline GMC and Seropositivity with Age Stratification
3.3. Baseline GMC and Positive Rate Analysis Across Age Groups
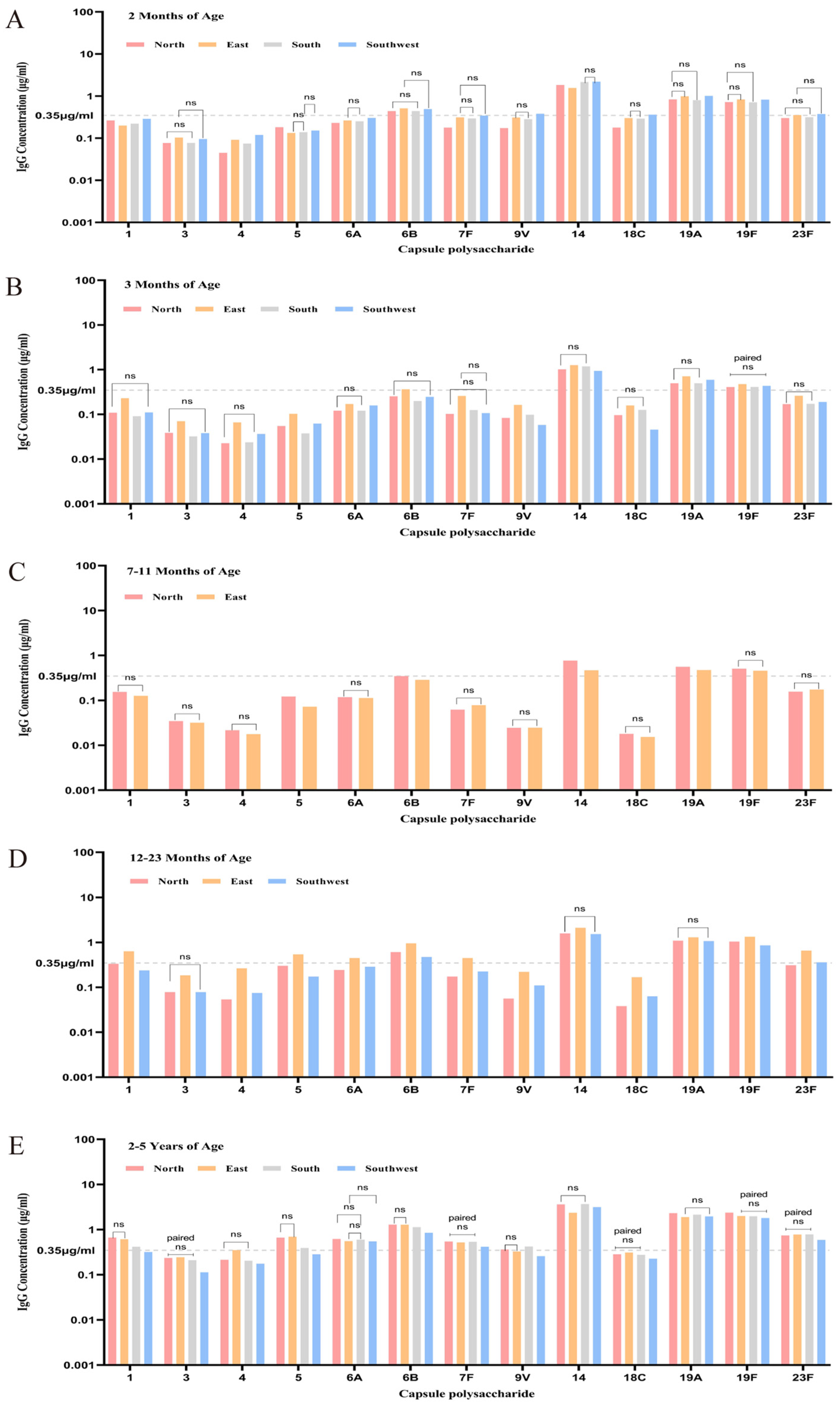
3.3.1. Overview of Baseline Antibody Levels and Positive Rates Across Five Age Groups
3.3.2. Baseline Antibody Levels and Positive Rates Across Age Groups Stratified by Region
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization; |
| NIPs | National immunization programs; |
| ELISA | Enzyme-linked immunosorbent assay; |
| PCV | Pneumococcal conjugate vaccine; |
| PPsV | Pneumococcal polysaccharide vaccine; |
| CPS | Capsule polysaccharide; |
| GMC | Geometric mean concentration; |
| CDC | Centers for Disease Control and Prevention |
| CWPS | Cell Wall Polysaccharide |
References
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef]
- Morimura, A.; Hamaguchi, S.; Akeda, Y.; Tomono, K. Mechanisms underlying pneumococcal transmission and factors influencing host-pneumococcus interaction: A review. Front. Cell. Infect. Microbiol. 2021, 11, 639450. [Google Scholar] [CrossRef]
- Feldman, C.; Anderson, R. Recent advances in the epidemiology and prevention of streptococcus pneumoniae infections. F1000Research 2020, 9, F1000 Faculty Rev-1338. [Google Scholar] [CrossRef]
- Ganaie, F.; Maruhn, K.; Li, C.; Porambo, R.J.; Elverdal, P.L.; Abeygunwardana, C.; van der Linden, M.; Duus, J.; Sheppard, C.L.; Nahm, M.H. Structural, genetic, and serological elucidation of streptococcus pneumoniae serogroup 24 serotypes: Discovery of a new serotype, 24c, with a variable capsule structure. J. Clin. Microbiol. 2021, 59, e0054021. [Google Scholar] [CrossRef]
- Ganaie, F.; Saad, J.S.; McGee, L.; van Tonder, A.J.; Bentley, S.D.; Lo, S.W.; Gladstone, R.A.; Turner, P.; Keenan, J.D.; Breiman, R.F.; et al. A new pneumococcal capsule type, 10d, is the 100th serotype and has a large cps fragment from an oral streptococcus. mBio 2020, 11, e00937-20. [Google Scholar] [CrossRef]
- Amerighi, F.; Valeri, M.; Donnarumma, D.; Maccari, S.; Moschioni, M.; Taddei, A.; Lapazio, L.; Pansegrau, W.; Buccato, S.; De Angelis, G.; et al. Identification of a monoclonal antibody against pneumococcal pilus 1 ancillary protein impairing bacterial adhesion to human epithelial cells. J. Infect. Dis. 2016, 213, 516–522. [Google Scholar] [CrossRef][Green Version]
- Roche, A.M.; Richard, A.L.; Rahkola, J.T.; Janoff, E.N.; Weiser, J.N. Antibody blocks acquisition of bacterial colonization through agglutination. Mucosal Immunol. 2015, 8, 176–185. [Google Scholar] [CrossRef]
- CDC. Global Pneumococcal. Disease and Vaccination. Available online: https://search.cdc.gov/search/?query=pneumococcal&dpage=1 (accessed on 5 November 2022).
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H.; et al. Burden of streptococcus pneumoniae and haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Glob. Health 2018, 6, e744–e757. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wahl, B.; Yu, W.; Xu, T.; Zhang, H.; Garcia, C.; Qin, Y.; Guo, Y.; Yin, Z.; Knoll, M.D.; et al. National, regional, and provincial disease burden attributed to streptococcus pneumoniae and haemophilus influenzae type b in children in China: Modelled estimates for 2010–17. Lancet Reg. Health West. Pac. 2022, 22, 100430. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Zhang, H.Y.; Ren, L.L.; Lu, Q.B.; Ren, X.; Zhang, C.H.; Wang, Y.F.; Lin, S.H.; Zhang, X.A.; Li, J.; et al. Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun. 2021, 12, 5026. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wang, Q.; Yao, K.; Xie, G.; Gao, W.; Huang, L.; Liu, X.; Zhu, C.; Chen, H.; Wang, H.; et al. The changing phenotypes and genotypes of invasive pneumococcal isolates from children in Shenzhen during 2013–2017. Vaccine 2019, 37, 7248–7255. [Google Scholar] [CrossRef]
- Zhang, H.; Lai, X.; Patenaude, B.N.; Jit, M.; Fang, H. Adding new childhood vaccines to China’s national immunization program: Evidence, benefits, and priorities. Lancet Public Health 2023, 8, e1016–e1024. [Google Scholar] [CrossRef]
- WHO. Pneumococcal conjugate vaccines in infants and children under 5 years of age: Who position paper—February 2019. Wkly. Epidemiol. Rec. 2019, 94, 85–104. [Google Scholar]
- Hanquet, G.; Krizova, P.; Dalby, T.; Ladhani, S.N.; Nuorti, J.P.; Danis, K.; Mereckiene, J.; Knol, M.J.; Winje, B.A.; Ciruela, P.; et al. Serotype replacement after introduction of 10-valent and 13-valent pneumococcal conjugate vaccines in 10 countries, Europe. Emerg. Infect. Dis. 2022, 28, 137–138. [Google Scholar] [CrossRef]
- Park, M.A.; Jenkins, S.M.; Smith, C.Y.; Pyle, R.C.; Sacco, K.A.; Ryu, E.; Hagan, J.B.; Joshi, A.Y.; Snyder, M.R.; Abraham, R.S. Pneumococcal serotype-specific cut-offs based on antibody responses to pneumococcal polysaccharide vaccination in healthy adults. Vaccine 2021, 39, 2850–2856. [Google Scholar] [CrossRef] [PubMed]
- Gaultier, G.N.; Nix, E.B.; Thorgrimson, J.; Boreham, D.; McCready, W.; Ulanova, M. Naturally acquired antibodies against 7 streptococcus pneumoniae serotypes in indigenous and non-indigenous adults. PLoS ONE 2022, 17, e0267051. [Google Scholar] [CrossRef] [PubMed]
- Balmer, P.; North, J.; Baxter, D.; Stanford, E.; Melegaro, A.; Kaczmarski, E.B.; Miller, E.; Borrow, R. Measurement and interpretation of pneumococcal igg levels for clinical management. Clin. Exp. Immunol. 2003, 133, 364–369. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, L.; Bai, S.; Zhao, W.; Zhang, A.; Li, J.; Zhang, J.N.; Zhou, S.S.; Qiu, R.; Huang, Z.; et al. Prevalence and serotype distribution of nasopharyngeal carriage of streptococcus pneumoniae among healthy children under 5 years of age in Hainan province, China. Infect. Dis. Poverty 2024, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, Z.; Zhang, L.; Kudinha, T.; An, H.; Qian, C.; Jiang, B.; Wang, Y.; Xu, Y.; Liu, Z.; et al. Serotype distribution, antimicrobial susceptibility, multilocus sequencing type and virulence of invasive streptococcus pneumoniae in China: A six-year multicenter study. Front. Microbiol. 2021, 12, 798750. [Google Scholar] [CrossRef]
- Shi, W.; Li, J.; Dong, F.; Qian, S.; Liu, G.; Xu, B.; Zhou, L.; Xu, W.; Meng, Q.; Wang, Q.; et al. Serotype distribution, antibiotic resistance pattern, and multilocus sequence types of invasive streptococcus pneumoniae isolates in two tertiary pediatric hospitals in Beijing prior to pcv13 availability. Expert Rev. Vaccines 2019, 18, 89–94. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Tang, W.; Yang, Q.; Wang, G.; Li, M. Clinical characteristics and risk factors for poor outcomes of invasive pneumococcal disease in pediatric patients in China. BMC Infect. Dis. 2024, 24, 602. [Google Scholar] [CrossRef]
- Ning, X.; Li, L.; Liu, J.; Wang, F.; Tan, K.; Li, W.; Zhou, K.; Jing, S.; Lin, A.; Bi, J.; et al. Invasive pneumococcal diseases in Chinese children: A multicentre hospital-based active surveillance from 2019 to 2021. Emerg. Microbes Infect. 2024, 13, 2332670. [Google Scholar] [CrossRef]
- Govindan, V.; Ganaie, F.A.; Ramakrishnan, S.M.; Ravindran, S.; Mavuppadi, A.M.; Ravikumar, K.L. Estimation of baseline igg antibody levels to 23 pneumococcal vaccine-type capsular polysaccharides in healthy vaccine naïve Indian adults. Vaccine 2023, 41, 4447–4452. [Google Scholar] [CrossRef]
- Ahmad, I.; Burton, R.; Nahm, M.; Ejaz, H.G.; Arshad, R.; Younis, B.B.; Mirza, S. Naturally acquired antibodies against 4 streptococcus pneumoniae serotypes in Pakistani adults with type 2 diabetes mellitus. PLoS ONE 2024, 19, e0306921. [Google Scholar] [CrossRef]
- The Leading Group Office of the State Council for the Seventh National Population Census. Zhongguo Renkou Pucha Nianjian-2020 (Shangce) [China Population Census Yearbook-2020 (Book 1)]; China Statistics Press: Beijing, China, 2021. [Google Scholar]
- Voysey, M.; Pollard, A.J.; Sadarangani, M.; Fanshawe, T.R. Prevalence and decay of maternal pneumococcal and meningococcal antibodies: A meta-analysis of type-specific decay rates. Vaccine 2017, 35, 5850–5857. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yamamoto, K.; Fukuda, Y.; Umemura, A.; Yoshida, M.; Ideguchi, S.; Ashizawa, N.; Hirayama, T.; Tashiro, M.; Takazono, T.; et al. An adult case of invasive pneumococcal disease due to serotype 12f-specific polysaccharide antibody failure following a 23-valent polysaccharide vaccination. Emerg. Microbes Infect. 2020, 9, 2266–2268. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Hong, L.; Xin, L.; Wang, F.; Zhou, Y. Serotype distribution and antimicrobial resistance of streptococcus pneumoniae in China among children under 14 years of age post-implementation of the pcv13: A systematic review and meta-analysis (2017–2024). Pneumonia 2024, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Cetron, M.S.; Farley, M.M.; Baughman, W.S.; Facklam, R.R.; Elliott, J.A.; Deaver, K.A.; Breiman, R.F. The prevalence of drug-resistant streptococcus pneumoniae in Atlanta. N. Engl. J. Med. 1995, 333, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.C.; Hofmann, J.; Cetron, M.S.; Elliott, J.A.; Facklam, R.R.; Breiman, R.F. The continued emergence of drug-resistant streptococcus pneumoniae in the united states: An update from the centers for disease control and prevention’s pneumococcal sentinel surveillance system. J. Infect. Dis. 1996, 174, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, W.P.; Bryant, J.; Paradiso, P.R.; Siber, G.R. Which pneumococcal serogroups cause the most invasive disease: Implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 2000, 30, 100–121. [Google Scholar] [CrossRef]
- Hocknell, R.E.; Cleary, D.W.; Srifeungfung, S.; Clarke, S.C. Serotype distribution of disease-causing streptococcus pneumoniae in Thailand: A systematic review. Vaccine 2019, 37, 3159–3166. [Google Scholar] [CrossRef] [PubMed]
- Arushothy, R.; Ahmad, N.; Amran, F.; Hashim, R.; Samsudin, N.; Azih, C.R.C. Pneumococcal serotype distribution and antibiotic susceptibility in Malaysia: A four-year study (2014–2017) on invasive paediatric isolates. Int. J. Infect. Dis. 2019, 80, 129–133. [Google Scholar] [CrossRef]
- Echaniz-Aviles, G.; Garza-González, E.; Román-Mancha, A.L.; Morfín-Otero, R.; Rodríguez-Noriega, E.; Ayala-Gaytán, J.J.; Guajardo-Lara, C.E.; Soto-Nogueron, A.; Carnalla-Barajas, M.N.; Camacho-Ortiz, A. Clinical and microbiological characteristics of community-acquired pneumonia associated with streptococcus pneumoniae in adult patients in Mexico. Rev. Argent. Microbiol. 2019, 51, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Vorobieva, S.J.V.; Furberg, A.S.; Slotved, H.C.; Bazhukova, T.; Haldorsen, B.; Caugant, D.A.; Sundsfjord, A.; Valentiner-Branth, P.; Simonsen, G.S. Epidemiological and molecular characterization of streptococcus pneumoniae carriage strains in pre-school children in Arkhangelsk, Northern European Russia, prior to the introduction of conjugate pneumococcal vaccines. BMC Infect. Dis. 2020, 20, 279. [Google Scholar]
- Maeda, H.; Morimoto, K. Global distribution and characteristics of pneumococcal serotypes in adults. Hum. Vaccin Immunother. 2025, 21, 2469424. [Google Scholar] [CrossRef]
- Brandão, A.P.; de Oliveira, T.C.; de Cunto Brandileone, M.C.; Gonçalves, J.E.; Yara, T.I.; Simonsen, V. Persistence of antibody response to pneumococcal capsular polysaccharides in vaccinated long term-care residents in Brazil. Vaccine 2004, 23, 762–768. [Google Scholar] [CrossRef]
- Sankilampi, U.; Honkanen, P.O.; Bloigu, A.; Herva, E.; Leinonen, M. Antibody response to pneumococcal capsular polysaccharide vaccine in the elderly. J. Infect. Dis. 1996, 173, 387–393. [Google Scholar] [CrossRef]
| Age Groups | Number of Subjects | Male | Female | Male-to-Female Ratio |
|---|---|---|---|---|
| 2 months | 6965 | 3570 | 3395 | 1.05:1 |
| 3–6 months | 5675 | 2598 | 2477 | 1.05:1 |
| 7–11 months | 1769 | 875 | 894 | 0.98:1 |
| 12–23 months | 2581 | 1344 | 1237 | 1.09:1 |
| 2–5 years | 4275 | 2183 | 2092 | 1.04:1 |
| Total | 21,265 | 10,884 | 10,381 | 1.05:1 |
| Geographical Region | Province/Municipality | Sample Time | Number of Subjects | Male | Female | Total Population (2020) | Sampling Ratio (‱) | |
|---|---|---|---|---|---|---|---|---|
| North China | Henan Province | 2016 2019 2020 2021 | 1072 1793 2007 3321 | 8193 | 4104 | 4089 | 99,365,519 | 0.82 |
| Shanxi Province | 2016 2022 | 839 1299 | 2138 | 1057 | 1081 | 34,915,616 | 0.61 | |
| Hebei Province | 2016 | 837 | 837 | 450 | 387 | 74,610,235 | 0.11 | |
| Beijing Municipality | 2016 | 83 | 83 | 40 | 43 | 21,893,095 | 0.04 | |
| East China | Jiangsu Province | 2017 2018 | 1200 1044 | 2244 | 1178 | 1066 | 84,748,016 | 0.26 |
| Zhejiang Province | 2020 2021 | 43 354 | 397 | 200 | 197 | 64,567,588 | 0.06 | |
| South China | Guangxi Zhuang Autonomous Region | 2022 | 3595 | 3595 | 1841 | 1754 | 50,126,804 | 0.72 |
| Southwest China | Yunnan Province | 2023 | 3778 | 3778 | 2014 | 1764 | 47,209,277 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, G.; Li, H.; Guo, L.; Yuan, L.; Chen, J.; Li, B.; Gou, J.; Yin, W.; Luo, S.; Ti, J.; et al. Baseline Analysis of Serotype-Specific IgG Antibody Levels for 13-Valent Pneumococcal Conjugate Vaccine in Healthy Chinese Individuals: A Multicenter Retrospective Study. Vaccines 2025, 13, 847. https://doi.org/10.3390/vaccines13080847
Shi G, Li H, Guo L, Yuan L, Chen J, Li B, Gou J, Yin W, Luo S, Ti J, et al. Baseline Analysis of Serotype-Specific IgG Antibody Levels for 13-Valent Pneumococcal Conjugate Vaccine in Healthy Chinese Individuals: A Multicenter Retrospective Study. Vaccines. 2025; 13(8):847. https://doi.org/10.3390/vaccines13080847
Chicago/Turabian StyleShi, Gang, Hong Li, Lina Guo, Lin Yuan, Jingjing Chen, Bin Li, Jinbo Gou, Weiyan Yin, Shuquan Luo, Jing Ti, and et al. 2025. "Baseline Analysis of Serotype-Specific IgG Antibody Levels for 13-Valent Pneumococcal Conjugate Vaccine in Healthy Chinese Individuals: A Multicenter Retrospective Study" Vaccines 13, no. 8: 847. https://doi.org/10.3390/vaccines13080847
APA StyleShi, G., Li, H., Guo, L., Yuan, L., Chen, J., Li, B., Gou, J., Yin, W., Luo, S., Ti, J., Duan, M., Cao, F., Xu, X., & Wang, B. (2025). Baseline Analysis of Serotype-Specific IgG Antibody Levels for 13-Valent Pneumococcal Conjugate Vaccine in Healthy Chinese Individuals: A Multicenter Retrospective Study. Vaccines, 13(8), 847. https://doi.org/10.3390/vaccines13080847






