Immunogenicity of DNA, mRNA and Subunit Vaccines Against Beak and Feather Disease Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Media, Bacterial Strains, Restriction Enzymes and PCR Reagents
2.2. Cloning and Transformation of E. coli and Agrobacterium
2.3. Agrobacterium Infiltration of N. benthamiana
2.4. Purification of TMV PsVs
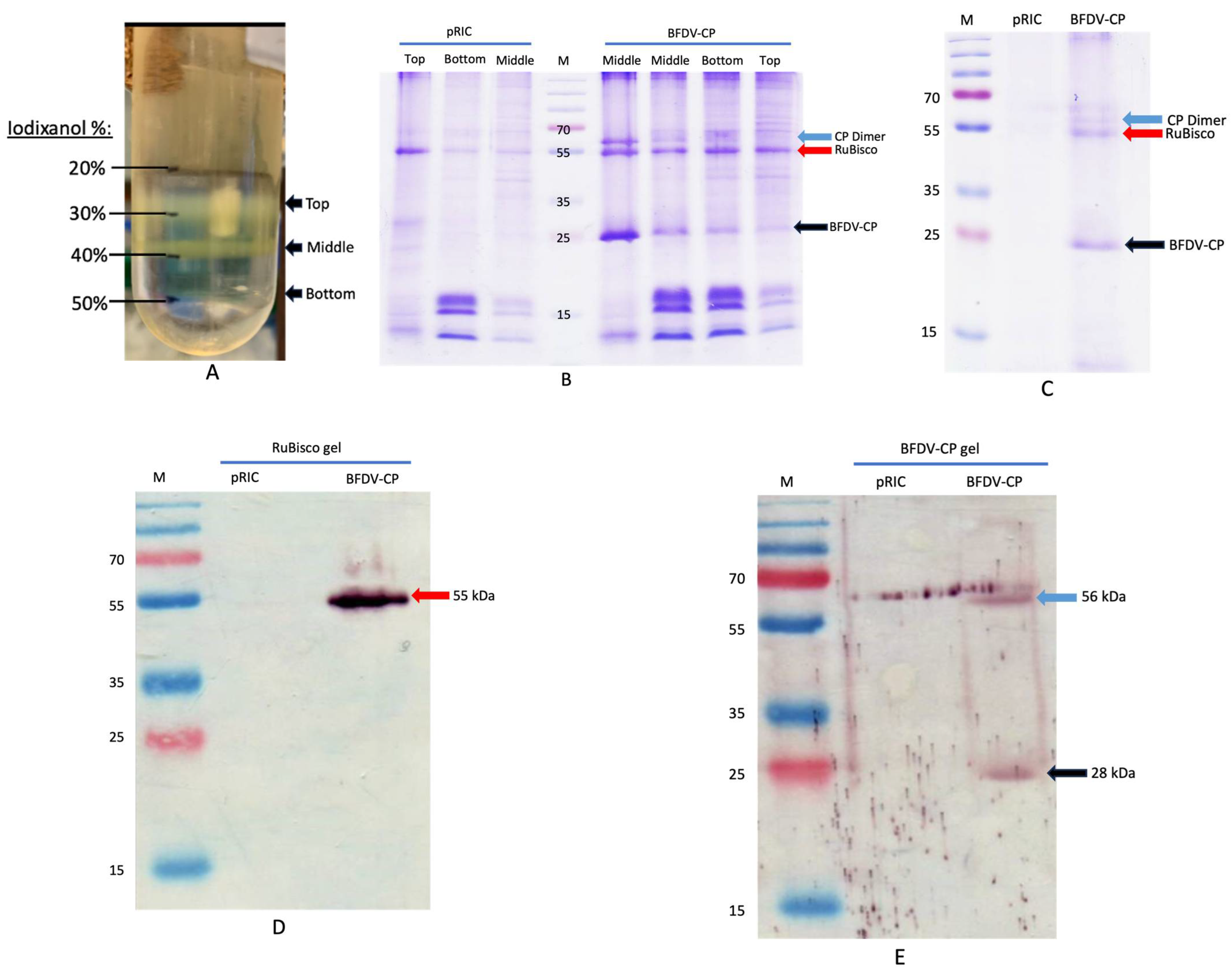
2.5. Purification of a BFDV CP Subunit Vaccine Candidate
2.6. SDS-PAGE, Coomassie Blue Staining, Protein Quantification and Western Blot Analysis
2.7. Transmission Electron Microscopy (TEM) to Confirm Presence of TMV PsVs
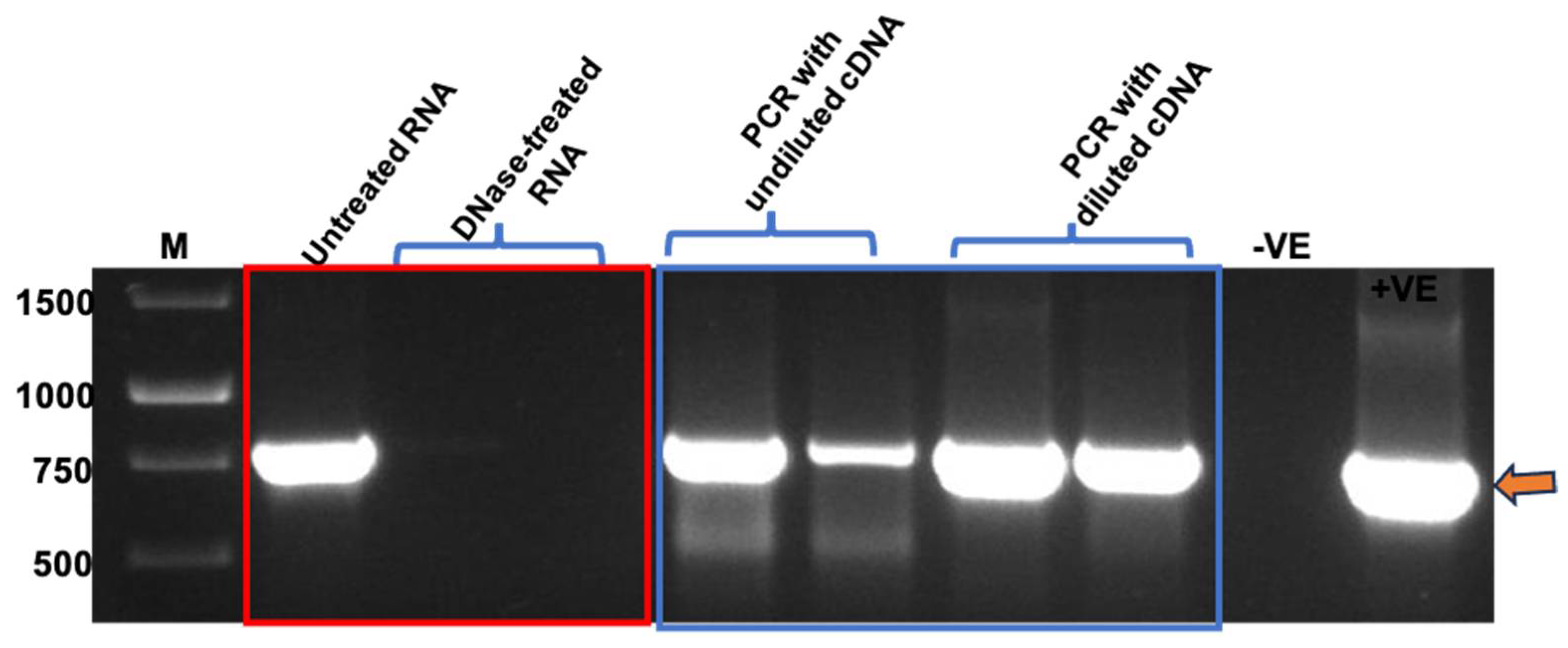
2.8. RNA Extraction and RT-PCR to Confirm BFDV-CP mRNA Encapsidation into TMV Rods
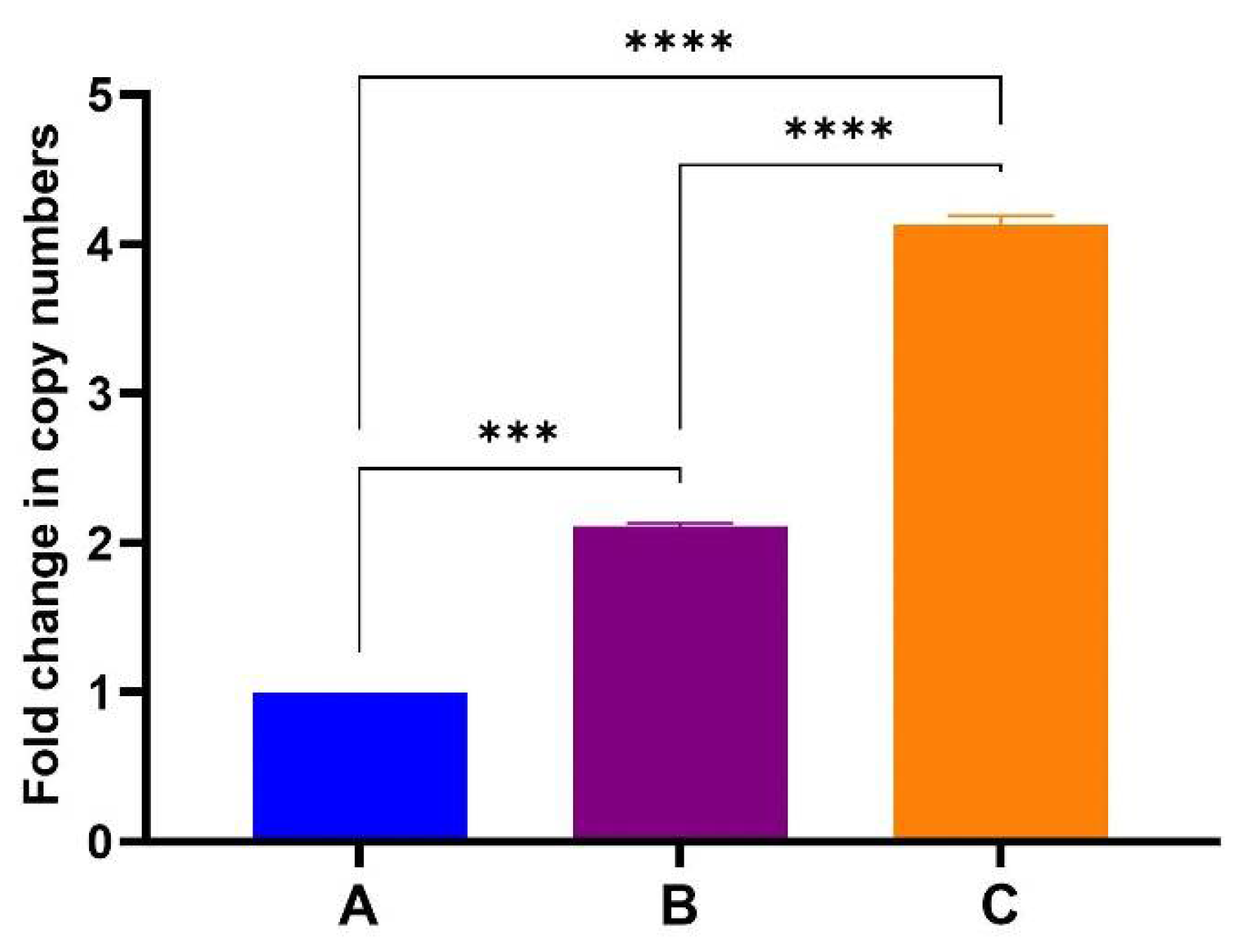
2.9. Real-Time qPCR (RT-qPCR) to Quantify Encapsidated mRNA
2.10. Large-Scale Production and Isolation of BFDV 1.1mer (DNA Vaccine Candidate)
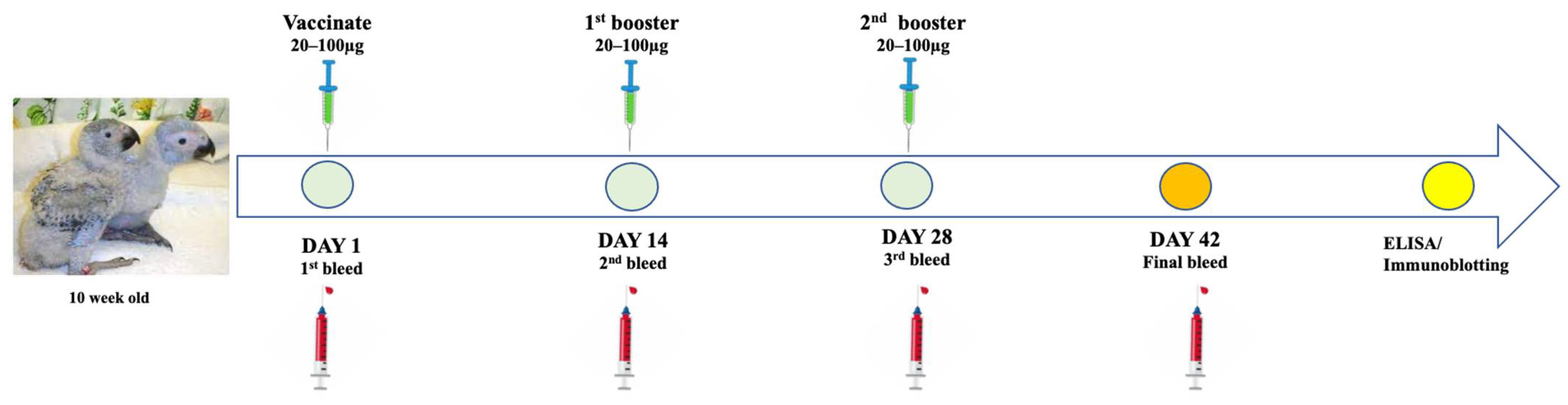
2.11. Immunisation of African Grey Parrots
2.12. Humoral Immune Response Analysis and Determination of Binding Titres Using Indirect ELISA
2.13. Statistical Analysis
3. Results
3.1. Transformation of BFDV-CP-OAS into E. coli and Agrobacterium Competent Cells
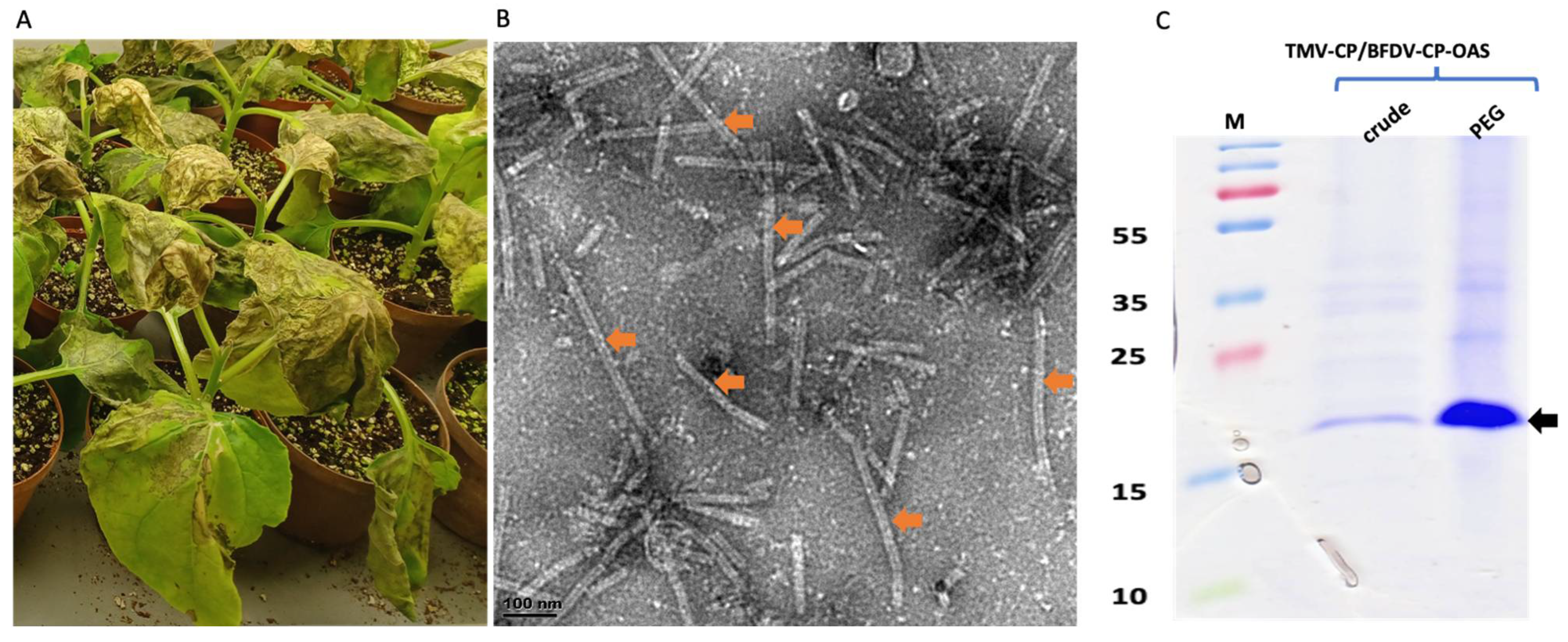
3.2. Co-Expression of TMV-CP with BFDV-CP-OAS
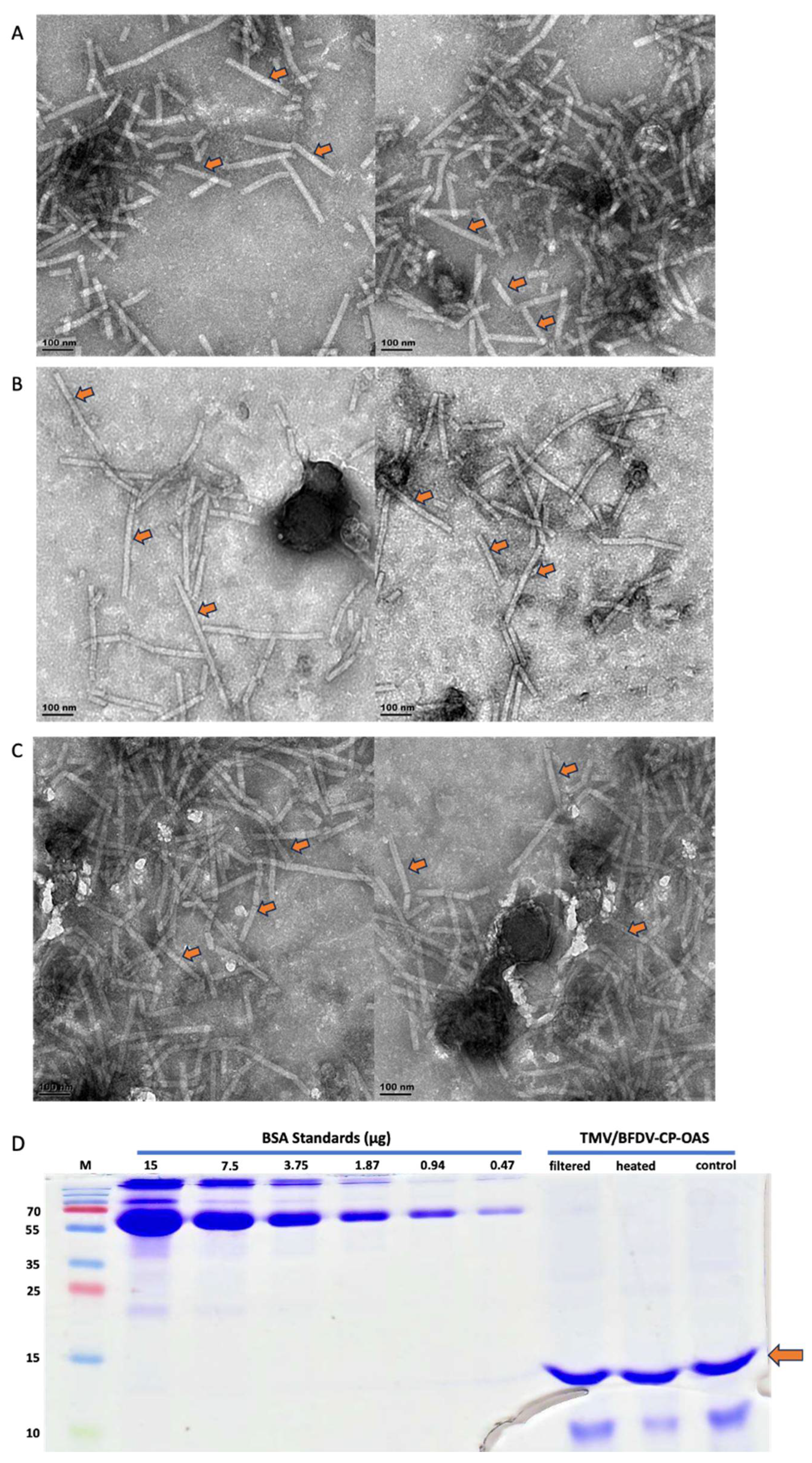
3.3. TEM Analyses, SDS-PAGE Separation and Quantification of Filter-Sterilised and Heat-Treated Samples
3.4. PCR and One-Step RT-qPCR Confirmed Encapsidation of Nucleic Acids into TMV Particles
3.5. Production of the BFDV-CP Subunit Vaccine Candidate
3.6. Production of the BFDV 1.1mer DNA Vaccine Candidate
3.7. Immunogenicity of the BFDV Vaccine Candidates in African Grey Parrot Chicks
3.7.1. Pre-Immunisation PCR to Confirm Absence of BFDV Infection
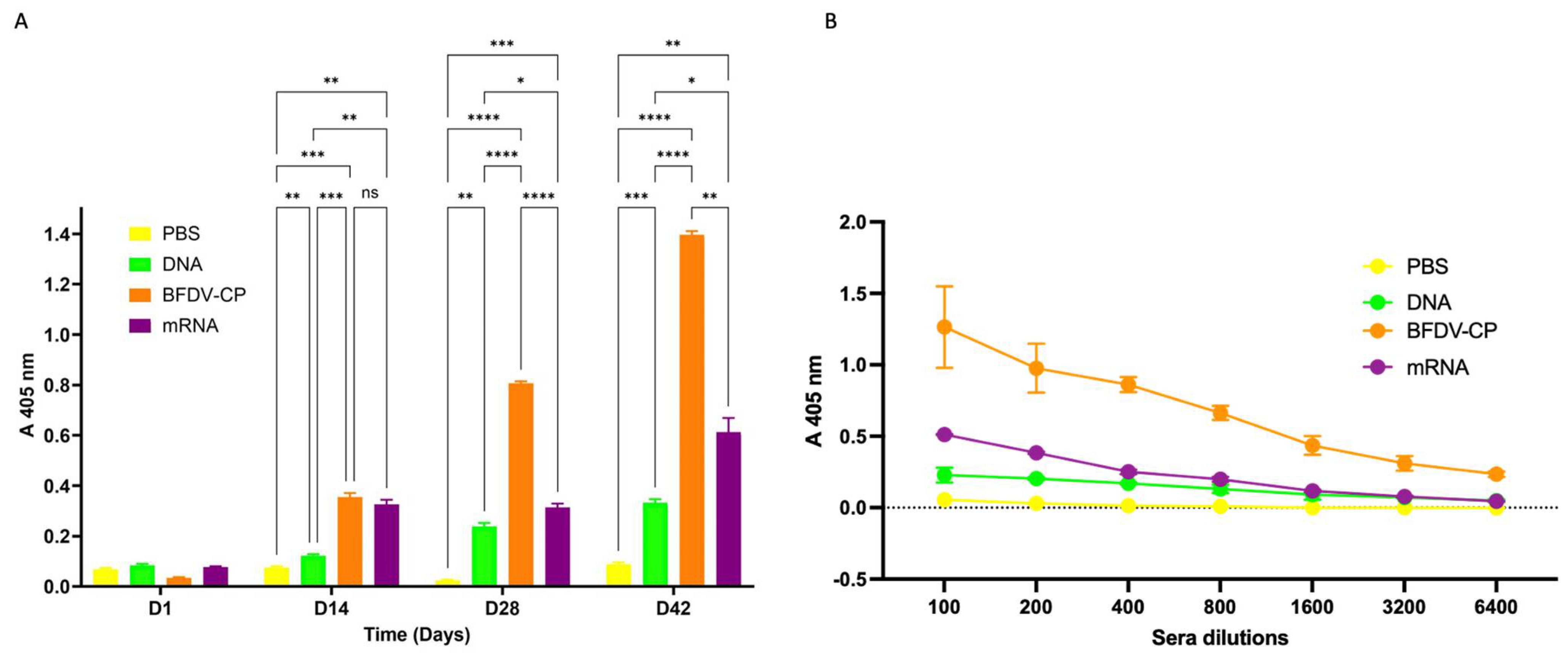
3.7.2. Indirect ELISA Analysis for Antigen Specific Antibodies and Determination of Binding Titres
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Amplification Plots Melt Peak and Standard Curve for pRIC4-BFDV-CP-OAS Plasmid DNA
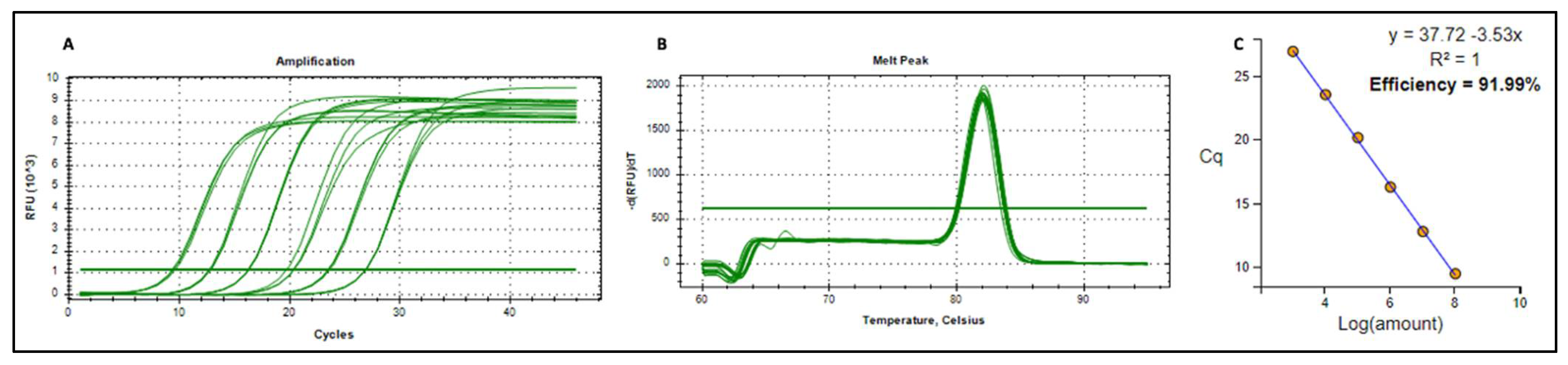
| Target | Sample Type | Sample | Cq | Average Cq | Absolute Copies |
|---|---|---|---|---|---|
| pRIC4-BFDV-CP-OAS | Standard | 1 ng | 9.60 | 9.47 | 110,034,823 |
| pRIC4-BFDV-CP-OAS | Standard | 1 ng | 9.45 | ||
| pRIC4-BFDV-CP-OAS | Standard | 1 ng | 9.35 | ||
| pRIC4-BFDV-CP-OAS | Standard | 0.1 ng | 12.84 | 12.78 | 11,003,482.3 |
| pRIC4-BFDV-CP-OAS | Standard | 0.1 ng | 12.68 | ||
| pRIC4-BFDV-CP-OAS | Standard | 0.1 ng | 12.82 | ||
| pRIC4-BFDV-CP-OAS | Standard | 10 pg | 16.28 | 16.27 | 1,100,348.23 |
| pRIC4-BFDV-CP-OAS | Standard | 10 pg | 16.29 | ||
| pRIC4-BFDV-CP-OAS | Standard | 10 pg | 16.23 | ||
| pRIC4-BFDV-CP-OAS | Standard | 1 pg | 19.72 | 20.15 | 110,034.823 |
| pRIC4-BFDV-CP-OAS | Standard | 1 pg | 20.40 | ||
| pRIC4-BFDV-CP-OAS | Standard | 1 pg | 20.33 | ||
| pRIC4-BFDV-CP-OAS | Standard | 0.1 pg | 23.63 | 23.53 | 11,003.4823 |
| pRIC4-BFDV-CP-OAS | Standard | 0.1 pg | 23.48 | ||
| pRIC4-BFDV-CP-OAS | Standard | 0.1 pg | 23.48 | ||
| pRIC4-BFDV-CP-OAS | Standard | 0.01 pg | 26.91 | 26.92 | 1100.34823 |
| pRIC4-BFDV-CP-OAS | Standard | 0.01 pg | 26.92 | ||
| pRIC4-BFDV-CP-OAS | Standard | 0.01 pg | 26.92 | ||
| pRIC4-BFDV-CP-OAS | Standard | 1 fg | 28.79 | 28.78 | 110.034823 |
| pRIC4-BFDV-CP-OAS | Standard | 1 fg | 28.96 | ||
| pRIC4-BFDV-CP-OAS | Standard | 1 fg | 28.61 | ||
| cDNA | Sample | A 1:100 | 13.78 | 13.88 | 566,945,200 |
| cDNA | Sample | A 1:100 | 14.00 | ||
| cDNA | Sample | A 1:100 | 13.86 | ||
| cDNA | Sample | A 1:1000 | 17.08 | 17.06 | 710,799,200 |
| cDNA | Sample | A 1:1000 | 17.05 | ||
| cDNA | Sample | A 1:1000 | 17.06 | ||
| cDNA | Sample | B 1:100 | 13.72 | 13.65 | 660,147,800 |
| cDNA | Sample | B 1:100 | 13.65 | ||
| cDNA | Sample | B 1:100 | 13.57 | ||
| cDNA | Sample | B 1:1000 | 17.00 | 16.98 | 748,875,800 |
| cDNA | Sample | B 1:1000 | 16.99 | ||
| cDNA | Sample | B 1:1000 | 16.96 | ||
| cDNA | Sample | C 1:100 | 13.93 | 13.85 | 576,893,100 |
| cDNA | Sample | C 1:100 | 13.87 | ||
| cDNA | Sample | C 1:100 | 13.76 | ||
| cDNA | Sample | C 1:1000 | 16.51 | 16.50 | 1,024,206,000 |
| cDNA | Sample | C 1:1000 | 16.53 | ||
| cDNA | Sample | C 1:1000 | 16.47 |
References
- Amery-Gale, J.; Marenda, M.S.; Owens, J.; Eden, P.A.; Browning, G.F.; Devlin, J.M. A high prevalence of beak and feather disease virus in non-psittacine Australian birds. J. Med. Microbiol. 2017, 66, 1005–1013. [Google Scholar] [CrossRef]
- Bonne, N.; Shearer, P.; Sharp, M.; Clark, P.; Raidal, S. Assessment of recombinant beak and feather disease virus capsid protein as a vaccine for psittacine beak and feather disease. J. Gen. Virol. 2009, 90, 640–647. [Google Scholar] [CrossRef]
- Sitinjak, M.; Chen, J.K.; Lee, M.Y.; Liu, H.J.; Wang, C.Y. Characterization of a novel reporter system for beak and feather disease virus. Gene 2023, 867, 147371. [Google Scholar] [CrossRef] [PubMed]
- Todd, D. Immunosuppressive threats to avian species. Avian Pathol. 2000, 29, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Last, R.D. Psittacine Beak and Feather Disease (PBFD). Refining Your Diagnostics in Aviary Birds; South African Veterinary Association: Pretoria, South Africa, 2022; pp. 1–4. [Google Scholar]
- Latimer, K.S.; Rakich, P.M.; Steffens, W.L.; Kircher, I.M.; Ritchie, B.W.; Niagro, F.D.; Lukert, P.D. A novel DNA virus associated with feather inclusions in psittacine beak and feather disease. Vet. Pathol. 1991, 28, 300–304. [Google Scholar] [CrossRef]
- Pass, D.A.; Perry, R.A. The pathology of psittacine beak and feather disease [birds]. Aust. Vet. J. 1984, 61, 69–74. [Google Scholar] [CrossRef]
- Ritchie, B.W.; Niagro, F.D.; Lukert, P.D.; Steffens, W.L., 3rd; Latimer, K.S. Characterization of a new virus from cockatoos with psittacine beak and feather disease. Virology 1989, 171, 83–88. [Google Scholar] [CrossRef]
- Regnard, G.L.; Rybicki, E.P.; Hitzeroth, I.I. Recombinant expression of beak and feather disease virus capsid protein and assembly of virus-like particles in Nicotiana benthamiana. Virol. J. 2017, 14, 174. [Google Scholar] [CrossRef]
- Sarker, S.; Terrón, M.C.; Khandokar, Y.; Aragão, D.; Hardy, J.M.; Radjainia, M.; Jiménez-Zaragoza, M.; de Pablo, P.J.; Coulibaly, F.; Luque, D.; et al. Structural insights into the assembly and regulation of distinct viral capsid complexes. Nat. Commun. 2016, 7, 13014. [Google Scholar] [CrossRef]
- De Kloet, E.; De Kloet, S.R. Analysis of the beak and feather disease viral genome indicates the existence of several genotypes which have a complex psittacine host specificity. Arch. Virol. 2004, 149, 2393–2412. [Google Scholar] [CrossRef]
- Sitinjak, M.C.; Chen, J.K.; Wang, C.Y. Characterization of novel cell-penetrating peptides derived from the capsid protein of beak and feather disease virus. Virus Res. 2023, 330, 199109. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Moylan, K.G.; Ghorashi, S.A.; Forwood, J.K.; Peters, A.; Raidal, S.R. Evidence of a deep viral host switch event with beak and feather disease virus infection in rainbow bee-eaters (Merops ornatus). Sci. Rep. 2015, 5, 14511. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Lloyd, C.; Forwood, J.; Raidal, S.R. Forensic genetic evidence of beak and feather disease virus infection in a Powerful Owl, Ninox strenua. Emu—Austral Ornithol. 2016, 116, 71–74. [Google Scholar] [CrossRef]
- Martens, J.M.; Stokes, H.S.; Berg, M.L.; Walder, K.; Raidal, S.R.; Magrath, M.J.L.; Bennett, A.T.D. Beak and feather disease virus (BFDV) prevalence, load and excretion in seven species of wild caught common Australian parrots. PLoS ONE 2020, 15, e0235406. [Google Scholar] [CrossRef]
- Varsani, A.; Regnard, G.L.; Bragg, R.; Hitzeroth, I.I.; Rybicki, E.P. Global genetic diversity and geographical and host-species distribution of beak and feather disease virus isolates. J. Gen. Virol. 2011, 92, 752–767. [Google Scholar] [CrossRef]
- Das, S.; Sarker, S.; Peters, A.; Raidal, S.R. Psittacine Beak and Feather Disease Virus in wild Orange-bellied Parrots. Assoc. Avian Vet. Australas. Comm. Ltd. Annu. Confer. 2015, 23, 18–27. [Google Scholar]
- Kundu, S.; Faulkes, C.G.; Greenwood, A.G.; Jones, C.G.; Kaiser, P.; Lyne, O.D.; Black, S.A.; Chowrimootoo, A.; Groombridge, J.J. Tracking Viral Evolution during a Disease Outbreak: The Rapid and Complete Selective Sweep of a Circovirus in the Endangered Echo Parakeet. J. Virol. 2012, 86, 5221–5229. [Google Scholar] [CrossRef]
- Ortiz-Catedral, L.; McInnes, K.; Hauber, M.E.; Brunton, D.H. First report of beak and feather disease virus (BFDV) in wild Red-fronted Parakeets (Cyanoramphus novaezelandiae) in New Zealand. Emu—Austral Ornithol. 2009, 109, 244–247. [Google Scholar] [CrossRef]
- Heath, L.; Martin, D.P.; Warburton, L.; Perrin, M.; Horsfield, W.; Kingsley, C.; Rybicki, E.P.; Williamson, A.-L. Evidence of Unique Genotypes of Beak and Feather Disease Virus in Southern Africa. J. Virol. 2004, 78, 9277–9284. [Google Scholar] [CrossRef]
- Raidal, S.; Firth, G.; Cross, G. Vaccination and challenge studies with psittacine beak and feather disease virus. Aust. Vet. J. 1993, 70, 437–441. [Google Scholar] [CrossRef]
- Bassami, M.R.; Berryman, D.; Wilcox, G.E.; Raidal, S.R. Psittacine beak and feather disease virus nucleotide sequence analysis and its relationship to porcine circovirus, plant circoviruses, and chicken anaemia virus. Virology 1998, 249, 453–459. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; Chen, K.; Zeng, X.; Yang, S.; Chang, W.; Tang, Y.; Chen, X.; Wang, S.; Chen, J.L. Identification and Characterization of a Distinct Strain of Beak and Feather Disease Virus in Southeast China. Virol. Sin. 2020, 35, 43–51. [Google Scholar] [CrossRef]
- Duvenage, L.; Hitzeroth, I.I.; Meyers, A.E.; Rybicki, E.P. Expression in tobacco and purification of beak and feather disease virus capsid protein fused to elastin-like polypeptides. J. Virol. Methods 2013, 191, 55–62. [Google Scholar] [CrossRef]
- Raidal; Cross, G. Control by vaccination of psittacine beak and feather disease in a mixed flock of Agapornis spp. Aust. Vet. Pract. 1994, 24, 178–180. [Google Scholar]
- Patterson, E.I.; Swarbrick, C.M.D.; Roman, N.; Forwood, J.K.; Raidal, S.R. Differential expression of two isolates of beak and feather disease virus capsid protein in Escherichia coli. J. Virol. Methods 2013, 189, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Ghorashi, S.A.; Swarbrick, C.M.D.; Khandokar, Y.B.; Himiari, Z.; Forwood, J.K.; Raidal, S.R. An efficient approach for recombinant expression and purification of the viral capsid protein from beak and feather disease virus (BFDV) in Escherichia coli. J. Virol. Methods 2015, 215, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sariya, L.; Prompiram, P. Expression of Recombinant Capsid Protein of Psittacine Beak and Feather Disease Virus Capsid Protein using Pichia pastoris system. J. Appl. Anim. Sci. 2014, 7, 35–44. [Google Scholar]
- Heath, L.; Williamson, A.-L.; Rybicki, E.P. The Capsid Protein of Beak and Feather Disease Virus Binds to the Viral DNA and Is Responsible for Transporting the Replication-Associated Protein into the Nucleus. J. Virol. 2006, 80, 7219–7225. [Google Scholar] [CrossRef]
- Stewart, M.E.; Bonne, N.; Shearer, P.; Khalesi, B.; Sharp, M.; Raidal, S.R. Baculovirus expression of beak and feather disease virus (BFDV) capsid protein capable of self-assembly and haemagglutination. J. Virol. Methods 2007, 141, 181–187. [Google Scholar] [CrossRef]
- Mulondo, G.; Buyse, M.L.R.; Labuschagne, K.; Jarvis, D.; van Zyl, A.; Rybicki, E.P.; Hitzeroth, I.I.; Mbewana, S. Production and immunogenicity of a plant-produced beak and feather disease virus vaccine in Japanese quails. Arch. Virol. 2025, 170, 163. [Google Scholar] [CrossRef]
- Heath, L.E. Molecular Studies on Beak and Feather Disease Virus. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2006. [Google Scholar]
- Buyse, M.R. Beak and Feather Disease Virus Vaccines and Challenge Material Produced in Plants and Cell Culture. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2022. [Google Scholar]
- de Moor, W.R.J.; Regnard, G.L.; Rybicki, E.P.; Williamson, A.L. Characterization of a dynamic self-replicating mammalian expression vector based on the circular ssDNA genome of beak and feather disease virus. J. Gen. Virol. 2022, 103, 001746. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Rouf, N.Z.; Biswas, S.; Tarannum, N.; Oishee, L.M.; Muna, M.M. Demystifying mRNA vaccines: An emerging platform at the forefront of cryptic diseases. RNA Biol. 2022, 19, 386–410. [Google Scholar] [CrossRef]
- Wolff, J.A.; Williams, P.; Chong, W.; Jani, A.; Acsadi, G. Direct Gene Transfer into Mouse Muscle in Vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.G.; Lévy, J.P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Conry, R.M.; LoBuglio, A.F.; Wright, M.; Sumerel, L.; Pike, M.J.; Johanning, F.; Benjamin, R.; Lu, D.; Curiel, D.T. Characterization of a Messenger RNA Polynucleotide Vaccine Vector. Cancer Res. 1995, 55, 1397–1400. [Google Scholar] [PubMed]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. Three decades of messenger RNA vaccine development. Nano Today 2019, 28, 100766. [Google Scholar] [CrossRef]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. Mrna vaccine era—Mechanisms, drug platform and clinical prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for mRNA Vaccines. In mRNA Vaccines; Current Topics in Microbiology and Immunology; Springer International Publishing: Cham, Switzerland, 2022; Volume 437, pp. 71–110. [Google Scholar]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Abramson, A.; Kirtane, A.R.; Shi, Y.; Zhong, G.; Collins, J.E.; Tamang, S.; Ishida, K.; Hayward, A.; Wainer, J.; Rajesh, N.U.; et al. Oral mRNA delivery using capsule-mediated gastrointestinal tissue injections. Matter 2022, 5, 975–987. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, M.; Hur, S.; Cho, Y.; Park, J.; Jung, H.; Seo, Y.; Woo, H.A.; Nam, K.T.; Lee, K.; et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 2021, 7, eabf4398. [Google Scholar] [CrossRef]
- Riley, R.S.; Kashyap, M.V.; Billingsley, M.M.; White, B.; Alameh, M.-G.; Bose, S.K.; Zoltick, P.W.; Li, H.; Zhang, R.; Cheng, A.Y.; et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 2021, 7, eaba1028. [Google Scholar] [CrossRef]
- Saunders, N.R.M.; Paolini, M.S.; Fenton, O.S.; Poul, L.; Devalliere, J.; Mpambani, F.; Darmon, A.; Bergère, M.; Jibault, O.; Germain, M.; et al. A Nanoprimer to Improve the Systemic Delivery of siRNA and mRNA. Nano Lett. 2020, 20, 4264–4269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Leal, J.; Soto, M.R.; Smyth, H.D.C.; Ghosh, D. Aerosolizable Lipid Nanoparticles for Pulmonary Delivery of mRNA through Design of Experiments. Pharmaceutics 2020, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of mRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef]
- Akufo-Addo, N. BioNTech Introduces First Modular mRNA Manufacturing Facility to Promote Scalable Vaccine Production in Africa; Global Data Point: London, UK, 2022. [Google Scholar]
- Yi, H.-G.; Kim, H.; Kwon, J.; Choi, Y.-J.; Jang, J.; Cho, D.-W. Application of 3D bioprinting in the prevention and the therapy for human diseases. Signal Transduct. Target. Ther. 2021, 6, 177. [Google Scholar] [CrossRef]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e1216. [Google Scholar] [CrossRef]
- Hema, M.; Vishnu Vardhan, G.P.; Savithri, H.S.; Murthy, M.R.N. Chapter 6—Emerging Trends in the Development of Plant Virus-Based Nanoparticles and Their Biomedical Applications. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 61–82. [Google Scholar]
- Rifkind, D.; Freeman, G.L. 11—Tobacco Mosaic Virus. In The Nobel Prize Winning Discoveries in Infectious Diseases; Rifkind, D., Freeman, G.L., Eds.; Academic Press: London, UK, 2005; pp. 81–84. [Google Scholar]
- Van Regenmortel, M.H.V.; Fraenkel-Conrat, H. The Plant Viruses: The Rod-Shaped Plant Viruses, Vol. 2; Kluwer Academic: Dordrecht, The Netherlands, 1986; Volume 2. [Google Scholar]
- Namba, K.; Pattanayek, R.; Stubbs, G. Visualization of protein-nucleic acid interactions in a virus: Refined structure of intact tobacco mosaic virus at 2.9 Å resolution by X-ray fiber diffraction. J. Mol. Biol. 1989, 208, 307–325. [Google Scholar] [CrossRef]
- Shah, S.N.; Saunders, K.; Thuenemann, E.C.; Evans, D.J.; Lomonossoff, G.P. Designer-length palladium nanowires can be templated by the central channel of tobacco mosaic virus nanorods. Virology 2022, 577, 155–162. [Google Scholar] [CrossRef]
- Rochon, D.A.; Siegel, A. Chloroplast DNA Transcripts are Encapsidated by Tobacco Mosaic Virus Coat Protein. Proc. Natl. Acad. Sci. USA 1984, 81, 1719–1723. [Google Scholar] [CrossRef]
- Siegel, A. Pseudovirions of tobacco mosaic virus. Virology 1971, 46, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.; Sleat, D.; Watts, J.; Turner, P.; Wilson, T. In vivo uncoating and efficient expression of foreign mRNAs packaged in TMV-like particles. Science 1987, 236, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Sleat, D.E.; Turner, P.C.; Finch, J.T.; Butler, P.J.G.; Wilson, T.M.A. Packaging of recombinant RNA molecules into pseudovirus particles directed by the origin-of-assembly sequence from tobacco mosaic virus RNA. Virology 1986, 155, 299–308. [Google Scholar] [CrossRef]
- Turner, P.C.; Watkins, P.A.C.; Zaitlin, M.; Wilson, T.M.A. Tobacco mosaic virus particles uncoat and express their rna in Xenopus laevis oocytes: Implications for early interactions between plant cells and viruses. Virology 1987, 160, 515–517. [Google Scholar] [CrossRef]
- Regnard, G.L. Development of a Potential Challenge Model and Plant-Produced Vaccine Candidate for Beak and Feather Disease Virus. Ph.D. Thesis, Univesrity of Cape Town, Cape Town, South Africa, 2015. [Google Scholar]
- Varsani, A.; de Villiers, G.K.; Regnard, G.L.; Bragg, R.R.; Kondiah, K.; Hitzeroth, I.I.; Rybicki, E.P. A unique isolate of beak and feather disease virus isolated from budgerigars (Melopsittacus undulatus) in South Africa. Arch. Virol. 2010, 153, 435–439. [Google Scholar] [CrossRef]
- Maclean, J.; Koekemoer, M.; Olivier, A.J.; Stewart, D.; Hitzeroth, I.I.; Rademacher, T.; Fischer, R.; Williamson, A.L.; Rybicki, E.P. Optimization of human papillomavirus type 16 (HPV-16) L1 expression in plants: Comparison of the suitability of different HPV-16 L1 gene variants and different cell-compartment localization. J. Gen. Virol. 2007, 88, 1460–1469. [Google Scholar] [CrossRef]
- Zhou, Y.; McCormick, A.A.; Kearney, C.M. Plant Expression of Trans-Encapsidated Viral Nanoparticle Vaccines with Animal RNA Replicons. Methods Mol. Biol. 2017, 1499, 77–86. [Google Scholar] [CrossRef]
- Regnard, G.L.; de Moor, W.R.J.; Hitzeroth, I.I.; Williamson, A.L.; Rybicki, E.P. Xenogenic rolling-circle replication of a synthetic beak and feather disease virus genomic clone in 293TT mammalian cells and Nicotiana benthamiana. J. Gen. Virol. 2017, 98, 2329–2338. [Google Scholar] [CrossRef]
- Thuenemann, E.C.; Byrne, M.J.; Peyret, H.; Saunders, K.; Castells-Graells, R.; Ferriol, I.; Santoni, M.; Steele, J.F.C.; Ranson, N.A.; Avesani, L.; et al. A Replicating Viral Vector Greatly Enhances Accumulation of Helical Virus-like Particles in Plants. Viruses 2021, 13, 885. [Google Scholar] [CrossRef]
- Saunders, K.; Thuenemann, E.C.; Shah, S.N.; Peyret, H.; Kristianingsih, R.; Lopez, S.G.; Richardson, J.; Lomonossoff, G.P. The Use of a Replicating Virus Vector for in Planta Generation of Tobacco Mosaic Virus Nanorods Suitable for Metallization. Front. Bioeng. Biotechnol. 2022, 10, 877361. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.; Thuenemann, E.C.; Peyret, H.; Lomonossoff, G.P. The Tobacco Mosaic Virus Origin of Assembly Sequence is Dispensable for Specific Viral RNA Encapsidation but Necessary for Initiating Assembly at a Single Site. J. Mol. Biol. 2022, 434, 167873. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mutoloki, S.; Evensen, Ø. Superior protection conferred by inactivated whole virus vaccine over subunit and DNA vaccines against salmonid alphavirus infection in Atlantic salmon (Salmo salar L.). Vaccine 2012, 30, 3918–3928. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, K. Development of DNA Vaccine for the Prevention of Psittacine Beak and Feather Disease. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2008. [Google Scholar]
- Crank, M.C.; Gordon, I.J.; Yamshchikov, G.V.; Sitar, S.; Hu, Z.; Enama, M.E.; Holman, L.A.; Bailer, R.T.; Pearce, M.B.; Koup, R.A.; et al. Phase 1 study of pandemic H1 DNA vaccine in healthy adults. PLoS ONE 2015, 10, e0123969. [Google Scholar] [CrossRef]
- Grunwald, T.; Ulbert, S. Improvement of DNA vaccination by adjuvants and sophisticated delivery devices: Vaccine-platforms for the battle against infectious diseases. Clin. Exp. Vaccine Res. 2015, 4, 1–10. [Google Scholar] [CrossRef]
- Li, L.; Petrovsky, N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines 2016, 15, 313–329. [Google Scholar] [CrossRef]
- Cui, Z. DNA Vaccine. Adv. Genet. 2005, 54, 257–289. [Google Scholar] [CrossRef]
- Kowalczyk, D.W.; Ertl, H.C.J. Immune responses to DNA vaccines. Cell. Mol. Life Sci. CMLS 1999, 55, 751–770. [Google Scholar] [CrossRef]
- Leifert, J.A.; Whitton, J.L. Immune Responses to DNA Vaccines: Induction of CD8 T Cells. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Philadelphia, PA, USA, 2013. [Google Scholar]
- Whitton, J.L.; Sheng, N.; Oldstone, M.B.A.; McKee, T.A. A “string-of-beads” vaccine, comprising linked minigenes, confers protection from lethal-dose virus challenge. J. Virol. 1993, 67, 348–352. [Google Scholar] [CrossRef]
- An, L.L.; Whitton, J.L. A multivalent minigene vaccine, containing B-cell, cytotoxic T-lymphocyte, and Th epitopes from several microbes, induces appropriate responses in vivo and confers protection against more than one pathogen. J. Virol. 1997, 71, 2292–2302. [Google Scholar] [CrossRef]
- An, L.L.; Whitton, J.L. Multivalent minigene vaccines against infectious disease. Curr. Opin. Mol. Ther. 1999, 1, 16–21. [Google Scholar]
- Volkmer, H.; Bertholet, C.; Jonjić, S.; Wittek, R.; Koszinowski, U.H. Cytolytic T lymphocyte recognition of the murine cytomegalovirus nonstructural immediate-early protein pp89 expressed by recombinant vaccinia virus. J. Exp. Med. 1987, 166, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Jonjic, S.; Del Val, M.; Keil, G.M.; Reddehase, M.J.; Koszinowski, U.H. A nonstructural viral protein expressed by a recombinant vaccinia virus protects against lethal cytomegalovirus infection. J. Virol. 1988, 62, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.W.; Jensen, C.M.; Holt, T.M.; Waldbillig, C.P.; Hathaway, D.K.; Jennings, N.J.; Ng, T.; Chu, H.J. Demonstration of efficacy of a West Nile virus DNA vaccine in foals. In Proceedings of the 51st Annual Convention of the American Association of Equine Practitioners, Seattle, WA, USA, 3–7 December 2005; pp. 183–190. [Google Scholar]
- Smith, L.R.; Wloch, M.K.; Ye, M.; Reyes, L.R.; Boutsaboualoy, S.; Dunne, C.E.; Chaplin, J.A.; Rusalov, D.; Rolland, A.P.; Fisher, C.L.; et al. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine 2010, 28, 2565–2572. [Google Scholar] [CrossRef]
- Trumpfheller, C.; Caskey, M.; Nchinda, G.; Longhi, M.P.; Mizenina, O.; Huang, Y.; Schlesinger, S.J.; Colonna, M.; Steinman, R.M. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc. Natl. Acad. Sci. USA 2008, 105, 2574–2579. [Google Scholar] [CrossRef]
- Tenbusch, M.; Ignatius, R.; Nchinda, G.; Trumpfheller, C.; Salazar, A.M.; Töpfer, K.; Sauermann, U.; Wagner, R.; Hannaman, D.; Tenner-Racz, K.; et al. Immunogenicity of DNA vaccines encoding Simian immunodeficiency virus antigen targeted to dendritic cells in rhesus macaques. PLoS ONE 2012, 7, e39038. [Google Scholar] [CrossRef]
- Fenaux, M.; Opriessnig, T.; Halbur, P.G.; Meng, X.J. Immunogenicity and Pathogenicity of Chimeric Infectious DNA Clones of Pathogenic Porcine Circovirus Type 2 (PCV2) and Nonpathogenic PCV1 in Weanling Pigs. J. Virol. 2003, 77, 11232–11243. [Google Scholar] [CrossRef]
- Karan, S.; Durán-Meza, A.L.; Chapman, A.; Tanimoto, C.; Chan, S.K.; Knobler, C.M.; Gelbart, W.M.; Steinmetz, N.F. In Vivo Delivery of Spherical and Cylindrical In Vitro Reconstituted Virus-like Particles Containing the Same Self-Amplifying mRNA. Mol. Pharm. 2024, 21, 2727–2739. [Google Scholar] [CrossRef]
- Ritchie, B.W.; Niagro, F.D.; Latimer, K.S.; Steffens, W.L.; Pesti, D.; Campagnoli, R.P.; Lukert, P.D. Antibody response to and maternal immunity from an experimental psittacine beak and feather disease vaccine. Am. J. Vet. Res. 1992, 53, 1512–1518. [Google Scholar] [CrossRef]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kang, K.W.; Lee, E.Y.; Seo, D.W.; Kim, H.L.; Kim, H.; Kwon, T.; Park, H.L.; Kim, H.; Lee, S.M.; et al. Heterologous prime-boost vaccination with adenoviral vector and protein nanoparticles induces both Th1 and Th2 responses against Middle East respiratory syndrome coronavirus. Vaccine 2018, 36, 3468–3476. [Google Scholar] [CrossRef]
- Zuo, F.; Abolhassani, H.; Du, L.; Piralla, A.; Bertoglio, F.; de Campos-Mata, L.; Wan, H.; Schubert, M.; Cassaniti, I.; Wang, Y.; et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat. Commun. 2022, 13, 2670. [Google Scholar] [CrossRef]

| Primer Name | Sequence (5′-3) | Tm (°C) | Purpose |
|---|---|---|---|
| pTRA-Fwd | CATTTCATTTGGAGAGGACACG | 60 | Sequencing and Colony PCR |
| pTRA-Rev | GAACTACTCACACATTATTCTGG | 60 | |
| Oligo1-BFDV-CP-Fwd | TATAAAAAAAAAAAAACATGTGGGGCACCTCTAACTGCG | 62.38 | In-Fusion cloning PCR/PCR |
| Oligo2-BFDV-CP-Rev | ATTTCTATAACTACCCTTCAGTTCTGGGATTATTG | 59.75 | |
| Oligo3-OriA-Fwd | TGAAGGGTAGTTATAGAAATAATATAAAATTAGG | 62.44 | In-Fusion cloning PCR/PCR |
| Oligo4-OriA-Rev | GCGGACTCTAGAGAGCTCGAGTACTACTATTTTTCCCTTTG | 67.5 | |
| BFDV_F | AGGAGCAAACGTCAAGCACTAC | 54.84 | qPCR |
| BFDV_R | GGGGCAAACTGACGGAATTGAA | 54.84 |
| Vaccine Group | Vaccine Doses(µg) | Vaccine/Polygen Adjuvant Ratio | No of Birds per Group |
|---|---|---|---|
| DNA Vaccine | 90 | 1:1 | 5 |
| BFDV CP Vaccine | 20 | 1:1 | 5 |
| mRNA Vaccine | 100 | 2:1 | 5 |
| Negative Control | DPBS Buffer | 1:1 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndlovu, B.; van Zyl, A.R.; Verwoerd, D.; Rybicki, E.P.; Hitzeroth, I.I. Immunogenicity of DNA, mRNA and Subunit Vaccines Against Beak and Feather Disease Virus. Vaccines 2025, 13, 762. https://doi.org/10.3390/vaccines13070762
Ndlovu B, van Zyl AR, Verwoerd D, Rybicki EP, Hitzeroth II. Immunogenicity of DNA, mRNA and Subunit Vaccines Against Beak and Feather Disease Virus. Vaccines. 2025; 13(7):762. https://doi.org/10.3390/vaccines13070762
Chicago/Turabian StyleNdlovu, Buyani, Albertha R. van Zyl, Dirk Verwoerd, Edward P. Rybicki, and Inga I. Hitzeroth. 2025. "Immunogenicity of DNA, mRNA and Subunit Vaccines Against Beak and Feather Disease Virus" Vaccines 13, no. 7: 762. https://doi.org/10.3390/vaccines13070762
APA StyleNdlovu, B., van Zyl, A. R., Verwoerd, D., Rybicki, E. P., & Hitzeroth, I. I. (2025). Immunogenicity of DNA, mRNA and Subunit Vaccines Against Beak and Feather Disease Virus. Vaccines, 13(7), 762. https://doi.org/10.3390/vaccines13070762









