Factors Associated with Acceptance of Vaccination Against Human Papillomavirus in eThekwini District of South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Sample Size
2.3. Sampling Procedure
2.4. Data Management and Analyses
2.5. Ethics Approval
3. Results
4. Discussion
4.1. Implications and Recommendations
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Adapted HPV Vaccination Survey Questionnaire
| Survey–Screening Good [morning/afternoon]. I am [interviewer’s name] with the University of Cape Town and the South African Medical Research Council (SAMRC). We would like to have a conversation with you with regards to the vaccine against human papillomavirus (HPV). HPV is a type of virus that causes infection and cancer of the cervix in women. We are interviewing parents from eThekwini district in KwaZulu-Natal to help improve the HPV vaccination services in our country. If a potential participant informs you that they do not have children, end the interview. I know you are busy, so this will take only 30 min. Your participation is completely voluntary and anonymous. If you do not want to answer a question or wish to stop the interview, just let me know.
If “No” to Q1: Thank you very much. End interview.
If “No” to Q9, participant is not eligible for survey, and you can thank the participant as follows: Thank you for answering those questions. Unfortunately, you are not eligible to participate in the survey since we are going to ask questions about children, and you are currently not having children. We would like to thank you very much for taking the time to answer my questions. End Interview.
The next questions are about you and about your youngest child who is 9 years old or older.
|
References
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human papillomavirus vaccines: WHO position paper (2022 update). Wkly. Epidemiol. Rec. 2022, 97, 645–672. [Google Scholar]
- Chesson, H.W.; Dunne, E.F.; Hariri, S.; Markowitz, L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 2014, 41, 660–664. [Google Scholar] [CrossRef]
- Rabil, M.J.; Tunc, S.; Bish, D.R.; Bish, E.K. Benefits of integrated screening and vaccination for infection control. PLoS ONE 2022, 17, e0267388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacDonald, N.E.; Butler, R.; Dubé, E. Addressing barriers to vaccine acceptance: An overview. Hum. Vaccin. Immunother. 2018, 14, 218–224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Gillison, M.L. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J. Clin. Oncol. 2018, 36, 262–267. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ledibane, T.D.; Ledibane, N.R.; Matlala, M. Performance of the school-based human papillomavirus vaccine uptake in Tshwane, South Africa. S. Afr. J. Infect. Dis. 2023, 38, 492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amponsah-Dacosta, E.; Blose, N.; Nkwinika, V.V.; Chepkurui, V. Human papillomavirus vaccination in South Africa: Programmatic challenges and opportunities for integration with other adolescent health services? Front. Public Health 2022, 10, 799984. [Google Scholar] [CrossRef]
- Orievulu, K.S.; Frampton, S.; Matthews, P.C.; Mpanza, N.; Mjilo, T.; Nxumalo, S.; Hordern, J.; Seeley, J. Infecting minds: Socio-contextual drivers of vaccine perceptions and attitudes among young and older adults living in urban and rural areas in KwaZulu-Natal, South Africa. BMC Public Health 2025, 25, 1086. [Google Scholar] [CrossRef]
- Iliyasu, R.; Etikan, I. Comparison of quota sampling and stratified random sampling. Biom. Biostat. Int. J. Rev. 2021, 10, 24–27. [Google Scholar] [CrossRef]
- Newman, M.; Gough, D. Systematic reviews in educational research: Methodology, perspectives and application. In Systematic Reviews in Educational Research: Methodology, Perspectives and Application; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–22. [Google Scholar]
- Kumar, R. Research Methodology: A Step-by-Step Guide for Beginners; SAGE Publications Limited: New York, NY, USA, 2019. [Google Scholar]
- Pett, M.; Lackey, N.; Sullivan, J. Making Sense of Factor Analysis; SAGE Publications Limited: New York, NY, USA, 2003. [Google Scholar]
- World Health Organization. Understanding the behavioural and social drivers of vaccine uptake: WHO position paper. Wkly. Epidemiol. Rec. 2022, 97, 209–224. [Google Scholar]
- Katoto, P.D.M.C.; Parker, S.; Coulson, N.; Pillay, N.; Cooper, S.; Jaca, A.; Mavundza, E.; Houston, G.; Groenewald, C.; Essack, Z.; et al. Predictors of COVID-19 Vaccine Hesitancy in South African Local Communities: The VaxScenes Study. Vaccines 2022, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, H.; Guo, J.; Fang, H.; Huang, Y.; Wang, J.; Lyu, Y.; Zhu, H.; Jing, R.; Lai, X. From COVID-19 Vaccination Intention to Actual Vaccine Uptake: A Longitudinal Study Among Chinese Adults After Six Months of a National Vaccination Campaign. Expert. Rev. Vaccines 2022, 21, 385–395. [Google Scholar] [CrossRef]
- Guan, B.; Lu, Y.; Mei, K.; Lu, J.; Lu, X.; Zhang, L. Gap between willingness and behavior in the vaccination against influenza, pneumonia, and herpes zoster among Chinese aged 50–69 years. Expert. Rev. Vaccines 2021, 20, 1147–1152. [Google Scholar] [CrossRef]
- Rodrigues, A.; Guerreiro, J.; Moura, S.; Romano, S.; Paulino, E. From hesitancy to decision: Shifts from intentions to uptake of Influenza and COVID-19 vaccines. Eur. J. Public Health 2024, 34, ckae144.1037. [Google Scholar] [CrossRef]
- Collini, F.; Bonaccorsi, G.; Del Riccio, M.; Bruschi, M.; Forni, S.; Galletti, G.; Gemmi, F.; Ierardi, F.; Lorini, C. Does Vaccine Confidence Mediate the Relationship between Vaccine Literacy and Influenza Vaccination? Exploring Determinants of Vaccination among Staff Members of Nursing Homes in Tuscany, Italy, during the COVID-19 Pandemic. Vaccines 2023, 11, 1375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grima, G.F.; Dublin, I.; The, C. Factors that influence the uptake and delivery of HPV vaccines in migrants: A systematic review. Eur. J. Public Health 2023, 33, ckad160.1630. [Google Scholar] [CrossRef]
- Xu, M.A.; Choi, J.; Capasso, A.; DiClemente, R.J. Improving HPV Vaccination Uptake Among Adolescents in Low Resource Settings: Sociocultural and Socioeconomic Barriers and Facilitators. Adolesc. Health Med. Ther. 2024, 15, 73–82. [Google Scholar] [CrossRef]
- Kutz, J.M.; Rausche, P.; Gheit, T.; Puradiredja, D.I.; Fusco, D. Barriers and facilitators of HPV vaccination in sub-saharan Africa: A systematic review. BMC Public Health 2023, 23, 974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deal, A.; Carter, J.; Aspray, N.; Clemente, S.; Bojang, S.; Webb, S.; Seedat, F.; Iwami, M.; Krishna, S.; Crawshaw, A.; et al. Defining drivers of human papillomavirus (HPV) vaccine uptake in migrant populations globally and strategies and interventions to improve coverage: A systematic review. medRxiv 2025. [Google Scholar] [CrossRef]
- Ortiz, R.R.; Smith, A.; Coyne-Beasley, T. A systematic literature review to examine the potential for social media to impact HPV vaccine uptake and awareness, knowledge, and attitudes about HPV and HPV vaccination. Hum. Vaccines Immunother. 2019, 15, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.D.; Enache, A.I.; Popa, I.V.; Antoniu, S.A.; Dragomir, R.A.; Burlacu, A. Determinants of the hesitancy toward COVID-19 vaccination in Eastern European countries and the relationship with health and vaccine literacy: A literature review. Vaccines 2022, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Lorini, C.; Collini, F.; Galletti, G.; Ierardi, F.; Forni, S.; Gatteschi, C.; Gemmi, F.; Stacchini, L.; Papini, S.; Velpini, B.; et al. Vaccine Literacy and Source of Information about Vaccination among Staff of Nursing Homes: A Cross-Sectional Survey Conducted in Tuscany (Italy). Vaccines 2022, 10, 682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Exploratory Variables | Summary Statistics | ||
|---|---|---|---|
| All | Accept HPV Vaccine | Do Not Accept HPV Vaccine | |
| Sample size | N = 713 (100%) | N = 667 (93.5%) | N = 46 (6.5%) |
| Mean age (standard deviation) | 42.6 (11.6) years | 42.5 (11.4) years | 44.5 (12.9) years |
| Sex: Male Female | 99 (13.9%) 614 (86.1%) | 88 (13.2%) 579 (86.8%) | 11 (23.9) 35 (76.1) |
| Education: Primary and below Secondary Tertiary and above Missing | 45 (6.3%) 552 (77.4%) 113 (15.8%) 5 (0.4%) | 44 (6.6%) 513 (76.9%) 105 (15.7%) 5 (0.7%) | 1 (2.2%) 38 (82.6%) 7 (15.2%) 0 (0.0%) |
| Household income: Less than ZAR 10,000 * ZAR 10,000 to 20,000 More than ZAR 20,000 | 656 (92.0%) 41 (5.8%) 16 (2.2%) | 613 (91.9%) 38 (5.7%) 14 (2.1%) | 41 (89.1%) 3 (6.52) 2 (4.3%) |
| Neighbourhood: Chatsworth Embo Wentworth Umlazi Missing | 182 (25.5%) 144 (20.2%) 190 (26.6%) 197 (27.6%) 2 (0.3%) | 169 (25.3%) 134 (20.1%) 166 (24.9%) 196 (29.4%) 2 (0.3%) | 13 (28.3%) 10 (21.7%) 23 (50.0%) 0 (0.0%) 0 (0.0%) |
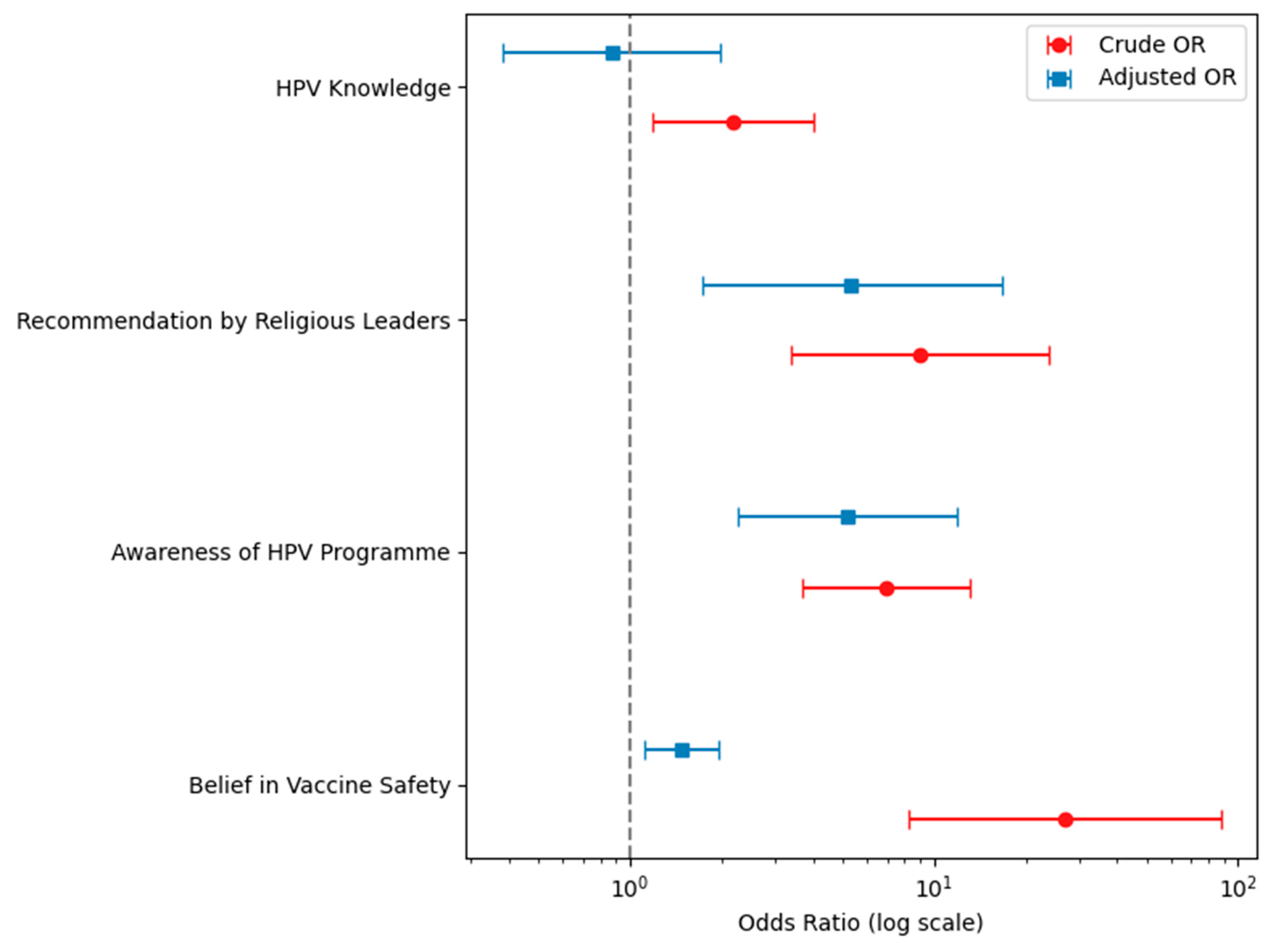
| Variable | N | Crude OR | 95% CI | p-Value | Adjusted OR | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|
| Sex of caregiver | 713 | ||||||
| Male | 99 | Baseline | |||||
| Female | 614 | 2.06 | 1.01–4.21 | 0.047 | 0.78 | 0.32–1.89 | 0.588 |
| Education | 710 | – | – | – | |||
| Primary | 45 | ||||||
| Secondary | 552 | 0.31 | 0.04–2.29 | 0.249 | |||
| Tertiary | 113 | 0.34 | 0.04–2.85 | 0.321 | |||
| Household income | 713 | – | – | – | |||
| <ZAR 3000 | 541 | Baseline | |||||
| ZAR 3000–10,000 | 115 | 1.26 | 0.52–3.07 | 0.609 | |||
| ZAR 100,001–20,000 | 41 | 0.88 | 0.26–2.99 | 0.837 | |||
| >ZAR 20,000 | 16 | 0.49 | 0.11–2.22 | 0.353 | |||
| Age | 711 | 0.99 | 0.96–1.01 | 0.254 | – | – | – |
| Received COVID-19 vaccine | 712 | ||||||
| No | 260 | Baseline | |||||
| Yes | 252 | 1.42 | 0.77–2.60 | 0.262 | – | – | – |
| HPV knowledge | 710 | ||||||
| Poor | 206 | Baseline | |||||
| Good | 504 | 2.19 | 1.19–4.00 | 0.011 | 0.84 | 0.33–2.11 | 0.710 |
| Recommendation of religious leaders on vaccination | 711 | ||||||
| Discourage | 20 | Baseline | |||||
| Encourage | 691 | 8.99 | 3.39–23.81 | <0.001 | 5.06 | 1.56–16.45 | 0.007 |
| Aware of school-based HPV vaccination programme | 700 | ||||||
| No | 98 | Baseline | |||||
| Yes | 602 | 6.96 | 3.67–13.17 | <0.001 | 5.22 | 2.01–13.56 | 0.001 |
| View on safety of vaccines | 708 | ||||||
| Sceptical | 90 | Baseline | |||||
| Safe | 618 | 26.89 | 8.24–87.71 | <0.001 | 19.69 | 5.86–66.15 | <0.001 |
| View on importance of vaccines | 708 | ||||||
| Not important | 90 | Baseline | |||||
| Important | 618 | 3.37 | 1.72–6.59 | <0.001 | 1.20 | 0.32–1.89 | 0.710 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhengu, P.; Wiysonge, C.S.; Katoto, P.D.M.C.; Ndwandwe, D.; Cooper, S.; Bhengu, S.; Mazingisa, A.V.; Saber, T.; Sithole, M.; Smith, D.; et al. Factors Associated with Acceptance of Vaccination Against Human Papillomavirus in eThekwini District of South Africa. Vaccines 2025, 13, 732. https://doi.org/10.3390/vaccines13070732
Bhengu P, Wiysonge CS, Katoto PDMC, Ndwandwe D, Cooper S, Bhengu S, Mazingisa AV, Saber T, Sithole M, Smith D, et al. Factors Associated with Acceptance of Vaccination Against Human Papillomavirus in eThekwini District of South Africa. Vaccines. 2025; 13(7):732. https://doi.org/10.3390/vaccines13070732
Chicago/Turabian StyleBhengu, Phelele, Charles S. Wiysonge, Patrick D. M. C. Katoto, Duduzile Ndwandwe, Sara Cooper, Sebenzile Bhengu, Akhona V. Mazingisa, Theresa Saber, Mandisi Sithole, Darian Smith, and et al. 2025. "Factors Associated with Acceptance of Vaccination Against Human Papillomavirus in eThekwini District of South Africa" Vaccines 13, no. 7: 732. https://doi.org/10.3390/vaccines13070732
APA StyleBhengu, P., Wiysonge, C. S., Katoto, P. D. M. C., Ndwandwe, D., Cooper, S., Bhengu, S., Mazingisa, A. V., Saber, T., Sithole, M., Smith, D., Tembe, L. G., Kuodi, P., & Shey, M. S. (2025). Factors Associated with Acceptance of Vaccination Against Human Papillomavirus in eThekwini District of South Africa. Vaccines, 13(7), 732. https://doi.org/10.3390/vaccines13070732







