Effects of Canine IL-12 on the Immune Response Against the Canine Parvovirus VP2 Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Cloning
2.2. Construction of Recombinant Baculovirus
2.3. Protein Expression and Purification
2.4. SDS-PAGE and Western Blotting Analysis
2.5. Evaluation of Biological Activity
2.6. Animals, Vaccination, and Challenge
2.7. Clinical Scores
2.8. Serum Neutralization Analysis
2.9. qPCR Quantitative Analysis of Rectal Swabs
2.10. Histopathological Analysis
2.11. Data Analysis
3. Results
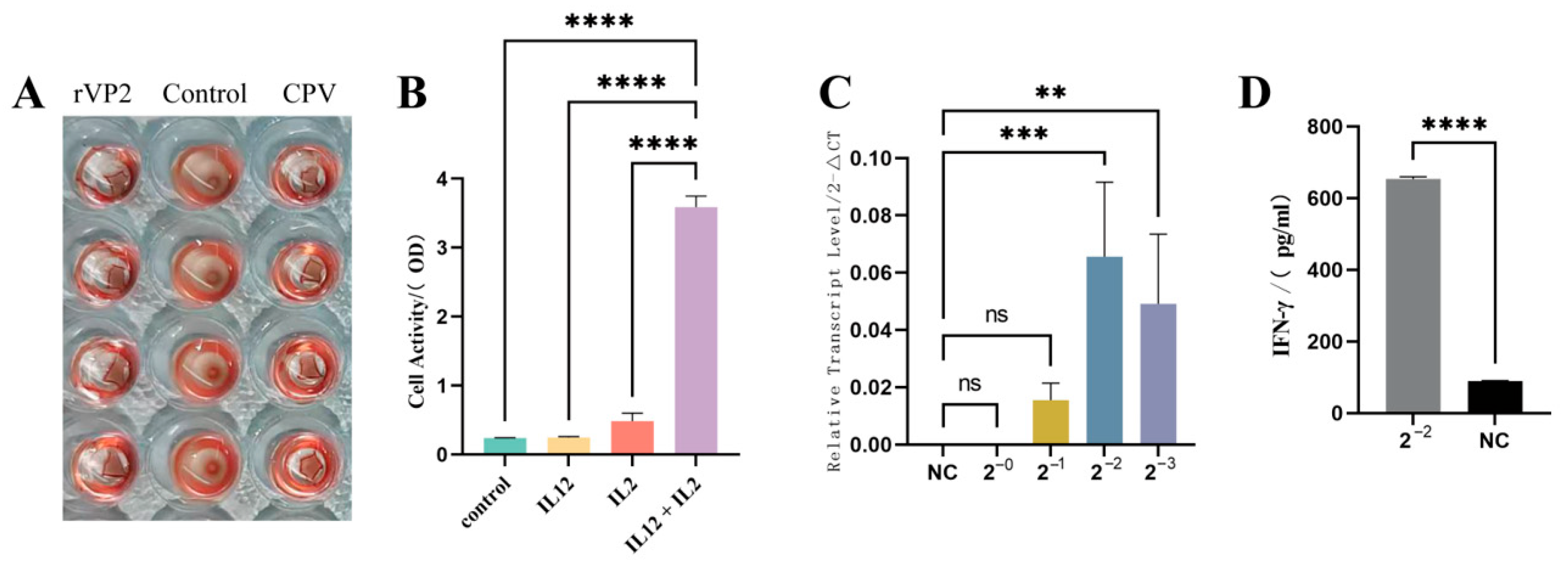
3.1. The Recombinant Baculovirus Was Successfully Constructed, Yielding Purified Recombinant rVP2 and rcIL-12 Proteins
3.2. The Two Recombinant Proteins Retained Biological Activities Comparable to Their Native Counterparts
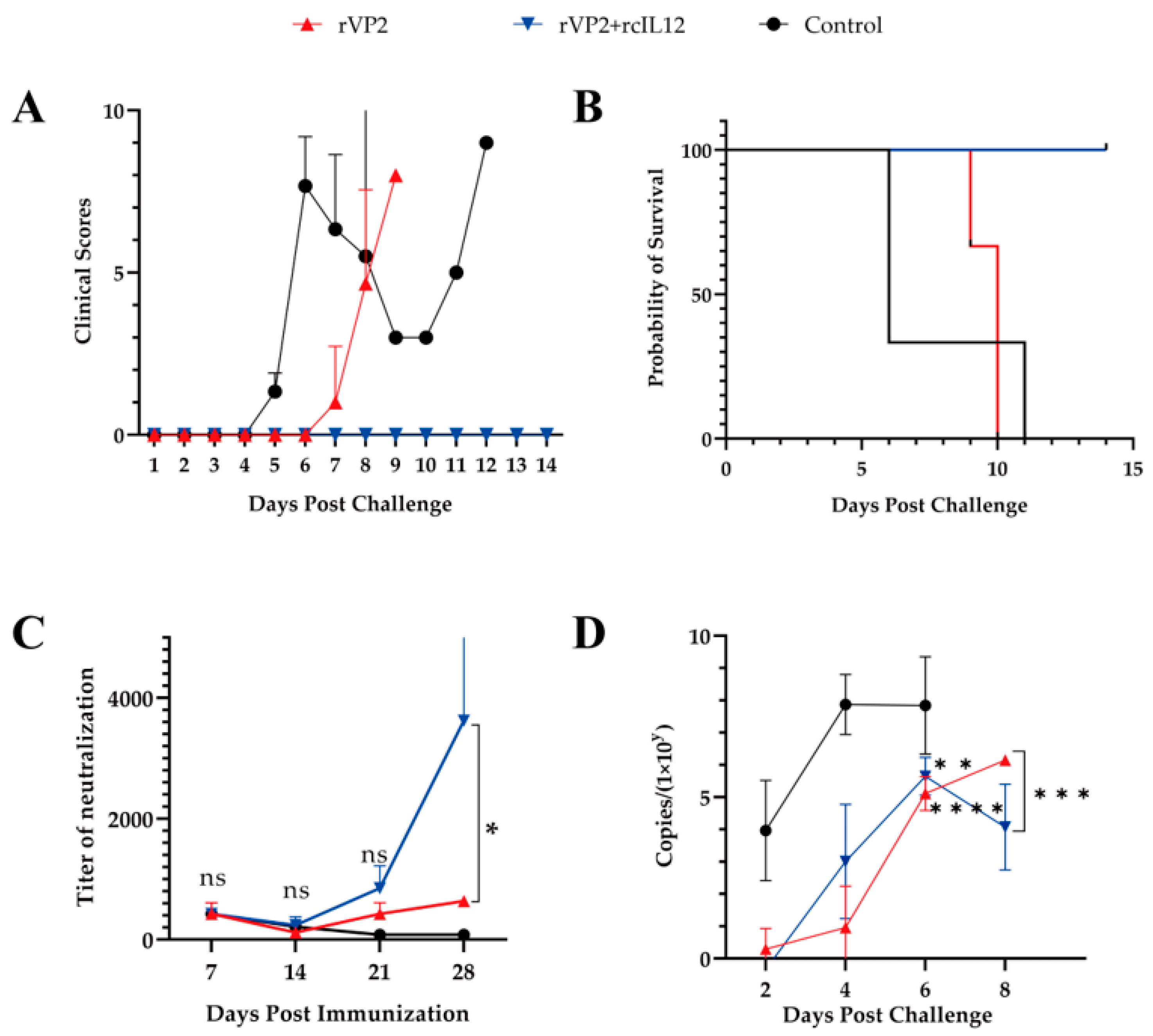
3.3. The Combined Immunization with rVP2 and rcIL-12 Demonstrated Superior Efficacy Compared to rVP2 Alone
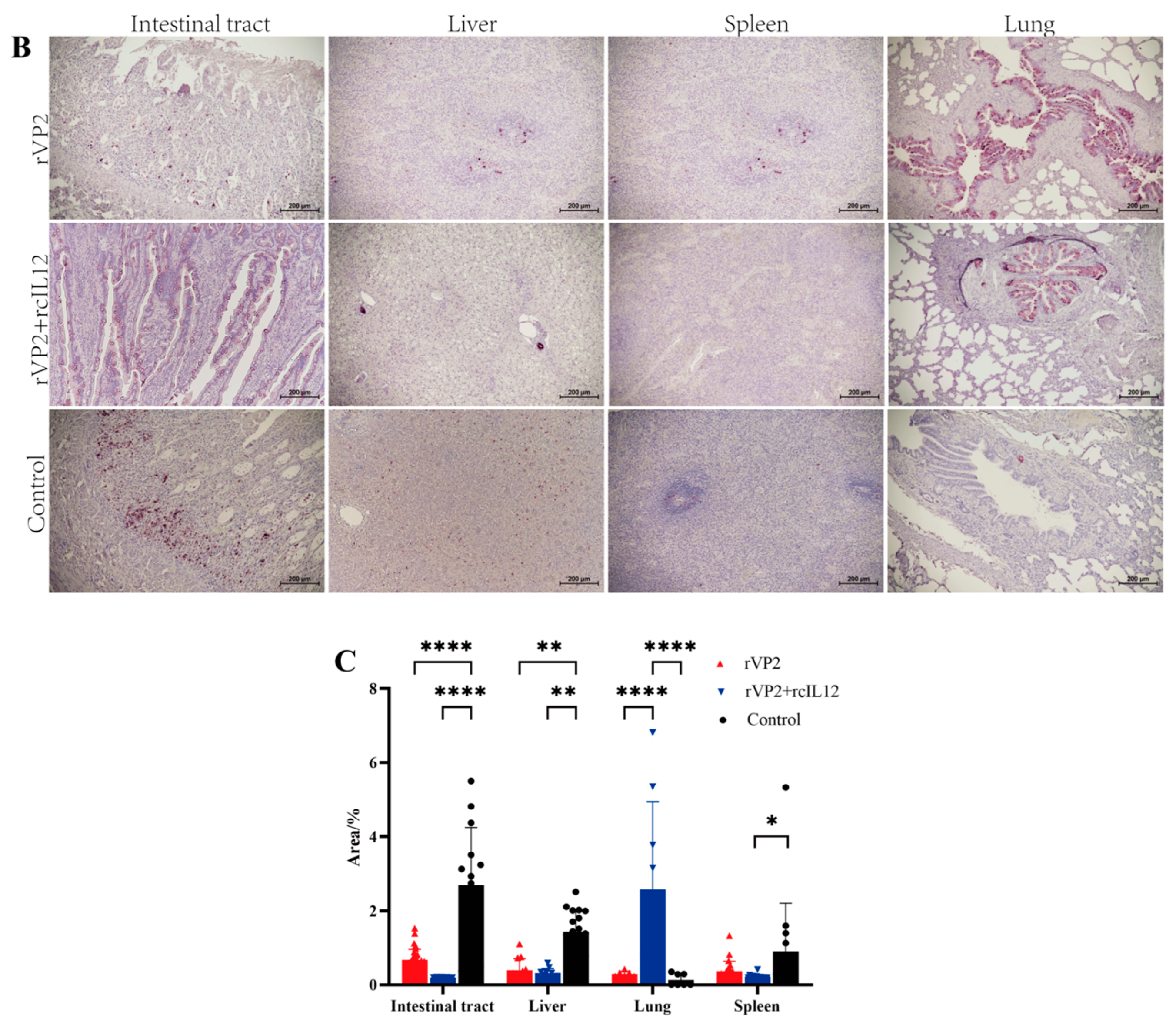
3.4. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Yin, C.; Cao, Z.; Zhang, S.; Zhang, J. Advances in prevention and control techniques for canine parvovirus disease. Chin. J. Vet. Drug 2024, 58, 86–94. [Google Scholar]
- Ahmed, N.; Riaz, A.; Zubair, Z.; Saqib, M.; Ijaz, S.; Nawaz-Ul-Rehman, M.S.; Al-Qahtani, A.; Mubin, M. Molecular analysis of partial VP-2 gene amplified from rectal swab samples of diarrheic dogs in Pakistan confirms the circulation of canine parvovirus genetic variant CPV-2a and detects sequences of feline panleukopenia virus (FPV). Virol. J. 2018, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Zhao, J.; Guo, D.; Sun, D. A Mini-Review on the Epidemiology of Canine Parvovirus in China. Front. Vet. Sci. 2020, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, L.; Hueffer, K.; Parrish, C.R.; Agbandje-McKenna, M. Structures of host range-controlling regions of the capsids of canine and feline parvoviruses and mutants. J. Virol. 2003, 77, 12211–12221. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Hu, G.Q.; Wang, H.L.; Liang, M.; Liang, H.; Guo, H.; Zhao, P.; Yang, Y.J.; Zheng, X.X.; Zhang, Z.F.; et al. Canine parvovirus VP2 protein expressed in silkworm pupae self-assembles into virus-like particles with high immunogenicity. PLoS ONE 2014, 9, e79575. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, H.C.; Wei, Y.Q.; Dong, H.; Han, S.C.; Ao, D.; Sun, D.H.; Wang, H.M.; Cao, S.Z.; Sun, S.Q. Self-assembly of virus-like particles of canine parvovirus capsid protein expressed from Escherichia coli and application as virus-like particle vaccine. Appl. Microbiol. Biotechnol. 2014, 98, 3529–3538. [Google Scholar] [CrossRef] [PubMed]
- Arulanandam, B.P.; O’Toole, M.; Metzger, D.W. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J. Infect. Dis. 1999, 180, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Bryan, S.A.; O’Connor, B.J.; Matti, S.; Leckie, M.J.; Kanabar, V.; Khan, J.; Warrington, S.J.; Renzetti, L.; Rames, A.; Bock, J.A.; et al. Effects of recombinant human interleukin-12 on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000, 356, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Rigopoulou, E.I.; Suri, D.; Chokshi, S.; Mullerova, I.; Rice, S.; Tedder, R.S.; Williams, R.; Naoumov, N.V. Lamivudine plus interleukin-12 combination therapy in chronic hepatitis B: Antiviral and immunological activity. Hepatology 2005, 42, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Carreño, V.; Zeuzem, S.; Hopf, U.; Marcellin, P.; Cooksley, W.G.; Fevery, J.; Diago, M.; Reddy, R.; Peters, M.; Rittweger, K.; et al. A phase I/II study of recombinant human interleukin-12 in patients with chronic hepatitis B. J. Hepatol. 2000, 32, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kalams, S.A.; Parker, S.D.; Elizaga, M.; Metch, B.; Edupuganti, S.; Hural, J.; De Rosa, S.; Carter, D.K.; Rybczyk, K.; Frank, I.; et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J. Infect. Dis. 2013, 208, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, M.; Boyaka, P.N.; Finkelman, F.D.; Kiyono, H.; Jackson, R.J.; Jirillo, E.; McGhee, J.R. Oral but not parenteral interleukin (IL)-12 redirects T helper 2 (Th2)-type responses to an oral vaccine without altering mucosal IgA responses. J. Exp. Med. 1997, 185, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, C.; Tian, C.; Li, Z.; Mai, J.; Cheng, S. Establishment and Application of a Duplex Real-Time PCR Method for Detecting Canine Coronavirus and Canine Parvovirus. Chin. J. Anim. Infect. Dis. 2023, 6, 1–9. [Google Scholar]
- Zhao, S.; Han, X.; Lang, Y.; Xie, Y.; Yang, Z.; Zhao, Q.; Wen, Y.; Xia, J.; Wu, R.; Huang, X.; et al. Development and efficacy evaluation of remodeled canine parvovirus-like particles displaying major antigenic epitopes of a giant panda derived canine distemper virus. Front. Microbiol. 2023, 14, 1117135. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.F.; Sgro, J.Y.; Parrish, C.R. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J. Virol. 1992, 66, 6858–6867. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Biancone, L.; Marasco, R.; Morrone, G.; Marasco, O.; Luzza, F.; Pallone, F. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology 1997, 112, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Hanagiri, T.; Takenoyama, M.; Yoshimatsu, T.; Hirashima, C.; Yoshino, I.; Nakanishi, K.; Nagashima, A.; Nomoto, K.; Yasumoto, K. Effects of interleukin-12 on in vitro culture with interleukin-2 of regional lymph node lymphocytes from lung cancer patients. Cancer Immunol. Immunother. 1996, 43, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, D.; Banu, K.; Mondal, B. Canine parvovirology—A brief updated review on structural biology, occurrence, pathogenesis, clinical diagnosis, treatment and prevention. Comp. Immunol. Microbiol. Infect. Dis. 2022, 82, 101765. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ho, M.G.; Hu, Y.J.; Shi, Y. Vaccine adjuvants: Current status, research and development, licensing, and future opportunities. J. Mater. Chem. B 2024, 12, 4118–4137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, H.; Hu, Y.; Xu, D.; Yin, C.; Han, Q.; Zhang, J. Chitosan Nanovaccines as Efficient Carrier Adjuvant System for IL-12 with Enhanced Protection Against HBV. Int. J. Nanomed. 2021, 16, 4913–4928. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Salas, A.; Sands, B.E.; Abraham, C.; Leibovitzh, H.; Neurath, M.F.; Vande Casteele, N. IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.Y.; Liu, Y.C.; Hsu, Y.C.; Chang, P.C.; Hsieh, M.K.; Shien, J.H.; Ou, S.C. Preparation of Chicken Anemia Virus (CAV) Virus-Like Particles and Chicken Interleukin-12 for Vaccine Development Using a Baculovirus Expression System. Pathogens 2019, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, S.; Janati-Fard, F.; Housaindokht, M.R. Designing a bioadjuvant candidate vaccine targeting infectious bursal disease virus (IBDV) using viral VP2 fusion and chicken IL-2 antigenic epitope: A bioinformatics approach. Comput. Biol. Med. 2023, 163, 107087. [Google Scholar] [CrossRef] [PubMed]
- Eldaghayes, I.; Rothwell, L.; Skinner, M.; Dayhum, A.; Kaiser, P. Efficacy of Fowlpox Virus Vector Vaccine Expressing VP2 and Chicken Interleukin-18 in the Protection against Infectious Bursal Disease Virus. Vaccines 2023, 11, 1716. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, Z.; Tao, Q.; Xu, L.; Gu, S.; Huang, Y.; Liu, Z.; Zhang, Y.; Wen, J.; Lai, S.; et al. Construction of recombinant pseudorabies virus expressing PCV2 Cap, PCV3 Cap, and IL-4: Investigation of their biological characteristics and immunogenicity. Front. Immunol. 2024, 15, 1339387. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Lang, P.A. Collagen-binding IL-12 inflames ‘cold’ tumours. Nat. Biomed. Eng. 2020, 4, 483–484. [Google Scholar] [CrossRef] [PubMed]


| Days | rVP2 Group | rVP2+rcIL-12 Group |
|---|---|---|
| Day 0 | 200 μg rVP2 protein | 200 μg rVP2 protein, 100 μg rcIL-12 protein |
| Day 14 | 200 μg rVP2 protein | 200 μg rVP2 protein, 100 μg rcIL-12 protein |
| Day 39 | 104 TCID50 units of CPV venom were administered by gavage and subcutaneously | |
| Day 53 | Survival dogs are euthanised | |
| Items of Observation | Standard of Scoring | Scores |
|---|---|---|
| Mental Status | Normal | 0 |
| Depressed | 1 | |
| Recumbent | 2 | |
| Death | 3 | |
| Appetite Status | Normal | 0 |
| Decreased | 1 | |
| Hyporexia (persistent ≥ 2d) | 2 | |
| Anorexia (persistent ≥ 2d) | 3 | |
| Fecal Consistency | Normal feces | 0 |
| Watery diarrhea | 1 | |
| Persistent watery diarrhea (≥2d) | 2 | |
| Blood-tinged feces | 3 | |
| Hemorrhagic diarrhea (≥2d) | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Jiao, W.; Zhao, D.; Gong, Y.; Ni, J.; Wu, H.; Du, J.; Wang, T.; Yin, C. Effects of Canine IL-12 on the Immune Response Against the Canine Parvovirus VP2 Protein. Vaccines 2025, 13, 758. https://doi.org/10.3390/vaccines13070758
Wang S, Jiao W, Zhao D, Gong Y, Ni J, Wu H, Du J, Wang T, Yin C. Effects of Canine IL-12 on the Immune Response Against the Canine Parvovirus VP2 Protein. Vaccines. 2025; 13(7):758. https://doi.org/10.3390/vaccines13070758
Chicago/Turabian StyleWang, Shiyan, Wenjie Jiao, Dannan Zhao, Yuzhu Gong, Jingying Ni, Huawei Wu, Jige Du, Tuanjie Wang, and Chunsheng Yin. 2025. "Effects of Canine IL-12 on the Immune Response Against the Canine Parvovirus VP2 Protein" Vaccines 13, no. 7: 758. https://doi.org/10.3390/vaccines13070758
APA StyleWang, S., Jiao, W., Zhao, D., Gong, Y., Ni, J., Wu, H., Du, J., Wang, T., & Yin, C. (2025). Effects of Canine IL-12 on the Immune Response Against the Canine Parvovirus VP2 Protein. Vaccines, 13(7), 758. https://doi.org/10.3390/vaccines13070758





