Abstract
Helicobacter pylori (H. pylori) is one of the most prevalent chronic bacterial infections globally, significantly contributing to gastritis, peptic ulcers, and gastric malignancies. Its pathogenesis involves a complex array of virulence factors—including cagA, vacA, and urease—which facilitate mucosal colonization, immune evasion, and persistent inflammation. A major challenge in vaccine development is the bacterium’s ability to manipulate both innate and adaptive immune responses, resulting in limited natural clearance and long-term persistence. This review synthesizes H. pylori pathogenesis and host immune dynamics, highlighting their implications for vaccine design. By elucidating the molecular and cellular mechanisms underlying host–pathogen interactions, we explore how these insights inform antigen selection, adjuvant optimization, and delivery strategies. By integrating basic science with translational objectives, this review aims to support the development of an effective H. pylori vaccine, addressing global health needs, particularly in regions with a high infection burden and limited access to treatment.
1. Introduction
Helicobacter pylori (H. pylori) is one of the most prevalent chronic bacterial infections globally, colonizing the gastric mucosa of over half the population worldwide [1]. While often asymptomatic, persistent infection is a known cause of chronic gastritis, peptic ulcer disease, mucosa-associated lymphoid tissue (MALT) lymphoma, and non-cardia gastric cancer. The International Agency for Research on Cancer (IARC) consistently evaluates biological and environmental carcinogens and has recently emphasized the need to identify and mitigate infectious and dietary risk factors for cancer [2].
Despite the substantial global burden of H. pylori infection, no licensed vaccine is currently available. Treatment continues to rely on multidrug antibiotic regimens, which are increasingly ineffective due to rising antimicrobial resistance (AMR) [3]. Resistance to essential antibiotics—clarithromycin, metronidazole, and levofloxacin—has significantly reduced eradication rates and limited therapeutic options worldwide [4,5]. According to SalahiNiri et al. [6], mean global resistance rates are 32.6% for clarithromycin, 35.3% for metronidazole, and 13.2% for levofloxacin, exhibiting considerable regional variation. In Europe and the United States, clarithromycin resistance exceeds 20%, while metronidazole resistance surpasses 70% [7].
In Arab countries, recent systematic analyses have highlighted a concerning increase in the prevalence of the mcr gene and other resistance determinants in both clinical and community isolates of H. pylori, threatening the effectiveness of last-resort antibiotics such as colistin [8]. Bakleh et al. [8] also documented significant regional variability in AMR gene dissemination across Arab nations, emphasizing the urgent need for enhanced antimicrobial stewardship and the development of alternative preventive measures. These escalating AMR trends contribute to persistent infection and increased rates of treatment failure and recurrence, particularly in high-burden regions where diagnostic capacity is limited [6,7]. The growing challenge of multidrug-resistant H. pylori underscores the critical need for an effective vaccine to reduce global dependence on antibiotics and mitigate long-term complications, including gastric malignancies.
The development of an H. pylori vaccine remains exceptionally challenging. H. pylori’s ability to evade host immunity allows it to persist long-term in the stomach’s harsh acidic environment. The bacterium actively suppresses immune responses, manipulates host signaling pathways, and avoids clearance through mechanisms such as antigenic variation, biofilm formation, and the modulation of T-cell responses [9,10]. Furthermore, natural infection rarely leads to sterilizing immunity, complicating the identification of correlates of protection. Additionally, substantial genetic heterogeneity, coupled with the difficulty of generating strong, durable mucosal immune responses, continues to hinder effective vaccine design.
Despite persistent challenges, recent scientific advances have reinvigorated research into H. pylori vaccines. Notably, Tu et al. [11] utilized an artificial intelligence-driven framework to identify conserved, immunodominant multiepitope sequences across diverse H. pylori strains. The in silico-designed vaccine construct demonstrated high antigenic affinity and favorable immune simulation profiles, offering a promising foundation for next-generation, precision-guided vaccine development. Simultaneously, Yunle et al. [12] provided a comprehensive review of emerging antigen candidates, including urease, vacA, and oipA, and highlighted the potential of novel adjuvants such as CpG oligodeoxynucleotides and cholera toxin B subunit derivatives. Their analysis further emphasized the importance of mucosal delivery strategies, particularly oral and intranasal routes, for eliciting robust local immune responses within the gastric mucosa.
These innovations, which encompass immunogen design, adjuvant selection, and advanced delivery platforms, collectively offer a renewed and realistic opportunity to overcome longstanding barriers in H. pylori vaccine development. As global health initiatives increasingly prioritize antimicrobial stewardship and cancer prevention, developing a safe and effective H. pylori vaccine remains a key scientific priority and a pressing public health need.
This review offers a comprehensive overview of the current vaccine platforms under investigation, which include subunit, DNA-based, live-attenuated, vector-based, epitope-based, and virus-like particle vaccines. Additionally, it explores promising mucosal adjuvants and innovative delivery systems. The review also discusses significant translational challenges, such as immune evasion, strain variability, and mucosal delivery barriers. Furthermore, it highlights emerging technologies relevant to H. pylori vaccine development, including AI-guided antigen selection, nanotechnology-based delivery systems, and advances in mucosal immunology.
2. Biology and Clinical Relevance of H. pylori
2.1. Microbiology and Structure of H. pylori
H. pylori is a Gram-negative, microaerophilic, spiral-shaped bacterium equipped with multiple unipolar flagella that facilitate motility within viscous gastric mucus [13]. Its helical shape enhances mucus penetration, enabling effective navigation toward the gastric epithelium rather than direct tissue invasion [14]. Acid resistance is primarily mediated by urease activity, which hydrolyses urea into ammonia and carbon dioxide, buffering the acidic environment and enabling bacterial survival in the stomach [15]. The outer membrane proteins (OMPs) of H. pylori, such as babA, sabA, and oipA, are essential for adhesion and colonization of the gastric epithelium [16]. Additionally, the cytotoxin-associated gene pathogenicity island (cagPAI), particularly the cagA gene, encodes a type IV secretion system (T4SS) that translocates the cagA protein into host epithelial cells, leading to aberrant signaling and inflammation [17]. Recent proteomic studies have revealed that more than 60 OMPs are involved in adhesion, immune evasion, and nutrient uptake, reflecting H. pylori’s complex interactions with the host [18]. Furthermore, electron microscopy has shown that its peptidoglycan structure supports its spiral morphology, potentially influencing host immune detection [19].
In addition to its interactions with host epithelial surfaces, H. pylori competes with the native gastric microbiota to establish a persistent niche. The stomach was once considered sterile, but it is now recognized to harbor a unique, although low-diversity, microbial community that contributes to mucosal homeostasis and pathogen resistance [20,21]. To overcome colonization resistance, H. pylori employs several mechanisms, including altering the gastric pH through urease activity, disrupting tight junctions, and producing antimicrobial peptides that suppress commensal competitors [22]. Moreover, H. pylori modifies mucosal glycan expression to selectively adhere to and outcompete resident microbes, a strategy that facilitates niche dominance [23].
The disruption of microbial equilibrium not only favors H. pylori persistence but also may contribute to gastric dysbiosis, with implications for disease progression and vaccine efficacy. Understanding these microbial dynamics is essential for developing targeted interventions that promote both microbial balance and effective immune priming. The key mechanisms underlying this competitive displacement are summarized in Figure 1, which illustrates how H. pylori modifies its environment and disrupts the microbial ecosystem to secure a colonization niche.
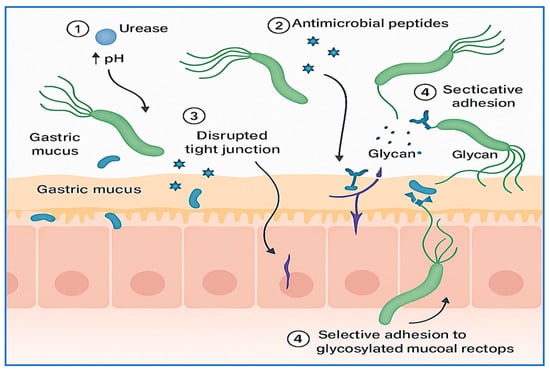
Figure 1.
Mechanisms of gastric microbiota displacement by H. pylori. This conceptual illustration depicts how H. pylori establishes colonization by competing with the native gastric microbiota. Key mechanisms include (1) urease-mediated alkalinization of the gastric environment, which reduces acid-dependent microbial competitors; (2) secretion of antimicrobial peptides targeting commensals; (3) disruption of epithelial tight junctions to facilitate mucosal infiltration; and (4) selective adhesion via glycan-binding adhesins to displace resident microbes. These strategies collectively promote gastric dysbiosis, enabling chronic infection and impairing immune priming, with potential consequences for host–pathogen interactions and vaccine efficacy.
2.2. Colonization and Survival Mechanisms
Once H. pylori enters the host, it must overcome multiple physical and biochemical barriers to colonize the gastric mucosa. Its spiral morphology and sheathed flagella enable efficient motility through viscous gastric mucus, guided by chemotactic responses to pH and urea gradients, which direct the bacterium toward the relatively neutral environment near the epithelial surface [24]. Upon reaching the gastric epithelium, H. pylori employs a repertoire of outer membrane adhesins—including babA, sabA, and hopQ—to bind specific host glycan structures and CEACAM receptors. This attachment not only anchors the organism securely within the gastric niche but also facilitates the translocation of virulence factors such as cagA into host cells via the type IV secretion system [25]. Figure 2 provides a schematic overview of these colonization and survival mechanisms, including motility, adhesion, acid neutralization, antioxidant defenses, biofilm formation, and antigenic variation.
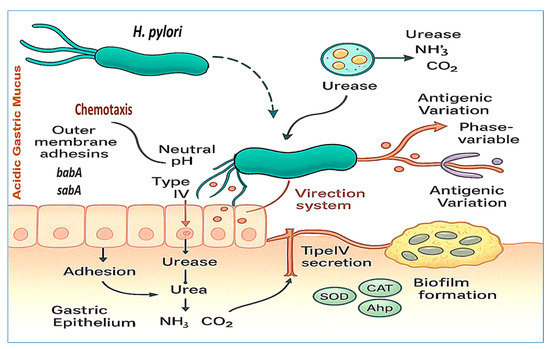
Figure 2.
H. pylori strategies for colonization and survival in the gastric environment. This figure illustrates key virulence factors, including the helical morphology and unipolar flagella, which facilitate motility through gastric mucus and chemotaxis toward urea and pH gradients. Acid neutralization is achieved through urease activity, which converts urea into ammonia and carbon dioxide. Outer membrane adhesins (e.g., babA and sabA) mediate adherence to the gastric epithelium, whereas the T4SS delivers effector proteins such as cagA into host cells, promoting inflammation and altering signaling pathways. Additional features include biofilm formation and antigenic variation, both of which contribute to immune evasion and persistent colonization.
A pivotal adaptation that enables H. pylori survival in the gastric environment is its production of urease, a nickel-dependent enzyme that hydrolyzes urea into ammonia and carbon dioxide. This biochemical reaction buffers the surrounding pH, effectively neutralizing gastric acid and protecting the bacterium from acid-mediated damage [26]. Additionally, H. pylori employs antioxidant defenses—such as superoxide dismutase, catalase, and alkyl hydroperoxide reductase—to detoxify reactive oxygen species generated by host immune cells [27]. Outer membrane vesicles (OMVs) have also emerged as significant contributors to H. pylori colonization and persistence. These vesicles encapsulate virulence factors—including vacA, urease, and adhesins—and are capable of modulating host cell signaling, promoting immune evasion, and enhancing biofilm development [28].
Furthermore, H. pylori forms biofilms—structured microbial communities embedded in a protective extracellular matrix—that shield the bacterium from host defenses and increase resistance to antibiotic therapies. Biofilm-associated cells exhibit reduced metabolic activity and altered gene expression patterns, rendering them more tolerant to treatment [29]. In addition to these physical defenses, H. pylori displays antigenic variation through phase-variable expression of OMPs such as oipA and hopZ. This reversible switch enables immune evasion by modulating the bacterial surface architecture in response to environmental pressures [30]. Further evidence suggests that OMVs actively contribute to biofilm development by transporting structural and regulatory molecules, thereby reinforcing H. pylori’s ability to persist long term within the human stomach [31].
2.3. Host Interaction and Inflammatory Pathways
The interaction between H. pylori and the gastric epithelium elicits a complex and dynamic inflammatory response, which is central to both the pathogenesis of gastric diseases and the persistence of infection. Upon colonization, H. pylori activates host pattern recognition receptors, such as toll-like receptors (TLR2, TLR4, and TLR9), initiating downstream signaling cascades via the NF-κB and MAPK pathways. This activation results in the production of pro-inflammatory mediators, including interleukin-8 (IL-8), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β) [32,33]. IL-8, in particular, serves as a potent chemoattractant for neutrophils and plays a central role in the chronic inflammatory milieu observed in infected gastric tissue. A hallmark of virulent H. pylori strains is the presence of cagPAI, which encodes a type IV secretion system (T4SS). This system allows the cagA protein to be translocated into host epithelial cells, where it becomes tyrosine-phosphorylated at EPIYA motifs by the Src and Abl kinases. Once phosphorylated, cagA interacts with a range of host signaling molecules, including SHP-2, leading to cytoskeletal reorganization, disruption of tight junctions, and dysregulation of cell proliferation and polarity [17]. CagA also modulates β-catenin signaling and promotes epithelial‒mesenchymal transition (EMT), linking chronic infection to gastric carcinogenesis [34].
In addition to cagA, vacA—present in virtually all H. pylori strains—plays a pivotal role in immune evasion. VacA forms pores in host cell membranes and targets mitochondria, inducing apoptosis in T-cells, B-cells, and epithelial cells while impairing antigen presentation by dendritic cells [35,36]. It also inhibits IL-2 signaling and T-cell activation, thereby weakening the adaptive immune response and allowing bacterial persistence [37]. The chronic inflammation induced by persistent H. pylori infection leads to the sustained infiltration of neutrophils, macrophages, dendritic cells, and lymphocytes into the gastric mucosa. These immune cells release reactive oxygen species, nitric oxide, and proteolytic enzymes, causing epithelial damage and amplifying the inflammatory loop [38,39]. Over time, this inflammatory microenvironment promotes gastric atrophy, intestinal metaplasia, dysplasia, and ultimately noncardia gastric cancer—particularly in individuals infected with cagA-positive and vacA s1/m1 strains [40,41].
Recent transcriptomic studies have revealed that H. pylori modulates host gene expression through noncoding RNAs and epigenetic reprogramming, influencing immune responses, promoting epithelial cell cycle progression, and even silencing tumor suppressor genes [42,43,44]. These findings underscore the multifaceted and long-term impact of H. pylori on gastric mucosal immunity and host cellular homeostasis. Figure 3 provides a schematic overview of the key host–pathogen interactions involved in H. pylori-induced inflammation, highlighting the roles of the toll-like receptors cagA and vacA virulence factors, immune cell recruitment, and downstream signaling events that contribute to chronic inflammation and gastric carcinogenesis.
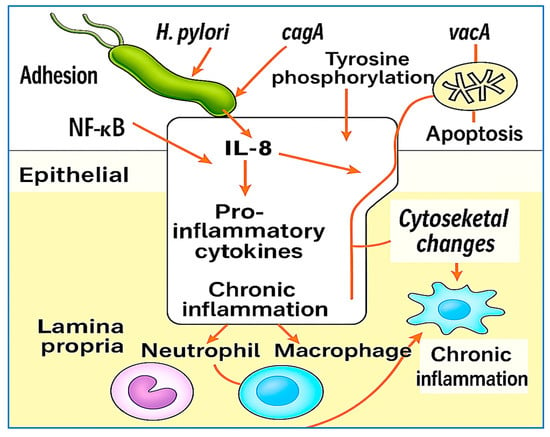
Figure 3.
Illustration of H. pylori-induced host interactions and inflammatory pathways. Upon colonization, H. pylori activates toll-like receptors (TLR2, TLR4, and TLR9), triggering the NF-κB and MAPK signaling pathways and the subsequent release of pro-inflammatory cytokines (IL-8, TNF-α, and IL-1β). The cagA protein is translocated into host epithelial cells via the T4SS, where it becomes phosphorylated and interferes with SHP-2 and β-catenin signaling, promoting tight junction disruption, EMT, and carcinogenesis. VacA induces mitochondrial dysfunction, apoptosis, and immune evasion. These events collectively recruit neutrophils, macrophages, and lymphocytes; amplify oxidative damage; and sustain chronic inflammation, which can progress to gastric atrophy and cancer.
2.4. Associated Diseases and Clinical Outcomes
H. pylori infection has been extensively linked to a wide range of gastrointestinal and extra-gastrointestinal diseases, with clinical outcomes shaped by bacterial virulence factors, host immune responses, and environmental exposures [45,46,47]. Among its most serious implications is its role in noncardia gastric adenocarcinoma [48]. Chronic H. pylori infection initiates the Correa cascade, beginning with chronic active gastritis and progressing through stages of atrophic gastritis, intestinal metaplasia, and dysplasia, ultimately leading to gastric carcinoma [49]. This pathway has been well documented in longitudinal and mechanistic studies, and the World Health Organization (WHO) has classified H. pylori as a Group I carcinogen. Recent meta-analyses have confirmed that eradication of the bacterium significantly reduces the risk of gastric cancer, particularly when intervention occurs before the onset of premalignant lesions [40,50].
In addition to gastric cancer, H. pylori is the primary causative agent of peptic ulcer disease and is responsible for the majority of duodenal and gastric ulcers. Through mucosal inflammation, disruption of protective mechanisms, and alterations in acid secretion, bacteria promote ulcer formation. The introduction of eradication therapies has dramatically reduced recurrence rates and transformed clinical management strategies for ulcer patients [51,52]. Another important gastric condition associated with H. pylori infection is MALT lymphoma. In the early stages, bacterial eradication can induce complete remission in many patients, highlighting the infectious etiology of this form of gastric lymphoma and underscoring the importance of timely detection [53,54].
In functional gastrointestinal disorders, H. pylori also plays a role in functional dyspepsia, particularly the epigastric pain subtype. Studies have demonstrated that eradication therapy leads to meaningful symptom improvement in a subset of patients, and as a result, testing and treatment for H. pylori are now included in standard guidelines for managing dyspepsia [55]. Increasing evidence also supports an association between H. pylori infection and systemic diseases beyond the gastrointestinal tract. Iron deficiency anemia—especially in patients unresponsive to iron supplementation—has been linked to H. pylori through mechanisms involving chronic mucosal bleeding, hepcidin modulation, and impaired iron absorption [56,57]. Similarly, a causal relationship has been proposed between H. pylori and idiopathic thrombocytopenic purpura, with several studies documenting platelet count recovery following bacterial eradication [58].
Emerging literature has also explored potential links between H. pylori infection and extra-gastrointestinal conditions, particularly neurological, metabolic, and immunotherapy-related outcomes. However, it is important to emphasize that these associations are based primarily on observational studies and should be considered preliminary or hypothesis generating rather than conclusive. For example, epidemiological data suggest a possible association between H. pylori and neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease, potentially mediated by systemic inflammation, molecular mimicry, and disruption of the gut‒brain axis [59]. Similarly, correlations have been reported between H. pylori infection and components of metabolic syndrome, including insulin resistance and type 2 diabetes mellitus; however, no definitive causal relationship has been established to date [60,61]. In the context of immunotherapy, early clinical data have revealed that H. pylori infection may negatively affect the efficacy of immune checkpoint inhibitors in patients with advanced gastric cancer. A multicenter study reported reduced progression-free and overall survival among H. pylori-positive individuals, possibly due to infection-mediated immune modulation [62]. While these findings are intriguing, further validation through prospective, mechanistic, and interventional research is essential before drawing firm conclusions or altering clinical practice.
Overall, the clinical significance of H. pylori infection extends well beyond the stomach. While its role in peptic ulcer disease and gastric cancer is well established, accumulating evidence highlights its contribution to hematologic, neurologic, and metabolic conditions. The growing body of literature underscores the importance of early detection, timely eradication, and a comprehensive understanding of H. pylori’s systemic effects. Figure 4 illustrates the major gastrointestinal outcomes associated with H. pylori infection, including chronic gastritis, peptic ulcer disease, MALT lymphoma, and noncardia gastric cancer. It distinguishes between duodenal ulcers—linked to high acid output in the antrum—and gastric ulcers, which arise in the corpus due to impaired mucosal defense. The figure summarizes the progression from chronic colonization to diverse pathological outcomes.
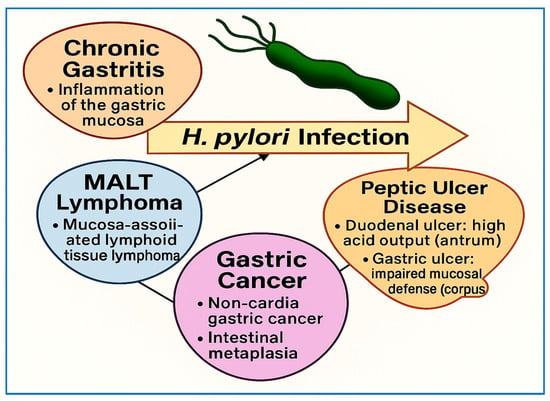
Figure 4.
Overview of major clinical outcomes associated with H. pylori infection. This information summarizes key gastrointestinal conditions associated with H. pylori infection, including chronic gastritis, peptic ulcer disease, MALT lymphoma, and gastric cancer. It distinguishes between duodenal ulcers—typically linked to high acid output in the antrum—and gastric ulcers, which arise in the corpus due to impaired mucosal defense. The figure highlights the progression from bacterial colonization to diverse gastric pathologies.
2.5. Epidemiology and Global Burden
H. pylori infection is one of the most prevalent chronic bacterial infections worldwide, affecting more than 50% of the global population [63]. Prevalence rates vary significantly by geographic region and are strongly influenced by socioeconomic, environmental, and hygiene-related factors. The highest infection rates are reported in developing countries, where limited access to clean water, inadequate sanitation, and crowded living conditions contribute to increased transmission [1]. In some regions of Africa, South America, and South Asia, the prevalence exceeds 70%, whereas rates in North America and Western Europe are generally less than 40% and continue to decline due to improved living standards and widespread antibiotic use [64].
Prevalence also varies by age, with higher rates typically observed in older individuals, reflecting cumulative exposure over time and early-life acquisition in endemic areas. Transmission occurs primarily through oral–oral and fecal–oral routes, often within households during early childhood. Key risk factors include close familial contact, poor hand hygiene, and unsafe water sources [22]. Although its prevalence remains high, its incidence (new infection rates) appears to be declining in many high-income countries as a result of improvements in public health and improved sanitation [65]. The WHO and the International Agency for Research on Cancer classify H. pylori as a Group I carcinogen, underscoring its established role in gastric carcinogenesis. It is estimated that H. pylori causes approximately 810,000 new gastric cancer cases annually, accounting for nearly 90% of all noncardia gastric cancers worldwide [65]. The population-attributable fraction (PAF) of gastric cancer caused by H. pylori exceeds 75% globally [66], highlighting its importance as a public health priority.
Additionally, H. pylori infection imposes a substantial economic burden because of the costs associated with diagnosis, eradication therapy, complications such as ulcers, and the treatment of malignancies. Despite its declining prevalence in some regions, eradication remains challenging because of increasing antibiotic resistance, inconsistent screening practices, and limited healthcare access in high-burden areas. Notably, resistance to clarithromycin and metronidazole has reduced the efficacy of standard triple therapy, prompting recommendations for region-specific treatment regimens on the basis of local resistance profiles [3,66,67,68,69,70]. Recent global strategies emphasize a multifaceted approach that combines hygiene promotion, education, early screening, and tailored eradication therapy. Targeted interventions in high-risk populations—particularly children and adults with known exposure—are essential for reducing transmission and preventing long-term complications. Given its persistence, oncogenic potential, and systemic effects, H. pylori remains a key priority for global infection control and cancer prevention initiatives.
2.6. Diagnostic Approaches for H. pylori
The accurate diagnosis of H. pylori infection is crucial for guiding treatment, preventing disease progression, and confirming eradication [71]. Diagnostic options are broadly classified into noninvasive and invasive tests. Among noninvasive tests, the urea breath test (UBT) is considered the most accurate, particularly for detecting active infection and for post-treatment follow-up [72]. The UBT measures labeled carbon dioxide in exhaled breath after the ingestion of urea tagged with ^13C or ^14C. Both the sensitivity and specificity exceed 90%. However, the recent use of antibiotics or proton pump inhibitors (PPIs) can reduce test accuracy; therefore, a medication-free window of at least two weeks is recommended before testing [73].
The stool antigen test (SAT) is another highly sensitive and cost-effective option that is suitable for both initial diagnosis and eradication confirmation [74]. Monoclonal antibody-based enzyme immunoassays outperform polyclonal formats. Like the UBT, the SAT accuracy is also diminished by recent antibiotic or PPI use [75]. Serological testing, typically enzyme-linked immunosorbent assay (ELISA) for H. pylori-specific IgG, is less clinically useful, as it cannot distinguish between active and past infections and is not appropriate for post-treatment evaluation [76].
Invasive tests, which are performed during upper gastrointestinal endoscopy, include histology, the rapid urease test (RUT), culture, and molecular diagnostics. Histology remains the gold standard for directly visualizing H. pylori and assessing mucosal pathology. To increase diagnostic yield, biopsy samples should be obtained from both the antrum and corpus, particularly in cases of atrophic or patchy gastritis [76]. The RUT provides rapid and inexpensive detection of urease activity in gastric tissue; however, its sensitivity is reduced in patients recently treated with antibiotics or PPIs or in cases of low bacterial density. Culture, although labor-intensive and not routinely performed, enables antimicrobial susceptibility testing—which is critical in regions with high resistance rates [3]. PCR-based assays allow for the simultaneous detection and genotyping of H. pylori and the identification of resistance mutations, particularly clarithromycin resistance (e.g., 23S rRNA gene mutations) [77,78]. Despite their high sensitivity, the widespread use of these assays is limited by cost and technical constraints.
Diagnostic strategies should be tailored to the clinical context, resource availability, and treatment objectives. Noninvasive tests are preferred in outpatient and follow-up settings, whereas invasive methods are reserved for endoscopic evaluations or resistance profiling. Current recommendations align with the Maastricht V/Florence Consensus Report, which provides evidence-based guidance on test selection, treatment algorithms, and antimicrobial stewardship [68]. Table 1 provides a structured comparison of the diagnostic methods used for detecting H. pylori, including both noninvasive and invasive techniques. The table outlines each method’s components, diagnostic accuracy, ability to detect active infection, and clinical advantages and limitations, supporting informed decision-making on the basis of patient condition, resource availability, and diagnostic goals.

Table 1.
Overview of diagnostic methods for H. pylori: characteristics, accuracy, and clinical utility.
2.7. Treatment and Management of H. pylori Infection
The eradication of H. pylori is a critical component of global strategies to prevent peptic ulcer disease, MALT lymphoma, and gastric cancer. The primary therapeutic goal is to achieve complete bacterial clearance, relieve symptoms, minimize recurrence, and prevent transmission. According to the Maastricht VI/Florence Consensus Report [75], eradication regimens should achieve a per-protocol success rate of ≥90%. The choice of therapy depends on several factors, including local antibiotic resistance rates, previous treatment history, patient adherence, and drug availability.
First-line treatment typically involves either bismuth-based quadruple therapy (BQT) or non-bismuth concomitant quadruple therapy. BQT combines a PPI, bismuth sub-citrate (120–240 mg four times daily), tetracycline (500 mg four times daily), and metronidazole (500 mg three or four times daily), which are administered for 10–14 days. This regimen remains effective even in regions with high clarithromycin resistance and is recommended as a first-line option in many settings [75]. Alternatively, concomitant therapy—consisting of a PPI, amoxicillin (1 g twice daily), clarithromycin (500 mg twice daily), and metronidazole (500 mg twice daily)—may be used in regions where dual resistance remains relatively low. Traditional triple therapy (PPI, clarithromycin, and amoxicillin) is no longer advised in areas where clarithromycin resistance exceeds 15% unless susceptibility testing supports its use [3,66].
Second-line therapies are typically employed after failure of initial treatment. These may include BQT (if not previously used) or levofloxacin-based triple therapy, which consists of a PPI, amoxicillin (1 g twice daily), and levofloxacin (500 mg once daily) for 10–14 days. However, increasing global resistance to fluoroquinolones has limited the utility of this approach in many regions [66]. Ideally, second-line treatment should be tailored on the basis of antimicrobial susceptibility testing, using culture or molecular diagnostics where available. For patients who have failed two prior eradication regimens, third-line or rescue therapy is recommended—again, ideally guided by susceptibility testing. In the absence of such data, the Maastricht VI Consensus Report endorses empiric use of rifabutin-based triple therapy or vonoprazan-based dual therapy [75].
Rifabutin-based triple therapy includes a PPI (standard dose twice daily), amoxicillin (1 g twice daily), and rifabutin (150 mg twice daily) for 10–14 days. This regimen may be effective even in heavily pre-treated patients, although caution is warranted because of rare but serious adverse effects such as myelotoxicity. Vonoprazan-based dual therapy has emerged as an effective alternative, particularly in areas with high clarithromycin resistance or where antibiotic stewardship is a priority. Compared with traditional PPIs, vonoprazan, a potent potassium-competitive acid blocker, offers greater stability and prolonged acid suppression. The regimen consists of vonoprazan 20 mg twice daily combined with amoxicillin 750 mg three times daily or 1 g two to three times daily, which is administered for 7–14 days. A randomized controlled trial by Furuta et al. demonstrated eradication rates as high as 94.4% with this combination, along with excellent tolerability and compliance [81].
The Maastricht VI guidelines now support vonoprazan–amoxicillin dual therapy as a valid option for first- or third-line treatment, depending on local resistance patterns and drug availability [75]. In parallel with antibiotic therapy, adjunctive strategies such as the use of probiotics have been investigated to increase treatment success and reduce side effects. Probiotics—particularly strains of Lactobacillus, Bifidobacterium, and Saccharomyces boulardii—have demonstrated benefits in mitigating gastrointestinal side effects (such as bloating and diarrhea) and in modestly improving eradication rates. Meta-analyses support the use of probiotics as a complementary therapy, especially in patients who experience antibiotic-associated intolerance [82,83].
In summary, the management of H. pylori infection should be individualized and evidence-based, taking into account regional resistance patterns, patient factors, and diagnostic access. The Maastricht VI/Florence Consensus provides comprehensive guidance on eradication strategies, emphasizing the importance of prolonged treatment duration (preferably 14 days), tailored antibiotic selection, and the integration of novel therapies such as vonoprazan-based dual regimens and third-line rifabutin-containing options. The adjunctive use of probiotics can further enhance treatment outcomes. Table 2 provides an updated comparative summary of current H. pylori eradication regimens, detailing components, treatment duration, intention-to-treat (ITT) and per-protocol (PP) efficacy from recent meta-analyses, resistance impact, guideline-based recommendations, and tolerability profiles. This overview facilitates evidence-based selection of therapy on the basis of patient history, local resistance data, and Maastricht VI recommendations.

Table 2.
Comparative summary of H. pylori eradication regimens: components, duration, updated ITT and PP efficacy, resistance impact, clinical use, and recent guideline recommendations.
2.8. Barriers to Vaccine Development and Translational Challenges
The development of a vaccine against H. pylori remains one of the most formidable challenges in modern bacterial vaccinology. Despite the pathogen’s well-established role in chronic gastritis, peptic ulcer disease, and noncardia gastric carcinoma, no licensed vaccine has yet reached clinical use. Numerous candidates have progressed to late-stage clinical trials but ultimately failed, underscoring the complex biological, immunological, technical, and translational barriers that must still be overcome.
2.8.1. Immune Evasion and Mucosal Immunity Defects
A central challenge in H. pylori vaccine development lies in its highly evolved immune evasion mechanisms. Although infection stimulates both humoral and cellular responses, these responses are largely ineffective in clearing the bacterium, which can persist for decades within the gastric mucosa. Persistence is driven by H. pylori’s ability to modulate host immunity. Khamri et al. [86] reported that H. pylori-exposed dendritic cells acquire a tolerogenic phenotype, marked by elevated IL-10 and TGF-β, promoting the expansion of regulatory T-cells (Tregs). These Tregs suppress effector responses and permit bacterial survival while limiting mucosal damage [87,88]. In addition, H. pylori structurally evades innate immune recognition. Rad et al. [89] demonstrated that its hypo-acylated lipopolysaccharide (LPS) weakly stimulates TLR4, dampening pro-inflammatory signaling. Furthermore, H. pylori releases OMVs that exert immunomodulatory effects.
Winter et al. [90] reported that OMVs can induce both pro- and anti-inflammatory cytokines and promote the apoptosis of Jurkat T-cells, contributing to immune suppression. Despite the presence of robust IgG and IgA responses during infection, these antibodies do not confer protective immunity, as reinfection post-eradication is common. Although Th1 and Th17 responses appear to mediate protection, inducing them effectively without excessive mucosal inflammation remains a challenge [91]. The absence of validated correlates of protection further impedes rational vaccine design. Overall, H. pylori’s ability to manipulate antigen presentation, modulate immune activation, and exploit OMVs renders it one of the most immune-evasive bacterial pathogens, requiring novel agents.
2.8.2. Antigenic Diversity and Strain Variability
The exceptional genomic plasticity of H. pylori presents another major obstacle to vaccine development. The bacterium displays extensive variability in key virulence factors, including cagA, vacA, babA, sabA, and oipA, all of which are associated with pathogenesis and immune evasion. The vacA gene, while universally present, presents polymorphisms in its signal (s), middle (m), and intermediate (i) regions. The s1/m1 genotype is linked to increased cytotoxicity and inflammation, whereas the s2/m2 strains are less pathogenic [92]. Similarly, the cagA gene encodes an oncoprotein delivered via a type IV secretion system and varies regionally—being present in 90% of strains in East Asia versus 50–70% in Western countries [17]. Polymorphisms in the EPIYA motif of cagA influence both its pathogenicity and immunogenicity. Additionally, adhesins such as babA, sabA, and oipA undergo phase variation, enabling immune evasion [18].
Comparative genomic analyses confirmed the rapid evolution of H. pylori through horizontal gene transfer and recombination. Thorell et al. [93] identified regional subpopulations with distinct virulence repertoires. This high antigenic diversity suggests that a single- or dual-antigen vaccine is unlikely to provide global protection. Although multivalent or region-specific vaccines may enhance efficacy, they introduce significant complexity in formulation, regulation, and deployment. The lack of validated immune correlates further complicates vaccine evaluation in clinical trials.
2.8.3. Technical Limitations of Mucosal Delivery
Effective vaccination against H. pylori requires robust mucosal immunity due to its ability to colonize the stomach. Oral immunization is an attractive strategy because it mimics natural infection and can induce both mucosal and systemic responses. However, the highly acidic stomach environment and proteolytic enzymes degrade protein antigens, limiting vaccine efficacy. The gastric mucosa also lacks inductive structures such as Peyer’s patches, impeding antigen presentation and adaptive priming [94].
To overcome these barriers, various delivery technologies have been explored. Acid-resistant coatings (e.g., HP55) and biodegradable nanoparticles can shield antigens and enhance mucosal uptake, promoting IgA responses and cytokine production in animal models [95]. Solid lipid nanoparticles have delivered DNA vaccines encoding urease subunits with promising reductions in bacterial burden [96]. Liposomes also offer potential, although their stability in acidic gastric conditions remains limited [97].
Despite progress, achieving effective and durable mucosal immunity remains difficult. Inconsistent antigen uptake and poor memory responses hamper success. Sublingual immunization shows potential but has yet to be translated into effective human application [98]. A lack of safe, potent mucosal adjuvants further limits vaccine efficacy [99]. Without such adjuvants, vaccines fail to elicit protective immunity. The absence of standardized preclinical models and validated correlates of protection exacerbates these challenges [100]. Future success will likely depend on mucoadhesive carriers, acid-stable formulations, and optimized adjuvant systems.
2.8.4. Translational Gaps Between Animal Models and Humans
Significant translational gaps remain between promising preclinical results and human clinical efficacy. Several key factors contribute:
Differences in the Immune Response
Murine and gerbil models, although valuable, do not fully capture human immune tolerance to H. pylori. While these models demonstrate immunogenicity and bacterial reduction, critical aspects of human immune regulation—particularly Treg-mediated tolerance—are not accurately modeled [101].
Genetic Diversity of H. pylori Strains
Preclinical models frequently utilize a limited set of H. pylori strains and fail to represent the global diversity in virulence profiles. This restricts the external validity of findings, as strain-specific differences significantly influence vaccine responsiveness [102].
Limitations of Preclinical Models
Anatomical and immunological differences between human and animal gastric mucosa reduce the predictive value of current models. Moreover, chronic infection spanning decades, as occurs in humans, is rarely replicated in animal studies [13,101].
Immune Modulation and Tolerance
The delicate balance between immune tolerance and inflammation in human infection, which is mediated by Tregs and other pathways, is not fully mirrored in standard animal models [100].
Need for Advanced Preclinical Models
Humanized mouse models, nonhuman primates, and gastric organoid systems may provide more accurate simulations of human immune responses and improve the translational potential of candidate vaccines [102].
Clinical Trial Discrepancies
Many vaccine candidates that show promise in animal models fail in human trials, often owing to antigenic mismatch and variability in human immune responses [103,104]. More predictive preclinical models are needed to guide vaccine development [13].
2.8.5. Regulatory, Logistical, and Economic Constraints
Systemic barriers further complicate H. pylori vaccine development. The long latency between infection and disease makes clinical trial design challenging and increases both ethical and financial burdens [105]. Most H. pylori-associated morbidity occurs in LMICs, where cold chain logistics and healthcare infrastructure are limited [106]. Pharmaceutical investment has been hampered by high costs, regulatory uncertainties, and a lack of validated immune correlates [10]. However, cost-effectiveness analyses indicate that an affordable vaccine could yield substantial public health benefits in high-burden regions [106]. Achieving oral stability, mucosal immunogenicity, and broad cross-strain protection remains technically challenging [10,107].
Recent advances in immunoinformatics and pangenomic analyses have offered new avenues for the design of multivalent vaccines, although these options remain at early stages [107]. Bibliometric reviews emphasize that international collaboration, sustainable funding, and public–private partnerships are critical for progress, especially in resource-limited settings [108]. While thermostable formulations have been developed for other enteric vaccines—such as rotavirus (Rotasiil®) and cholera (Shanchol™)—no thermostable H. pylori vaccine candidates are yet available. This represents a key barrier to equitable global deployment, particularly in LMICs where maintaining cold chains is difficult [109,110].
Table 3 summarizes the major biological, technical, and translational barriers to H. pylori vaccine development and their implications for vaccine design, providing a framework for addressing immune evasion, antigenic diversity, mucosal delivery limitations, and implementation challenges.

Table 3.
Key Barriers to H. pylori Vaccine Development and Their Implications for Design.
2.9. Current Progress and Emerging Strategies in H. pylori Vaccine Development
Despite the formidable challenges outlined previously, significant progress has been made in recent years in the quest for an effective H. pylori vaccine. Researchers have explored a wide spectrum of vaccine platforms—including subunit, DNA, live-attenuated, vector-based, and multivalent formulations—each offering distinct immunological advantages and logistical trade-offs. Advances in genomics, bioinformatics, and mucosal immunology have further reinvigorated vaccine development, enabling rational antigen selection, optimized delivery, and enhanced immune response design.
2.9.1. Subunit Vaccines
Subunit vaccines remain among the most extensively studied approaches owing to their favorable safety profiles and antigen specificity. Multiple antigens—including urease subunits (ureA, ureB), heat shock proteins (hspA, hspB), vacA, and cagA—have shown immunogenicity and protective potential in preclinical studies. Del Giudice et al. [94] demonstrated that recombinant urease B subunit immunization elicited significant protective immunity in mice. Sun et al. [111] reported that subcutaneous immunization with the H. pylori urease B subunit increased both systemic (serum IgG) and mucosal (gastric) anti-urease B antibodies, alongside IL-4 and IFN-γ production, indicating the ability to induce both systemic and local responses.
Similarly, Zhang et al. [112] demonstrated that intranasal immunization with hspA and γ-glutamyl transpeptidase (GGT), especially when combined as a fusion protein (rHspA-GGT) with the mucosal adjuvant LTB, reduced gastric bacterial loads by stimulating balanced Th1/Th2 responses. Corthésy-Theulaz et al. [113] reported that oral immunization with recombinant urease B induced protection and clearance of infection in mice. Guo et al. [114] advanced this strategy further by formulating a multivalent vaccine combining the cholera toxin B subunit with multiple H. pylori antigens (ureA, hpaA, HSP60, ureB), which resulted in significantly reduced colonization. While combining multiple antigens has improved protection in animal models by targeting redundant virulence mechanisms, subunit vaccines generally require potent adjuvants to achieve effective mucosal immunity—a key area of ongoing research.
2.9.2. DNA and Vector-Based Vaccines
DNA vaccines encoding H. pylori antigens (primarily urease subunits) have been evaluated in murine models with mixed outcomes. Bégué et al. [115] demonstrated that a DNA vaccine encoding urease B induced a balanced Th1/Th2 response in mice, whereas Zavala-Spinetti et al. [116] reported limited protective efficacy, highlighting the need for further optimization. Hatzifoti et al. [117] showed that mucosal delivery of a urease B DNA vaccine could stimulate innate and cellular responses. Recent innovations have focused on enhancing DNA vaccine efficacy via novel delivery systems. Francis et al. [96] employed solid lipid nanoparticles to deliver a urease alpha subunit DNA vaccine, achieving high levels of antigen-specific antibodies and gastric CD4+ T-cell responses.
Nikzad-Chaleshtori et al. [118] used a urease E subunit-based DNA vaccine, which induced strong IgG, IFN-γ, IL-4, and IL-17 responses in mice and achieved 87.5% protection against H. pylori challenge. Recombinant bacterial and viral vectors (e.g., Salmonella and adenovirus) engineered to express H. pylori antigens have also been explored. Ghasemi et al. [119] developed a protective immunity-enhanced Salmonella vaccine vector expressing multiple antigens (hpaA, Hp-nap, ureA, ureB), which provided sterile protection in 70% of mice. Nie et al. [120] utilized an influenza A virus vector expressing napA and achieved robust mucosal and Th1/Th17 responses, with significant reductions in bacterial colonization.
2.9.3. Live-Attenuated Vaccines
Live-attenuated bacterial vectors, particularly Salmonella and Lactobacillus, have shown promise for inducing mucosal immunity. These platforms mimic natural infection pathways and promote durable systemic and mucosal responses. Early clinical trials demonstrated safety: Angelakopoulos and Hohmann [121] reported that an attenuated Salmonella Typhimurium vector expressing urease was well tolerated in humans, eliciting urease-specific immunity. DiPetrillo et al. [122] and Bumann et al. [123] reported similar findings with Salmonella vectors expressing urease A/B subunits. Recent studies have enhanced vector performance. Ghasemi et al. [119] employed a multiantigen Salmonella vector that significantly reduced colonization and inflammation, whereas Nie et al. [120] demonstrated that an intranasal influenza A virus vector expressing napA induced potent mucosal immunity. These findings underscore the potential of multiantigen live-attenuated strategies, although further evaluation of biosafety, scalability, and regulatory hurdles is needed.
2.9.4. Epitope-Based and Multiepitope Vaccines
Immunoinformatics-driven epitope-based vaccines represent a precision strategy to overcome antigenic diversity. Jebali et al. [124] designed a lipid nanoparticle-based multiepitope vaccine targeting conserved epitopes from urease, cagA, hopE, sabA, and babA, achieving promising immunogenicity. Cui et al. [107] and Wang et al. [125] similarly developed multiepitope vaccines targeting essential proteins, with encouraging preclinical outcomes. Urrutia-Baca et al. [126] and Ghosh et al. [127] used in silico design to develop oral multiepitope candidates with strong immunogenic profiles. While these approaches show promise, clinical validation remains necessary.
2.9.5. Novel Adjuvants and Delivery Technologies
Potent adjuvants and innovative delivery platforms are essential for generating protective mucosal immunity. Jiang et al. [128] demonstrated that codelivery of H. pylori antigens with the cholera toxin B subunit and CpG oligodeoxynucleotides enhanced systemic and mucosal responses in mice. Hatzifoti et al. [117] also reported the synergistic effect of CpG motifs with DNA vaccines. Advanced delivery systems such as SLNs and chitosan nanoparticles have shown promise. Francis et al. [96] achieved enhanced immunity with SLN-based urease A DNA vaccines. Amaral et al. [129] demonstrated that chitosan nanoparticles carrying a multiepitope vaccine induced robust IL-17A and IFN-γ responses in mice. While these technologies offer exciting potential, further work is needed to optimize adjuvant safety and scalability for human use.
2.9.6. Clinical Trial Landscape
Although many H. pylori vaccine candidates have shown preclinical promise, only a few have progressed to clinical trials. The most notable advancement came from a phase 3 trial in China by Zeng et al. [130], which demonstrated 71.8% efficacy in preventing infection within one year and 55% efficacy at three years using an oral recombinant urease B/heat-labile toxin B vaccine in more than 4,400 children. The vaccine was well tolerated, with no serious adverse events. Other trials, such as those by Michetti et al. [131], demonstrated the safety and immunogenicity of oral recombinant urease vaccines in adults but failed to achieve sterilizing immunity. Current limitations include the lack of correlates of protection, antigen variability among strains and challenges in achieving strong mucosal responses via noninvasive routes.
Future progress will depend on optimized antigen selection, improved delivery technologies, and standardized immunological end points. Large-scale, multinational trials are essential for evaluating vaccine generalizability and long-term efficacy. Table 4 presents a comparative overview of current H. pylori vaccine platforms, summarizing antigen composition, delivery routes, adjuvants, and development stages. Subcutaneous administration remains primarily used in preclinical settings with strong adjuvants, whereas the oral and intranasal routes require potent mucosal adjuvants. Live-attenuated vaccines have demonstrated safety and immunogenicity in phase I trials but lack proven human efficacy. Epitope-based approaches remain largely computational or confined to animal studies.

Table 4.
Summary of H. pylori vaccine platforms, status, and delivery strategies.
2.10. Future Perspectives and Recommendations
Despite decades of research, the development of an effective H. pylori vaccine remains elusive. However, the convergence of recent advances in immunology, biotechnology, and computational biology offers promising new avenues for overcoming longstanding barriers. Future efforts should prioritize several strategic directions. First, rational design approaches grounded in pangenomics, and reverse vaccinology must be expanded to identify conserved, immunogenic antigens capable of eliciting broad protection across diverse H. pylori strains. Particular attention should be given to antigens involved in adhesion, colonization, and immune evasion. The incorporation of multiple virulence factors into multiepitope constructs may enhance cross-protective efficacy while minimizing the risk of immune escape [126,132].
Second, effective delivery of antigens to the gastric mucosa remains a formidable challenge. Innovative carriers—including biodegradable nanoparticles, solid lipid nanoparticles, mucoadhesive hydrogels, and enteric-coated formulations—hold promise for enhancing mucosal targeting. These delivery systems should be combined with next-generation mucosal adjuvants, such as CpG oligodeoxynucleotides and cholera toxin B subunit derivatives, to induce robust local and systemic immune responses without compromising mucosal integrity [129,133].
Third, translational progress requires the adoption of sophisticated preclinical models that more accurately reflect human gastric physiology. Humanized mouse models, gastric organoids, and nonhuman primates can yield deeper insights into immunological mechanisms and correlates of protection. Harmonizing immunological endpoints between animal and clinical studies will further improve comparability and streamline regulatory approval processes [134,135].
Future clinical trials must also be rigorously designed to evaluate both short- and long-term efficacy. Greater emphasis should be placed on measuring mucosal immunity, reducing bacterial loads, and increasing the durability of immune memory. Adaptive trial designs and broader geographical representations—particularly in endemic regions—are essential for improving the robustness and global applicability of clinical outcomes [130].
Given the global burden of H. pylori infection and its causal link to gastric cancer, coordinated international efforts are urgently needed. Public‒private partnerships, governmental funding agencies, and global health organizations must increase H. pylori vaccine development as a priority within broader antimicrobial resistance and cancer prevention strategies. A comprehensive roadmap aligning scientific, clinical, and policy efforts will be critical to achieving sustainable progress [136].
Finally, successful integration of an H. pylori vaccine into national immunization programs will require careful planning around cost-effectiveness, target populations (e.g., school-aged children in endemic areas), delivery logistics, and health system readiness. Modeling studies are vital for supporting strategic planning and forecasting the long-term public health impact of various implementation scenarios [107]. In summary, while formidable challenges remain, the rapid evolution of molecular, immunological, and bioengineering technologies offers renewed optimism for realizing an effective H. pylori vaccine. This goal will demand collaborative, interdisciplinary efforts and sustained investment.
3. Conclusions
Although decades of scientific effort have yet to yield a licensed H. pylori vaccine, its development is increasingly within reach. This review highlights how the integration of modern vaccinology, systems biology, and nanotechnology is expanding the field’s possibilities. The growing array of vaccine platforms—ranging from subunit and epitope-based constructs to DNA and live-attenuated vectors—demonstrates a field that is both evolving and accelerating. Advances in mucosal delivery systems, antigen design through immunoinformatics, and next-generation adjuvants are challenging traditional vaccine paradigms. Success hinges on overcoming the translational barriers that separate promising preclinical results from meaningful clinical outcomes. Key steps include standardizing immune correlates, optimizing human-relevant models, and ensuring broad trial representation across endemic regions. An H. pylori vaccine must be viewed not only as a scientific achievement but also as a vital public health intervention with the potential to reduce antibiotic resistance and prevent gastric cancer in vulnerable populations. With the convergence of political will, strategic investment, and scientific innovation, the question of realizing an H. pylori vaccine is now a matter of timing rather than feasibility. The next phase of H. pylori vaccinology requires bold, cross-disciplinary collaboration, and that effort must commence immediately.
Author Contributions
Conceptualization, A.E., E.M. and A.A.; Writing—original draft preparation, A.E., E.M. and A.A.; writing—review and editing, A.E., E.M. and A.A.; visualization, A.E., E.M. and A.A.; supervision, A.E. and E.M.; project administration, A.E., E.M. and A.A.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2025).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Hooi, J.K.; Lai, W.Y.; Ng, W.K.; Suen, M.M.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.; Wu, J.C. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- IARC Working Group on the Identification of Carcinogenic Hazards to Humans. Aspartame, methyleugenol, and isoeugenol. In IARC Monographs on the Identification of Carcinogenic Hazards to Humans; International Agency for Research on Cancer: Lyon, France, 2024; Volume 134, pp. 1–402. [Google Scholar]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef] [PubMed]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.; Crowe, S.; Valasek, M. The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Dore, M.P.; Graham, D.Y. Diagnosis and treatment of Helicobacter pylori infection. Annu. Rev. Med. 2022, 73, 183–195. [Google Scholar] [CrossRef]
- Salahi-Niri, A.; Nabavi-Rad, A.; Monaghan, T.M.; Rokkas, T.; Doulberis, M.; Sadeghi, A.; Zali, M.R.; Yamaoka, Y.; Tacconelli, E.; Yadegar, A. Global prevalence of Helicobacter pylori antibiotic resistance among children in the world health organization regions between 2000 and 2023: A systematic review and meta-analysis. BMC Med. 2024, 22, 598. [Google Scholar] [CrossRef]
- Mégraud, F.; Graham, D.Y.; Howden, C.W.; Trevino, E.; Weissfeld, A.; Hunt, B.; Smith, N.; Leifke, E.; Chey, W.D. Rates of antimicrobial resistance in Helicobacter pylori isolates from clinical trial patients across the US and Europe. Off. J. Am. Coll. Gastroenterol. 2023, 118, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Bakleh, M.Z.; Kohailan, M.; Marwan, M.; Alhaj Sulaiman, A. A Systematic Review and Comprehensive Analysis of mcr Gene Prevalence in Bacterial Isolates in Arab Countries. Antibiotics 2024, 13, 958. [Google Scholar] [CrossRef]
- Backert, S.; Tegtmeyer, N. Type IV secretion and signal transduction of Helicobacter pylori CagA through interactions with host cell receptors. Toxins 2017, 9, 115. [Google Scholar] [CrossRef]
- Sutton, P.; Chionh, Y.T. Why can’t we make an effective vaccine against Helicobacter pylori? Expert Rev. Vaccines 2013, 12, 433–441. [Google Scholar] [CrossRef]
- Tu, Z.; Wang, Y.; Liang, J.; Liu, J. Helicobacter pylori-targeted AI-driven vaccines: A paradigm shift in gastric cancer prevention. Front. Immunol. 2024, 15, 1500921. [Google Scholar] [CrossRef]
- Yunle, K.; Tong, W.; Jiyang, L.; Guojun, W. Advances in Helicobacter pylori vaccine research: From candidate antigens to adjuvants—A review. Helicobacter 2024, 29, e13034. [Google Scholar] [CrossRef] [PubMed]
- Kusters, J.G.; Van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Keates, S.; Ghiran, I.; Kelly, C.P.; Ewoldt, R.H.; McKinley, G.H.; Therefore, P.; Erramilli, S. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc. Natl. Acad. Sci. USA 2009, 106, 14321–14326. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.R.; Marcus, E.A.; Wen, Y.; Oh, J.; Sachs, G. Gene expression in vivo shows that Helicobacter pylori colonizes an acidic niche on the gastric surface. Proc. Natl. Acad. Sci. USA 2007, 104, 7235–7240. [Google Scholar] [CrossRef]
- Matsuo, Y.; Kido, Y.; Yamaoka, Y. Helicobacter pylori outer membrane protein-related pathogenesis. Toxins 2017, 9, 101. [Google Scholar] [CrossRef]
- Hatakeyama, M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc. Jpn. Acad. Ser. B 2017, 93, 196–219. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori infection, its laboratory diagnosis, and antimicrobial resistance: A perspective of clinical relevance. Clin. Microbiol. Rev. 2022, 35, e00258-21. [Google Scholar] [CrossRef]
- Bonis, M.; Ecobichon, C.; Guadagnini, S.; Prévost, M.C.; Boneca, I.G. A M23B family metallopeptidase of Helicobacter pylori required for cell shape, pole formation and virulence. Mol. Microbiol. 2010, 78, 809–819. [Google Scholar] [CrossRef]
- Bik, E.M.; Eckburg, P.B.; Gill, S.R.; Nelson, K.E.; Purdom, E.A.; Francois, F.; Perez-Perez, G.; Blaser, M.J.; Relman, D.A. Molecular analysis of the bacterial microbiota in the human stomach. Proc. Natl. Acad. Sci. USA 2006, 103, 732–737. [Google Scholar] [CrossRef]
- Delgado, S.; Cabrera-Rubio, R.; Mira, A.; Suárez, A.; Mayo, B. Microbiological survey of the human gastric ecosystem using culturing and pyrosequencing methods. Microb. Ecol. 2013, 65, 763–772. [Google Scholar] [CrossRef]
- Vale, F.; Vítor, J. Transmission pathway of Helicobacter pylori: Does food play a role in rural and urban areas? Int. J. Food Microbiol. 2010, 138, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, Y.; Dieye, Y.; Poh, B.H.; Ng, C.G.; Loke, M.F.; Goh, K.L.; Vadivelu, J. Culturable bacterial microbiota of the stomach of Helicobacter pylori positive and negative gastric disease patients. Sci. World J. 2014, 2014, 610421. [Google Scholar] [CrossRef]
- Oleastro, M.; Ménard, A. The role of Helicobacter pylori outer membrane proteins in adherence and pathogenesis. Biology 2013, 2, 1110–1134. [Google Scholar] [CrossRef] [PubMed]
- Moonens, K.; Hamway, Y.; Neddermann, M.; Reschke, M.; Tegtmeyer, N.; Kruse, T.; Kammerer, R.; Mejías-Luque, R.; Singer, B.B.; Backert, S. Helicobacter pylori adhesin HopQ disrupts trans dimerization in human CEACAM s. EMBO J. 2018, 37, e98665. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.L. Urease. In Helicobacter pylori: Physiology and Genetics; ASM Press: Washington, DC, USA, 2001; pp. 177–191. [Google Scholar]
- Olbermann, P.; Josenhans, C.; Moodley, Y.; Uhr, M.; Stamer, C.; Vauterin, M.; Suerbaum, S.; Achtman, M.; Linz, B. A global overview of the genetic and functional diversity in the Helicobacter pylori cag pathogenicity island. PLoS Genet. 2010, 6, e1001069. [Google Scholar] [CrossRef]
- Li, L.; Ren, Y.; Wang, Z.; Niu, Y.; Zhao, Y.; Aihaiti, X.; Ji, Y.; Li, M. Association of Helicobacter pylori Infection with Depression and Anxiety: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2024, 2024, 9247586. [Google Scholar] [CrossRef]
- Krzyżek, P.; Grande, R.; Migdał, P.; Paluch, E.; Gościniak, G. Biofilm formation as a complex result of virulence and adaptive responses of Helicobacter pylori. Pathogens 2020, 9, 1062. [Google Scholar] [CrossRef]
- Xu, C.; Soyfoo, D.M.; Wu, Y.; Xu, S. Virulence of Helicobacter pylori outer membrane proteins: An updated review. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1821–1830. [Google Scholar] [CrossRef]
- Ahmed, A.A.Q.; Besio, R.; Xiao, L.; Forlino, A. Outer membrane vesicles (OMVs) as biomedical tools and their relevance as immune-modulating agents against H. pylori infections: Current status and future prospects. Int. J. Mol. Sci. 2023, 24, 8542. [Google Scholar] [CrossRef]
- Salama, N.R.; Hartung, M.L.; Müller, A. Life in the human stomach: Persistence strategies of the bacterial pathogen Helicobacter pylori. Nat. Rev. Microbiol. 2013, 11, 385–399. [Google Scholar] [CrossRef]
- Liu, M.; Hu, Z.; Wang, C.; Zhang, Y. The TLR/MyD88 signaling cascade in inflammation and gastric cancer: The immune regulatory network of Helicobacter pylori. J. Mol. Med. 2023, 101, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Korona-Głowniak, I.; Forma, A.; Maani, A.; Sitarz, E.; Rahnama-Hezavah, M.; Radzikowska, E.; Portincasa, P. Mechanisms of the epithelial–mesenchymal transition and tumor microenvironment in Helicobacter pylori-induced gastric cancer. Cells 2020, 9, 1055. [Google Scholar] [CrossRef]
- Foegeding, N.J.; Caston, R.R.; McClain, M.S.; Ohi, M.D.; Cover, T.L. An overview of Helicobacter pylori VacA toxin biology. Toxins 2016, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Tay, A.C.Y.; Marshall, B.J.; Jain, U. Helicobacter pylori VacA, a distinct toxin exerts diverse functionalities in numerous cells: An overview. Helicobacter 2019, 24, e12544. [Google Scholar] [CrossRef]
- Amieva, M.; Peek Jr, R.M. Pathobiology of Helicobacter pylori–induced gastric cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef]
- Franco, A.T.; Johnston, E.; Krishna, U.; Yamaoka, Y.; Israel, D.A.; Nagy, T.A.; Wroblewski, L.E.; Piazuelo, M.B.; Correa, P.; Peek Jr, R.M. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008, 68, 379–387. [Google Scholar] [CrossRef]
- Backert, S.; Blaser, M.J. The role of CagA in the gastric biology of Helicobacter pylori. Cancer Res. 2016, 76, 4028–4031. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Peek Jr, R.M.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef]
- de Sablet, T.; Piazuelo, M.B.; Shaffer, C.L.; Schneider, B.G.; Asim, M.; Chaturvedi, R.; Bravo, L.E.; Sicinschi, L.A.; Delgado, A.G.; Mera, R.M. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut 2011, 60, 1189–1195. [Google Scholar] [CrossRef]
- Noto, J.M.; Peek Jr, R.M. The gastric microbiome, its interaction with Helicobacter pylori, and its potential role in the progression to stomach cancer. PLoS Pathog. 2017, 13, e1006573. [Google Scholar] [CrossRef]
- Liu, A.-R.; Yan, Z.-W.; Jiang, L.-Y.; Lv, Z.; Li, Y.-K.; Wang, B.-G. The role of noncoding RNA in the diagnosis and treatment of Helicobacter pylori-related gastric cancer, with a focus on inflammation and immune response. Front. Med. 2022, 9, 1009021. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, K.; Shi, M.; Gong, H.; Han, M.; Tian, W.; Wang, X.; Zhang, D. MicroRNAs in Helicobacter pylori-infected gastric cancer: Function and clinical application. Pharmacol. Res. 2024, 205, 107216. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Zhao, J. The role and mechanisms of Helicobacter pylori outer membrane vesicles in the pathogenesis of extragastrointestinal diseases. Microb. Pathog. 2025, 200, 107312. [Google Scholar] [CrossRef]
- Fiorani, M.; Tohumcu, E.; Del Vecchio, L.E.; Porcari, S.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The influence of Helicobacter pylori on human gastric and gut microbiota. Antibiotics 2023, 12, 765. [Google Scholar] [CrossRef]
- Nabavi-Rad, A.; Sadeghi, A.; Asadzadeh Aghdaei, H.; Yadegar, A.; Smith, S.M.; Zali, M.R. The double-edged sword of probiotic supplementation on gut microbiota structure in Helicobacter pylori management. Gut Microbes 2022, 14, 2108655. [Google Scholar] [CrossRef]
- Xie, S.; Wang, S.; Xue, L.; Middleton, D.R.; Guan, C.; Hao, C.; Wang, J.; Li, B.; Chen, R.; Li, X. Helicobacter pylori is associated with precancerous and cancerous lesions of the gastric cardia mucosa: Results of a large population-based study in China. Front. Oncol. 2020, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-C.; Yang, Y.-J.; Yang, H.-B.; Tsai, Y.-C.; Chang, W.-L.; Wu, C.-T.; Kuo, H.-Y.; Yu, Y.-T.; Yang, E.-H.; Cheng, W.-C. Evolution of the Correa’s cascade steps: A long-term endoscopic surveillance among nonulcer dyspepsia and gastric ulcer after H. pylori eradication. J. Formos. Med. Assoc. 2023, 122, 400–410. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chiang, T.-H.; Chou, C.-K.; Tu, Y.-K.; Liao, W.-C.; Wu, M.-S.; Graham, D.Y. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef]
- Ali, A.; AlHussaini, K.I. Helicobacter pylori: A contemporary perspective on pathogenesis, diagnosis and treatment strategies. Microorganisms 2024, 12, 222. [Google Scholar] [CrossRef]
- Lemos, F.F.B.; de Castro, C.T.; Calmon, M.S.; Luz, M.S.; Pinheiro, S.L.R.; Dos Santos, C.F.S.M.; Santos, G.L.C.; Marques, H.S.; Delgado, H.A.; Teixeira, K.N. Effectiveness of Helicobacter pylori eradication in the treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma: An up-to-date meta-analysis. World J. Gastroenterol. 2023, 29, 2202. [Google Scholar] [CrossRef] [PubMed]
- Matysiak-Budnik, T.; Priadko, K.; Bossard, C.; Chapelle, N.; Ruskoné-Fourmestraux, A. Clinical management of patients with gastric MALT lymphoma: A gastroenterologist’s point of view. Cancers 2023, 15, 3811. [Google Scholar] [CrossRef]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis. Gut 2020, 69, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Gold, B.D.; Kato, A. Helicobacter pylori-associated iron deficiency anemia in childhood and adolescence-pathogenesis and clinical management strategy. J. Clin. Med. 2022, 11, 7351. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C.; Camaschella, C. How I treat unexplained refractory iron deficiency anemia. Blood J. Am. Soc. Hematol. 2014, 123, 326–333. [Google Scholar] [CrossRef]
- Săsăran, M.O.; Mărginean, C.O.; Koller, A.M. Impact of Helicobacter pylori Infection upon the Evolution and Outcome of Pediatric Immune Thrombocytopenic Purpura: A Comprehensive Review. Diagnostics 2023, 13, 3205. [Google Scholar] [CrossRef]
- Sulashvili, N.; Patsia, L.; El-Hakeem, A.; Ayaan, S.; Hizomi, A.; Agarwal, S.; Sulashvili, M. Exploring the Gut-Brain Axis: The Role of the Microbiome in Modulating Brain Function and Its Implications in Neurodegenerative Disorders Like Parkinson’s and Alzheimer’s and Pharmacotherapy Treatment Strategies. Georgian Sci. 2025, 7, 329–353. [Google Scholar] [CrossRef]
- Azami, M.; Baradaran, H.R.; Dehghanbanadaki, H.; Kohnepoushi, P.; Saed, L.; Moradkhani, A.; Moradpour, F.; Moradi, Y. Association of Helicobacter pylori infection with the risk of metabolic syndrome and insulin resistance: An updated systematic review and meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 145. [Google Scholar] [CrossRef]
- Xie, Q.; He, Y.; Zhou, D.; Jiang, Y.; Deng, Y.; Li, R. Recent research progress on the correlation between metabolic syndrome and Helicobacter pylori infection. PeerJ 2023, 11, e15755. [Google Scholar] [CrossRef]
- Magahis, P.T.; Maron, S.B.; Cowzer, D.; King, S.; Schattner, M.; Janjigian, Y.; Faleck, D.; Laszkowska, M. Impact of Helicobacter pylori infection status on outcomes among patients with advanced gastric cancer treated with immune checkpoint inhibitors. J. Immunother. Cancer 2023, 11, e007699. [Google Scholar] [CrossRef]
- Hassan, M.N.; Arif, A.; Shahzad, M.S.; Ibrahim, M.; Rahman, H.A.; Razaq, M.A.; Ahmed, R. 88. Global prevalence of Helicobacter pylori and its effect on human health. Pure Appl. Biol. 2020, 9, 936–948. [Google Scholar] [CrossRef]
- Magalhães Queiroz, D.M.; Luzza, F. Epidemiology of Helicobacter pylori infection. Helicobacter 2006, 11, 1–5. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori infection and antibiotic resistance—From biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef]
- Rocha, G.R.; Lemos, F.F.B.; de Oliveira Silva, L.G.; Luz, M.S.; Santos, G.L.C.; Pinheiro, S.L.R.; Calmon, M.S.; de Melo, F.F. Overcoming antibiotic-resistant Helicobacter pylori infection: Current challenges and emerging approaches. World J. Gastroenterol. 2025, 31, 102289. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Abalkhail, A.; Anagrayyah, S.; Anajirih, N.; Almuzaini, A.M.; Rawway, M.; Alfadhel, A.; Draz, A. Helicobacter pylori infection: Current status and future prospects on diagnostic, therapeutic and control challenges. Antibiotics 2023, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Abalkhail, A.; Anajirih, N.; Alkhamisi, F.; Aldamegh, M.; Alramzi, A.; AlShaqi, R.; Alotaibi, N.; Aljuaid, A.; Alzahrani, H. Helicobacter pylori: Routes of Infection, Antimicrobial Resistance, and Alternative Therapies as a Means to Develop Infection Control. Diseases 2024, 12, 311. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Abalkhail, A.; Sindi, W.; Alzahrani, Y.; Alhifani, S.; Alshehri, T.; Anajirih, N.A.; ALMutairi, T.; Alsaedi, A. Pivotal role of Helicobacter pylori virulence genes in pathogenicity and vaccine development. Front. Med. 2025, 11, 1523991. [Google Scholar] [CrossRef]
- Umar, Z.; Tang, J.-W.; Marshall, B.J.; Tay, A.C.Y.; Wang, L. Rapid diagnosis and precision treatment of Helicobacter pylori infection in clinical settings. Crit. Rev. Microbiol. 2025, 51, 369–398. [Google Scholar] [CrossRef]
- Sousa, C.; Ferreira, R.; Santos, S.B.; Azevedo, N.F.; Melo, L.D. Advances on diagnosis of Helicobacter pylori infections. Crit. Rev. Microbiol. 2023, 49, 671–692. [Google Scholar] [CrossRef]
- Said, Z.N.A.; El-Nasser, A.M. Evaluation of urea breath test as a diagnostic tool for Helicobacter pylori infection in adult dyspeptic patients. World J. Gastroenterol. 2024, 30, 2302. [Google Scholar] [CrossRef] [PubMed]
- Cardos, A.I.; Maghiar, A.; Zaha, D.C.; Pop, O.; Fritea, L.; Miere, F.; Cavalu, S. Evolution of diagnostic methods for Helicobacter pylori infections: From traditional tests to high technology, advanced sensitivity and discrimination tools. Diagnostics 2022, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Bang, C.S.; Lee, J.J.; Baik, G.H. Artificial intelligence for the prediction of Helicobacter pylori infection in endoscopic images: Systematic review and meta-analysis of diagnostic test accuracy. J. Med. Internet Res. 2020, 22, e21983. [Google Scholar] [CrossRef]
- Pohl, D.; Keller, P.M.; Bordier, V.; Wagner, K. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J. Gastroenterol. 2019, 25, 4629. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, R.; Ebrahimi, A.; Sahebkar, A. Helicobacter pylori infection: Conventional and molecular strategies for bacterial diagnosis and antibiotic resistance testing. Curr. Pharm. Biotechnol. 2023, 24, 647–664. [Google Scholar] [CrossRef]
- Best, L.M.; Takwoingi, Y.; Siddique, S.; Selladurai, A.; Gandhi, A.; Low, B.; Yaghoobi, M.; Gurusamy, K.S. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst. Rev. 2018, CD012080. [Google Scholar] [CrossRef]
- Gisbert, J.; De la Morena, F.; Abraira, V. Accuracy of Monoclonal Stool Antigen Test for the Diagnosis of Helicobacter pylori Infection: A Systematic Review and Meta-analysisAbstract no.: 10·02. Helicobacter 2006, 11, 380. [Google Scholar]
- Zhang, M.-M.; Qian, W.; Qin, Y.-Y.; He, J.; Zhou, Y.-H. Probiotics in Helicobacter pylori eradication therapy: A systematic review and meta-analysis. World J. Gastroenterol. 2015, 21, 4345. [Google Scholar] [CrossRef]
- Ford, A.C.; Delaney, B.; Forman, D.; Moayyedi, P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst. Rev. 2006, 19, CD003840. [Google Scholar]
- Furuta, T.; Yamade, M.; Kagami, T.; Uotani, T.; Suzuki, T.; Higuchi, T.; Tani, S.; Hamaya, Y.; Iwaizumi, M.; Miyajima, H. Dual therapy with vonoprazan and amoxicillin is as effective as triple therapy with vonoprazan, amoxicillin and clarithromycin for eradication of Helicobacter pylori. Digestion 2020, 101, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-Z.; Threapleton, D.E.; Wang, J.-Y.; Xu, J.-M.; Yuan, J.-Q.; Zhang, C.; Li, P.; Ye, Q.-L.; Guo, B.; Mao, C. Comparative effectiveness and tolerance of treatments for Helicobacter pylori: Systematic review and network meta-analysis. BMJ 2015, 351, h4052. [Google Scholar] [CrossRef]
- Gatta, L.; Nyssen, O.P.; Fiorini, G.; Saracino, I.M.; Pavoni, M.; Romano, M.; Gravina, A.G.; Granata, L.; Pellicano, R.; Gasbarrini, A. Effectiveness of first and second-line empirical treatment in Italy: Results of the European registry on Helicobacter pylori management. United Eur. Gastroenterol. J. 2023, 11, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Khamri, W.; Walker, M.M.; Clark, P.; Atherton, J.C.; Thursz, M.R.; Bamford, K.B.; Lechler, R.I.; Lombardi, G. Helicobacter pylori stimulates dendritic cells to induce interleukin-17 expression from CD4+ T lymphocytes. Infect. Immun. 2010, 78, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Smythies, L.E.; Waites, K.B.; Lindsey, J.R.; Harris, P.R.; Ghiara, P.; Smith, P.D. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-γ, gene-deficient mice. J. Immunol. 2000, 165, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, A.; Suri-Payer, E.; Enarsson, K.; Svennerholm, A.-M.; Lundin, B.S. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 2003, 71, 1755–1762. [Google Scholar] [CrossRef]
- Rad, R.; Schmid, R.M.; Prinz, C. Helicobacter pylori, iron deficiency, and gastric autoimmunity. Blood 2006, 107, 4969–4970. [Google Scholar] [CrossRef]
- Winter, J.; Letley, D.; Rhead, J.; Atherton, J.; Robinson, K. Helicobacter pylori membrane vesicles stimulate innate pro-and anti-inflammatory responses and induce apoptosis in Jurkat T cells. Infect. Immun. 2014, 82, 1372–1381. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, J.; Xu, H. Strategies of Helicobacter pylori in evading host innate and adaptive immunity: Insights and prospects for therapeutic targeting. Front. Cell. Infect. Microbiol. 2024, 14, 1342913. [Google Scholar] [CrossRef]
- Yamaoka, Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 629–641. [Google Scholar] [CrossRef]
- Thorell, K.; Yahara, K.; Berthenet, E.; Lawson, D.J.; Mikhail, J.; Kato, I.; Mendez, A.; Rizzato, C.; Bravo, M.M.; Suzuki, R. Rapid evolution of distinct Helicobacter pylori subpopulations in the Americas. PLoS Genet. 2017, 13, e1006546. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Covacci, A.; Telford, J.L.; Montecucco, C.; Rappuoli, R. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 2001, 19, 523–563. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, W.; Liu, H.; Li, C.; Zhang, Y.; Meng, X.; Tang, T.; Xi, T.; Xing, Y. Oral Helicobacter pylori vaccine-encapsulated acid-resistant HP55/PLGA nanoparticles promote immune protection. Eur. J. Pharm. Biopharm. 2017, 111, 33–43. [Google Scholar] [CrossRef]
- Francis, J.E.; Skakic, I.; Majumdar, D.; Taki, A.C.; Shukla, R.; Walduck, A.; Smooker, P.M. Solid lipid nanoparticles delivering a DNA vaccine encoding Helicobacter pylori Urease A Subunit: Immune analyses before and after a mouse model of infection. Int. J. Mol. Sci. 2024, 25, 1076. [Google Scholar] [CrossRef]
- Corthésy, B.; Bioley, G. Lipid-based particles: Versatile delivery systems for mucosal vaccination against infection. Front. Immunol. 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Ostberg, A.K.; Flach, C.-F.; Ekman, A.; Blomquist, M.; Czerkinsky, C.; Holmgren, J. Sublingual immunization protects against Helicobacter pylori infection and induces T and B-cell responses in the stomach. Infect. Immun. 2010, 78, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Anggraeni, R.; Ana, I.D.; Wihadmadyatami, H. Development of mucosal vaccine delivery: An overview on the mucosal vaccines and their adjuvants. Clin. Exp. Vaccine Res. 2022, 11, 235. [Google Scholar] [CrossRef]
- Gong, J.; Wang, Q.; Chen, X.; Lu, J. Helicobacter pylori Vaccine: Mechanism of Pathogenesis, Immune Evasion and Analysis of Vaccine Types. Vaccines 2025, 13, 526. [Google Scholar] [CrossRef]
- Czinn, S.J.; Blanchard, T. Vaccinating against Helicobacter pylori infection. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 133–140. [Google Scholar] [CrossRef]
- Graham, D.; Opekun, A.; Osato, M.; El-Zimaity, H.; Lee, C.; Yamaoka, Y.; Qureshi, W.; Cadoz, M.; Monath, T. Challenge model for Helicobacter pylori infection in human volunteers. Gut 2004, 53, 1235–1243. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Huang, X.; Liu, Q. Animal Models of Helicobacter pylori Infection and Vaccines: Current Status and Future Prospects. Helicobacter 2024, 29, e13119. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, F.H.; Menbari, S.; Mohammadzadeh, R.; Pishdadian, A.; Farsiani, H. Developing a potent vaccine against Helicobacter pylori: Critical considerations and challenges. Expert Rev. Mol. Med. 2024, 27, 1–61. [Google Scholar]
- Malfertheiner, P.; Mégraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y. Management of Helicobacter pylori infection—The Maastricht V/Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- de Vries, R.; Klok, R.M.; Brouwers, J.R.; Postma, M.J. Cost-effectiveness of a potential future Helicobacter pylori vaccine in the Netherlands: The impact of varying the discount rate for health. Vaccine 2009, 27, 846–852. [Google Scholar] [CrossRef]
- Cui, M.; Ji, X.; Guan, F.; Su, G.; Du, L. Design of a Helicobacter pylori multiepitope vaccine based on immunoinformatics. Front. Immunol. 2024, 15, 1432968. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Liu, X.; Du, Q.; Li, Y. Bibliometric analysis of Helicobacter pylori vaccine development from 1993 to 2023. Front. Microbiol. 2025, 16, 1479195. [Google Scholar] [CrossRef] [PubMed]
- Isanaka, S.; Guindo, O.; Langendorf, C.; Matar Seck, A.; Plikaytis, B.D.; Sayinzoga-Makombe, N.; McNeal, M.M.; Meyer, N.; Adehossi, E.; Djibo, A. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. N. Engl. J. Med. 2017, 376, 1121–1130. [Google Scholar] [CrossRef]
- Chen, W.H.; Cohen, M.B.; Kirkpatrick, B.D.; Brady, R.C.; Galloway, D.; Gurwith, M.; Hall, R.H.; Kessler, R.A.; Lock, M.; Haney, D. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with Vibrio cholerae O1 El Tor. Clin. Infect. Dis. 2016, 62, 1329–1335. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.-Q.; Zhang, Y.-T.; Zhao, S.-G. Evaluating the immune responses of mice to subcutaneous immunization with Helicobacter pylori urease B subunit. J. Anim. Sci. Biotechnol. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Yang, F.; Wu, W.; Sun, H.; Xie, Q.; Si, W.; Zou, Q.; Yang, Z. Immunization with heat shock protein A and γ-glutamyl transpeptidase induces reduction on the Helicobacter pylori colonization in mice. PLoS ONE 2015, 10, e0130391. [Google Scholar] [CrossRef]
- Corthésy-Theulaz, I.; Porta, N.; Glauser, M.; Saraga, E.; Vaney, A.-C.; Haas, R.; Kraehenbuhl, J.-P.; Blum, A.; Michetti, P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology 1995, 109, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, H.; Tang, F.; Yin, R.; Liu, H.; Gong, X.; Wei, J.; Zhang, Y.; Xu, G.; Liu, K. Oral immunization with a multivalent epitope-based vaccine, based on NAP, urease, HSP60, and HpaA, provides therapeutic effect on H. pylori infection in Mongolian gerbils. Front. Cell. Infect. Microbiol. 2017, 7, 349. [Google Scholar] [CrossRef]
- Bégué, R.E.; Cruz, A.R.; Ramgoolam, A.; Breslin, M.B. Immunogenicity of Helicobacter pylori urease B protein and DNA vaccines in a mouse model. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Spinetti, L.; Breslin, M.B.; Correa, H.; Bégué, R.E. Development and evaluation of a DNA vaccine based on Helicobacter pylori urease B: Failure to prevent experimental infection in the mouse model. Helicobacter 2006, 11, 517–522. [Google Scholar] [CrossRef]
- Hatzifoti, C.; Roussel, Y.; Harris, A.G.; Wren, B.W.; Morrow, J.W.; Bajaj-Elliott, M. Mucosal immunization with a urease B DNA vaccine induces innate and cellular immune responses against Helicobacter pylori. Helicobacter 2006, 11, 113–122. [Google Scholar] [CrossRef]
- Nikzad-Chaleshtori, M.; Asgari, M.; Rezaeizadeh, G.; Aali, F.; Doosti, A. The urease E subunit vaccine stimulate the immune response versus Helicobacter pylori in animal model. Immunol. Res. 2025, 73, 74. [Google Scholar] [CrossRef]
- Ghasemi, A.; Wang, S.; Sahay, B.; Abbott, J.R.; Curtiss III, R. Protective immunity enhanced Salmonella vaccine vectors delivering Helicobacter pylori antigens reduce H. pylori stomach colonization in mice. Front. Immunol. 2022, 13, 1034683. [Google Scholar] [CrossRef]
- Nie, L.; Huang, Y.; Cheng, Z.; Luo, H.; Zhan, Y.; Dou, K.; Ma, C.; Yu, C.; Luo, C.; Liu, Z. An intranasal influenza virus vector vaccine protects against Helicobacter pylori in mice. J. Virol. 2024, 98, e01923-23. [Google Scholar] [CrossRef] [PubMed]
- Angelakopoulos, H.; Hohmann, E.L. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect. Immun. 2000, 68, 2135–2141. [Google Scholar] [CrossRef]
- DiPetrillo, M.D.; Tibbetts, T.; Kleanthous, H.; Killeen, K.P.; Hohmann, E.L. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine 1999, 18, 449–459. [Google Scholar] [CrossRef]
- Bumann, D.; Metzger, W.G.; Mansouri, E.; Palme, O.; Wendland, M.; Hurwitz, R.; Haas, G.; Aebischer, T.; Von Specht, B.-U.; Meyer, T.F. Safety and immunogenicity of live recombinant Salmonella enterica serovar Typhi Ty21a expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine 2001, 20, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Jebali, A.; Esmaeilzadeh, A.; Esmaeilzadeh, M.K.; Shabani, S. Immunoinformatics design and synthesis of a multiepitope vaccine against Helicobacter pylori based on lipid nanoparticles. Sci. Rep. 2024, 14, 17910. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pan, X.; Wang, H.; Zhou, Y.; Zhu, J.; Yang, J.; Li, W. Immunological response of recombinant H. pylori multiepitope vaccine with different vaccination strategies. Int. J. Clin. Exp. Pathol. 2014, 7, 6559. [Google Scholar]
- Urrutia-Baca, V.H.; Gomez-Flores, R.; De La Garza-Ramos, M.A.; Tamez-Guerra, P.; Lucio-Sauceda, D.G.; Rodríguez-Padilla, M.C. Immunoinformatics approach to design a novel epitope-based oral vaccine against Helicobacter pylori. J. Comput. Biol. 2019, 26, 1177–1190. [Google Scholar] [CrossRef]
- Ghosh, P.; Bhakta, S.; Bhattacharya, M.; Sharma, A.R.; Sharma, G.; Lee, S.-S.; Chakraborty, C. A novel multiepitopic peptide vaccine candidate against Helicobacter pylori: In-silico identification, design, cloning and validation through molecular dynamics. Int. J. Pept. Res. Ther. 2021, 27, 1149–1166. [Google Scholar] [CrossRef]
- Jiang, W.; Baker, H.J.; Smith, B.F. Mucosal immunization with helicobacter, CpG DNA, and cholera toxin is protective. Infect. Immun. 2003, 71, 40–46. [Google Scholar] [CrossRef]
- Amaral, R.; Concha, T.; Vítor, J.; Almeida, A.J.; Calado, C.; Gonçalves, L.M. Chitosan Nanoparticles for Enhanced Immune Response and Delivery of Multi-Epitope Helicobacter pylori Vaccines in a BALB/c Mouse Model. Pharmaceutics 2025, 17, 132. [Google Scholar] [CrossRef]
- Zeng, M.; Mao, X.-H.; Li, J.-X.; Tong, W.-D.; Wang, B.; Zhang, Y.-J.; Guo, G.; Zhao, Z.-J.; Li, L.; Wu, D.-L. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Michetti, P.; Kreiss, C.; Kotloff, K.L.; Porta, N.; Blanco, J.L.; Bachmann, D.; Herranz, M.; Saldinger, P.F.; Corthésy–Theulaz, I.; Losonsky, G. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori–infected adults. Gastroenterology 1999, 116, 804–812. [Google Scholar] [CrossRef]
- Ru, Z.; Yu, M.; Zhu, Y.; Chen, Z.; Zhang, F.; Zhang, Z.; Ding, J. Immmunoinformatics-based design of a multi-epitope vaccine with CTLA-4 extracellular domain to combat Helicobacter pylori. FASEB J. 2022, 36, e22252. [Google Scholar] [CrossRef]
- Huang, T.-T.; Cao, Y.-X.; Cao, L. Novel therapeutic regimens against Helicobacter pylori: An updated systematic review. Front. Microbiol. 2024, 15, 1418129. [Google Scholar] [CrossRef] [PubMed]
- Bergin, I.L.; Sheppard, B.J.; Fox, J.G. Helicobacter pylori infection and high dietary salt independently induce atrophic gastritis and intestinal metaplasia in commercially available outbred Mongolian gerbils. Dig. Dis. Sci. 2003, 48, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Idowu, S.; Bertrand, P.P.; Walduck, A.K. Gastric organoids: Advancing the study of H. pylori pathogenesis and inflammation. Helicobacter 2022, 27, e12891. [Google Scholar] [CrossRef] [PubMed]
- Kickbusch, I.; Szabo, M.M.C. A new governance space for health. Glob. Health Action 2014, 7, 23507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).