An Evaluation of the Safety, Immunogenicity, and Protective Efficacy of a Combined Diphtheria–Tetanus–Acellular Pertussis, Haemophilus influenzae Type b, and ACYW135 Meningococcal Conjugate Vaccine in Murine and Rat Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Vaccine Preparation
2.1.2. Animal Models
2.1.3. Bacteria Strains
2.2. Methods
2.2.1. Safety Analysis
Acute Toxicity Test
Repeated-Dose Toxicity Studies
2.2.2. Immunogenicity Analysis
Immunization Procedure
Detection of Serum-Specific IgG Antibodies by ELISA
2.2.3. Protective Efficacy Analysis
Potency Test of DTaP
Potency Test of Pertussis
Potency Test of Tetanus Toxoid
Potency Test of Diphtheria Toxoid
Passive Infant Rat Protection Studies of Hib
Serum Bactericidal Assay (SBA) of MCV4
2.2.4. Statistical Analysis
3. Results
3.1. Safety Results
3.1.1. Acute Toxicity Test
3.1.2. Repeated-Dose Toxicity Studies
3.2. Immunogenicity Results
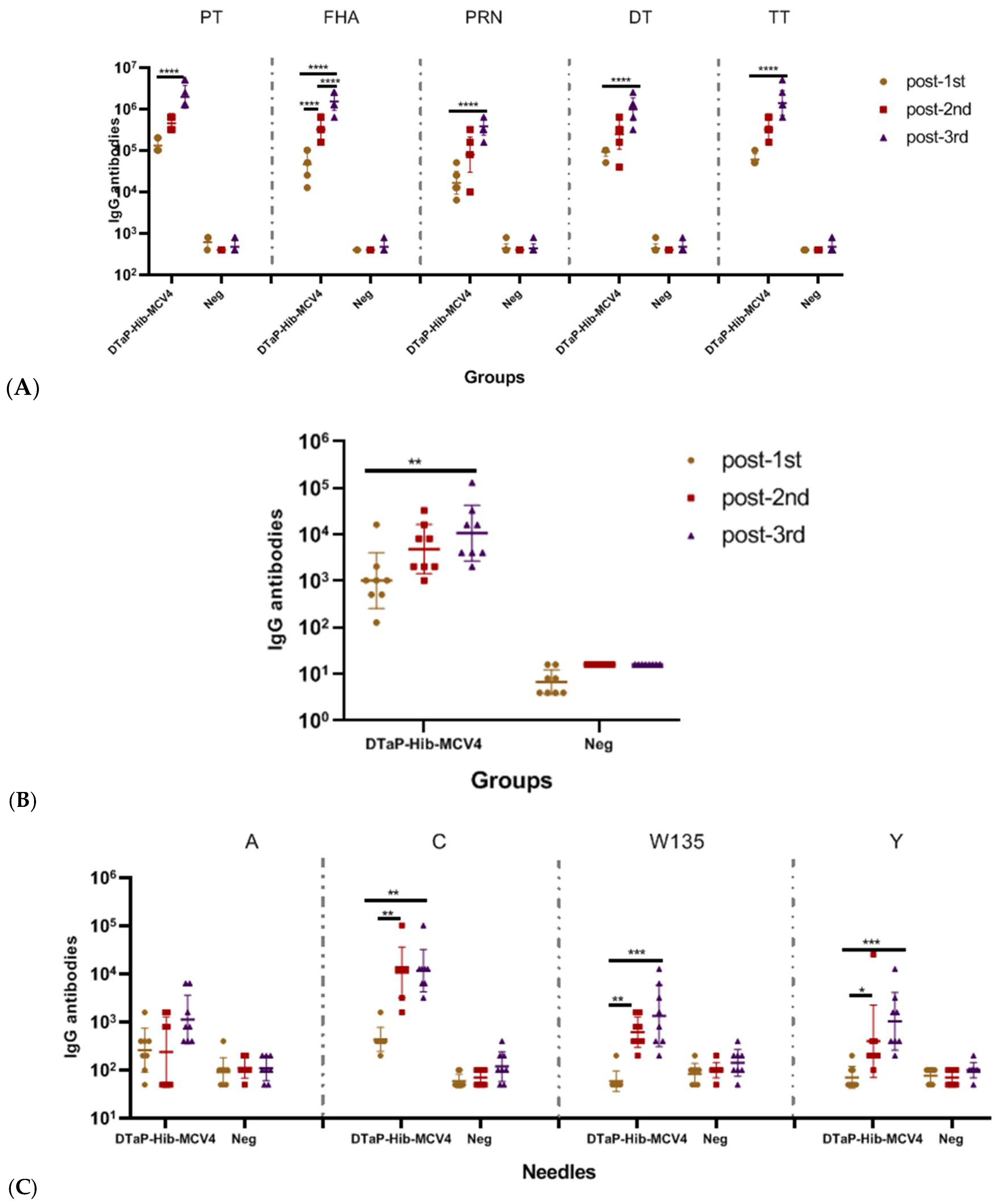
3.2.1. Immunogenicity of DTaP-Hib-MCV4 Vaccine in a Mouse Model
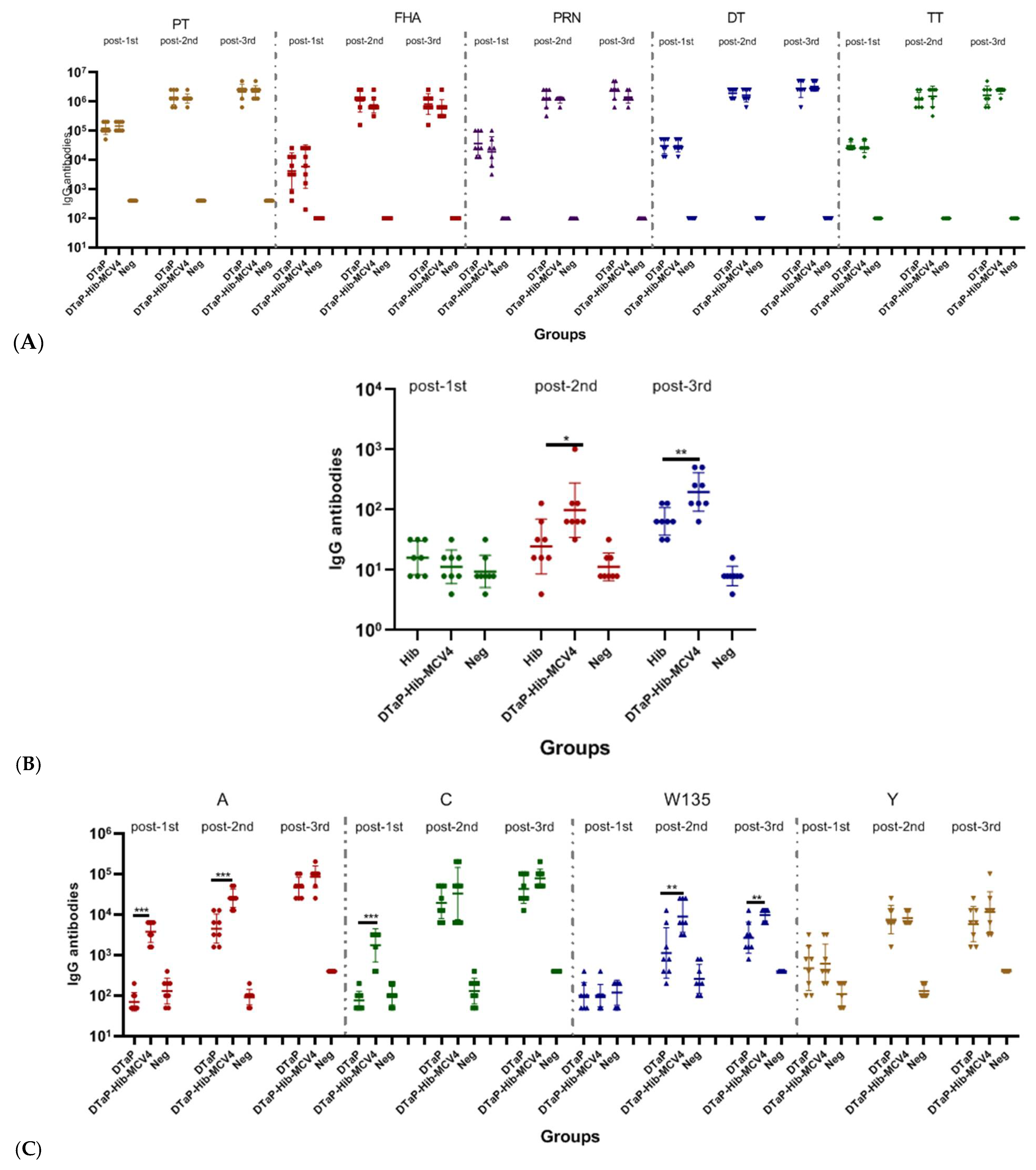
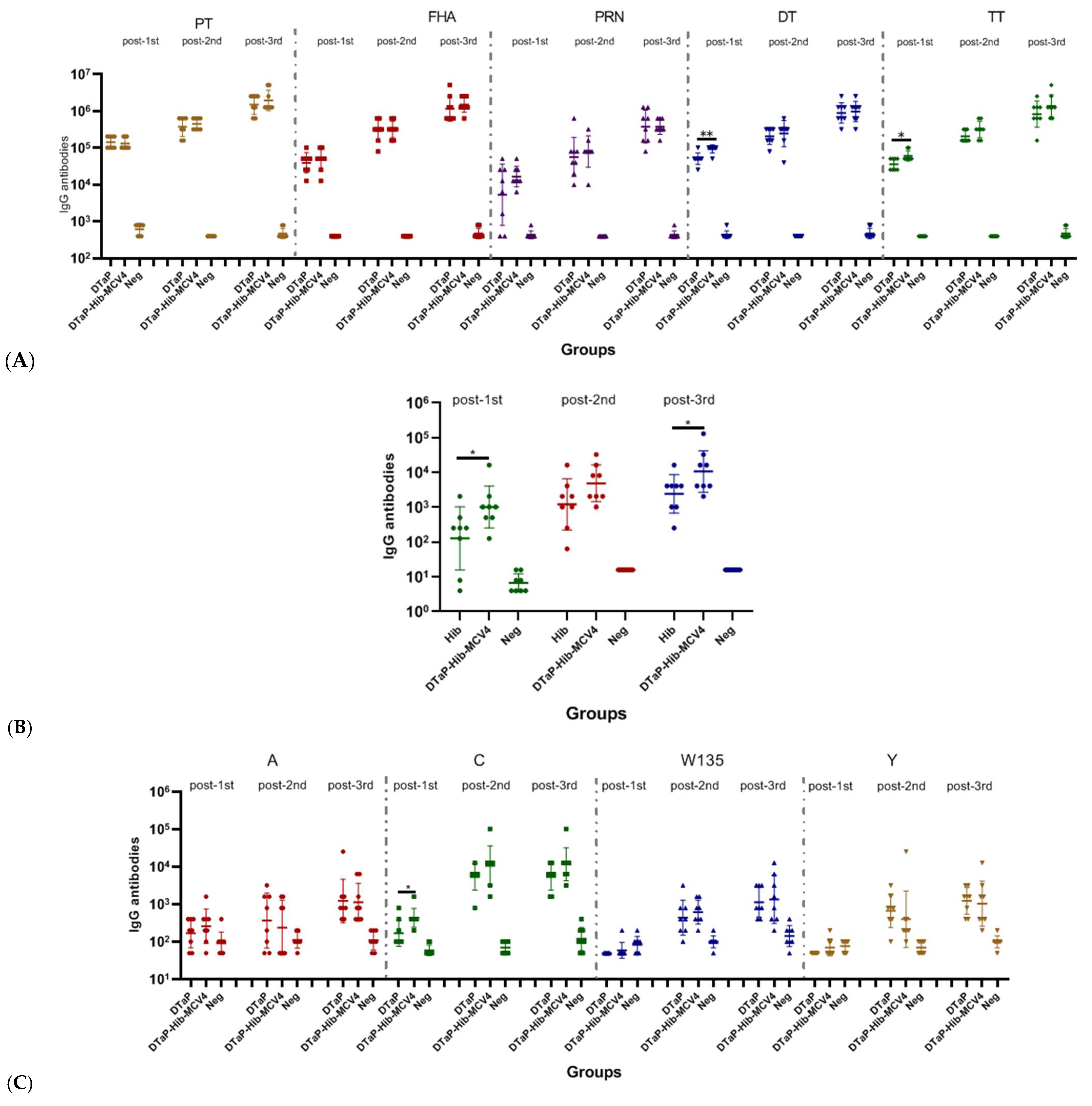
3.2.2. The Immunogenicity of the DTaP-Hib-MCV4 Vaccine in a Rat Model
3.2.3. Immunogenicity of DTaP-Hib-MCV4 Combination Vaccine vs. Each Individual Vaccine in Murine Models
3.3. Protective Efficacy Results
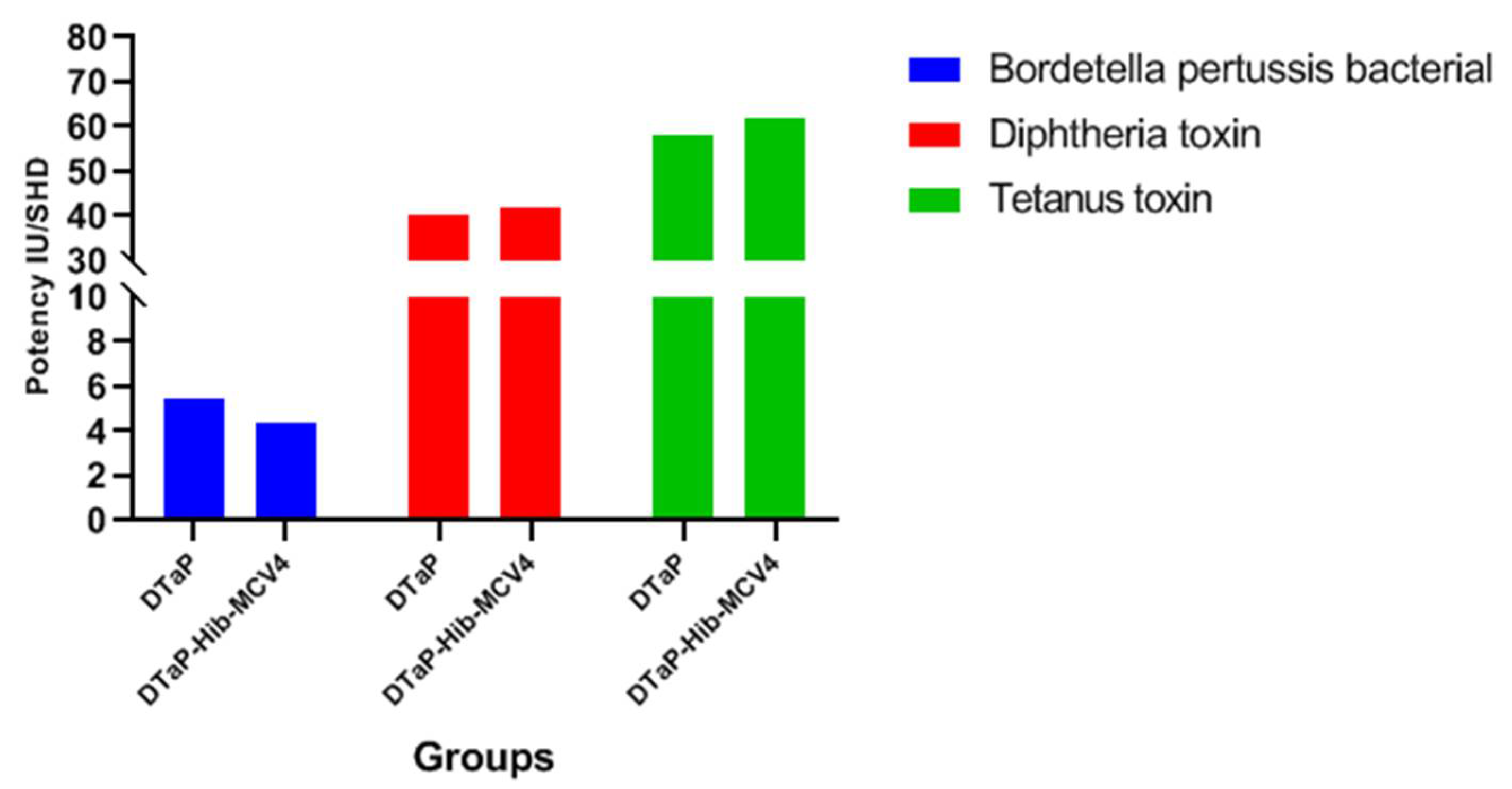
3.3.1. Potency Test of DTaP
3.3.2. Passive Infant Rat Protection Studies of Hib
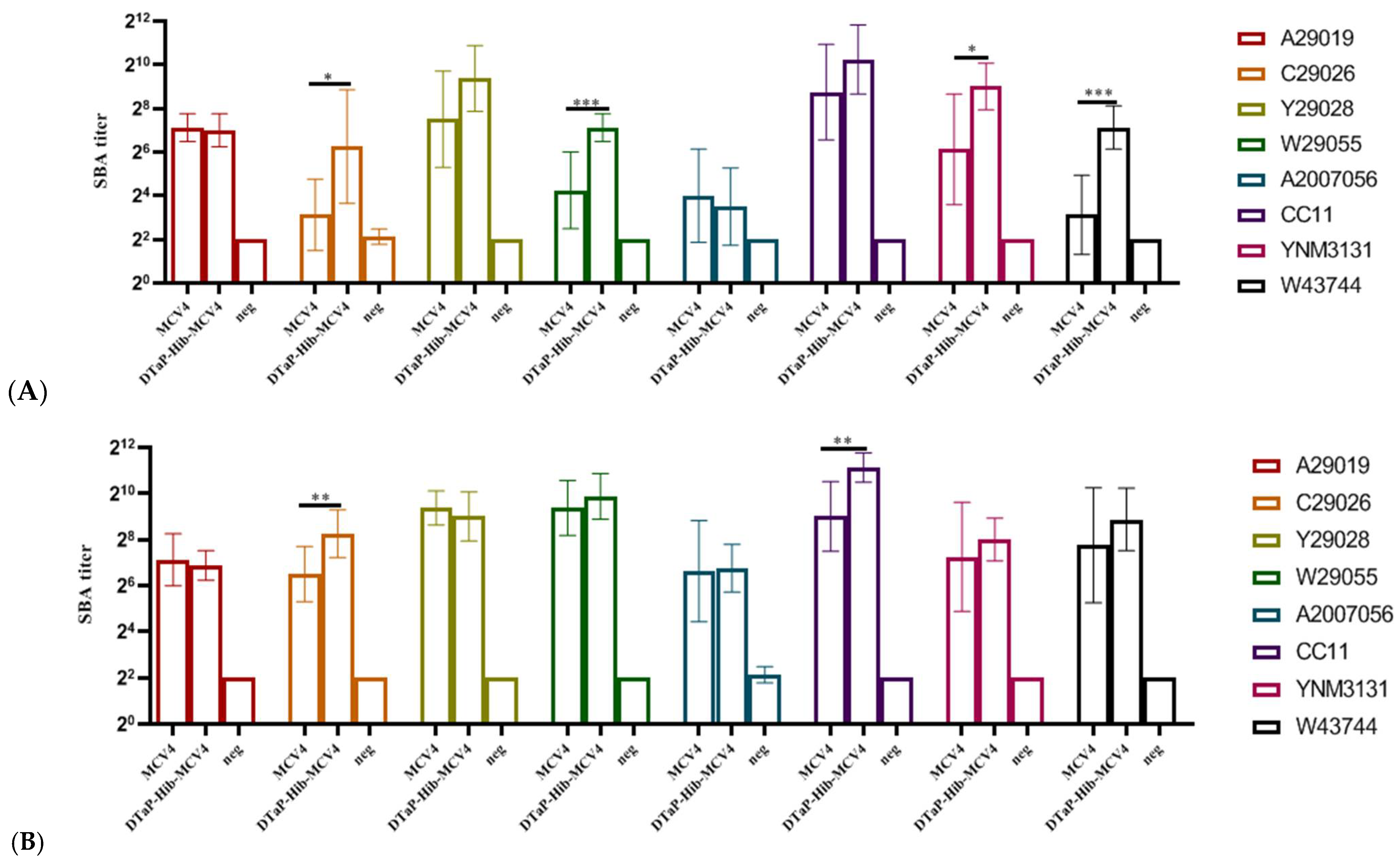
3.3.3. Serum Bactericidal Assay (SBA) of MCV4
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Guo, Y.; Dong, Y.; Liu, Y.; Zhao, Y.; Yu, S.; Li, S.; Wu, C.; Yang, B.; Li, W.; et al. Safety and immunogenicity of a combined DTacP-sIPV-Hib vaccine in animal models. Hum. Vaccines Immunother. 2022, 18, 2160158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, L.; Wang, Y.; Wang, J.; Wang, Y.; Li, J.; Wang, L.; Guo, Y. Adverse events following immunization of co- and separate administration of DTaP-IPV/Hib vaccines: A real-world comparative study. Hum. Vaccines Immunother. 2024, 20, 2372884. [Google Scholar] [CrossRef]
- Maman, K.; Zöllner, Y.; Greco, D.; Duru, G.; Sendyona, S.; Remy, V. The value of childhood combination vaccines: From beliefs to evidence. Hum. Vaccines Immunother. 2015, 11, 2132–2141. [Google Scholar] [CrossRef]
- Marshall, G.S.; Happe, L.E.; Lunacsek, O.E.; Szymanski, M.D.; Woods, C.R.; Zahn, M.; Russell, A. Use of combination vaccines is associated with improved coverage rates. Pediatr. Infect. Dis. J. 2007, 26, 496–500. [Google Scholar] [CrossRef]
- World Health Organization. DT-Based Combined Vaccines; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Kang, J.H.; Lee, H.J.; Kim, K.H.; Oh, S.H.; Cha, S.H.; Lee, J.; Kim, N.H.; Eun, B.W.; Kim, C.H.; Hong, Y.J.; et al. The Immunogenicity and Safety of a Combined DTaP-IPV//Hib Vaccine Compared with Individual DTaP-IPV and Hib (PRP~T) Vaccines: A Randomized Clinical Trial in South Korean Infants. J. Korean Med. Sci. 2016, 31, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.Y.; Zhou, J.J.; Li, M.S.; Zhang, Q.; Yan, T.T.; Wu, D.; Zheng, H. Disease burden and vaccine immunization status of epidemic cerebrospinal meningitis in infants. Chin. Prev. Med. 2025, 26, 252–256. [Google Scholar]
- Huang, G.S.; Liu, M.Z.; Ke, B.X.; Jiang, X. Etiology and epidemiological analysis on the first ST⁃1655 complex serogroup Y Neisseria meningitidis isolated from patient in Shantou. J. Trop. Med. 2022, 22, 724–726. [Google Scholar]
- Mo, Q.Q.; Chen, L.F.; Huang, F. Investigation and Analysis of the First Case of Group Y Meningococcal Meningitis in Maoming City in 2020. Strait J. Prev. Med. 2024, 30, 73–75. [Google Scholar]
- Zhang, N.; Cheng, X.; Zhu, Y.; Mo, O.; Yu, H.; Zhu, L.; Zhang, J.; Kuang, L.; Gao, Y.; Cao, R.; et al. Multi-valent mRNA vaccines against monkeypox enveloped or mature viron surface antigens demonstrate robust immune response and neutralizing activity. Sci. China Life Sci. 2023, 66, 2329–2341. [Google Scholar] [CrossRef]
- Eskola, J.; Ward, J.; Dagan, R.; Goldblatt, D.; Zepp, F.; Siegrist, C.A. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet 1999, 354, 2063–2068. [Google Scholar] [CrossRef]
- Trotter, C.L.; Ramsay, M.E.; Slack, M.P. Rising incidence of Haemophilus influenzae type b disease in England and Wales indicates a need for a second catch-up vaccination campaign. Commun. Dis. Public Health 2003, 6, 55–58. [Google Scholar] [PubMed]
- Chokephaibulkit, K. Combination vaccines. J. Med. Assoc. Thail. = Chotmaihet Thangphaet 2002, 85 (Suppl. 2), S694–S699. [Google Scholar]
- Verdier, F. Non-clinical vaccine safety assessment. Toxicology 2002, 174, 37–43. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Non-Clinical Evaluation of Vaccines, Annex 1; TRS No. 927; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Mawas, F.; Newman, G.; Burns, S.; Corbel, M.J. Suppression and modulation of cellular and humoral immune responses to Haemophilus influenzae type B (Hib) conjugate vaccine in hib-diphtheria-tetanus toxoids-acellular pertussis combination vaccines: A study in a rat model. J. Infect. Dis. 2005, 191, 58–64. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Group A and Group C Meningococcal Conjugate Vaccine. In Chinese Pharmacopoeia 3.5.4 Potency Assay; Chinese Pharmacopoeia Commission: Beijing, China, 2025. [Google Scholar]
- Chinese Pharmacopoeia Commission. Diphtheria, tetanus and pertussis vaccines. In Chinese Pharmacopoeia 3.2.4 Potency Assay; Chinese Pharmacopoeia Commission: Beijing, China, 2025. [Google Scholar]
- Loosmore, S.M.; Yang, Y.P.; Coleman, D.C.; Shortreed, J.M.; England, D.M.; Klein, M.H. Outer membrane protein D15 is conserved among Haemophilus influenzae species and may represent a universal protective antigen against invasive disease. Infect. Immun. 1997, 65, 3701–3707. [Google Scholar] [CrossRef]
- Maslanka, S.E.; Gheesling, L.L.; Libutti, D.E.; Donaldson, K.B.; Harakeh, H.S.; Dykes, J.K.; Arhin, F.F.; Devi, S.J.; Frasch, C.E.; Huang, J.C.; et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin. Diagn. Lab. Immunol. 1997, 4, 156–167. [Google Scholar] [CrossRef]
- Borrow, R.; Balmer, P.; Miller, E. Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine 2005, 23, 2222–2227. [Google Scholar] [CrossRef]
- Rappuoli, R.; Mandl, C.W.; Black, S.; De Gregorio, E. Vaccines for the twenty-first century society. Nat. Rev. Immunol. 2011, 11, 865–872. [Google Scholar] [CrossRef]
- Former State Food and Drug Administration of the People’s Republic of China. Technical Guidance for Single-Dose Toxicity Tests of Drugs (Announcement No. 4 of 2014); Former State Food and Drug Administration of the People’s Republic of China: Beijing, China, 2014. [Google Scholar]
- Former State Food and Drug Administration of the People’s Republic of China. Technical Guidance for Repeated Dose Toxicity Studies of Drugs (Announcement No. 4 of 2014); Former State Food and Drug Administration of the People’s Republic of China: Beijing, China, 2014. [Google Scholar]
- Hogenesch, H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front. Immunol. 2012, 3, 406. [Google Scholar] [CrossRef]
- Lu, F.; Hogenesch, H. Kinetics of the inflammatory response following intramuscular injection of aluminum adjuvant. Vaccine 2013, 31, 3979–3986. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Tan, H.; Zhang, C.; Chen, J.; Ni, L.; Yun, X.; Huang, Y.; Wang, W. Safety of pentavalent DTaP-IPV/Hib combination vaccine in post-marketing surveillance in Guangzhou, China, from 2011 to 2017. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 99, 149–155. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, C.; Ye, C.; Zhu, J.; Shen, J.; Zhu, C.; Yang, P.; Liu, T.; Xu, Y. Surveillance for adverse events following immunization with DTaP-containing combination vaccines in Linping, China, 2019-2022. Front. Public Health 2024, 12, 1278513. [Google Scholar] [CrossRef] [PubMed]
- Ga, E.; Kang, J.A.; Hwang, J.; Moon, S.; Choi, J.; Bae, E.; Seol, H.; Mun, Y.; Song, D.; Jeong, D.G.; et al. Assessment of the immune interference effects of multivalent vaccine for influenza epidemic strain in 2022-2023 and evaluation of its efficacy. Heliyon 2024, 10, e28326. [Google Scholar] [CrossRef]
- González-Domínguez, I.; Abdeljawad, A.; Lai, T.Y.; Boza, M.; McCroskery, S.; Lemus, N.; Slamanig, S.; Singh, G.; Warang, P.; Yellin, T.; et al. Mucosal multivalent NDV-based vaccine provides cross-reactive immune responses against SARS-CoV-2 variants in animal models. Front. Immunol. 2025, 16, 1524477. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Jia, Z.; Luo, X.X.; Fu, S.Y.; Yang, Y.; Xing, H. Meta-analysis of safety and immunogenicity of DTaP-IPV-Hib-HepB hexavaccine. Chin. J. Biol. 2024, 2, 195–201. [Google Scholar]
- Wang, X.; Gao, N.; Wen, J.; Li, J.; Ma, Y.; Sun, M.; Liang, J.; Shi, L. Immunogenicity of a Candidate DTacP-sIPV Combined Vaccine and Its Protection Efficacy against Pertussis in a Rhesus Macaque Model. Vaccines 2021, 10, 47. [Google Scholar] [CrossRef]
- Findlow, J.; Balmer, P.; Borrow, R. A review of complement sources used in serum bactericidal assays for evaluating immune responses to meningococcal ACWY conjugate vaccines. Hum. Vaccines Immunother. 2019, 15, 2491–2500. [Google Scholar] [CrossRef]
- Denoël, P.A.; Goldblatt, D.; de Vleeschauwer, I.; Jacquet, J.M.; Pichichero, M.E.; Poolman, J.T. Quality of the Haemophilus influenzae type b (Hib) antibody response induced by diphtheria-tetanus-acellular pertussis/Hib combination vaccines. Clin. Vaccine Immunol. CVI 2007, 14, 1362–1369. [Google Scholar] [CrossRef]
- Zimba, B.; Mpinganjira, S.; Msosa, T.; Bickton, F.M. The urban-poor vaccination: Challenges and strategies in low-and-middle income countries. Hum. Vaccines Immunother. 2024, 20, 2295977. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Sun, Y.; Liu, Z.; Dang, Y.; Huang, Y. Improving Innovation and Access to Combination Vaccines for Childhood Immunization in China. Int. J. Environ. Res. Public Health 2022, 19, 15557. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.R.; Lan, H.; Xiang, M.J.; Yang, R.P.; Wang, Q.X. Immunogenicity and immune persistence of adsorbed diphtheria, tetanus and acellular pertussis combined vaccine. Chin. J. Biol. 2013, 26, 1805–1808+1811. [Google Scholar]
- Sun, X.; Stefanetti, G.; Berti, F.; Kasper, D.L. Polysaccharide structure dictates mechanism of adaptive immune response to glycoconjugate vaccines. Proc. Natl. Acad. Sci. USA 2019, 116, 193–198. [Google Scholar] [CrossRef]
- Fatima, M.; Hong, K.J. Innovations, Challenges, and Future Prospects for Combination Vaccines Against Human Infections. Vaccines 2025, 13, 335. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, X.; Shao, Z.; Ji, Y.; Wang, H.; Xu, Q.; Wei, B.; Duan, Z.; Wang, C.; Yang, Y.; Zhao, J.; et al. An Evaluation of the Safety, Immunogenicity, and Protective Efficacy of a Combined Diphtheria–Tetanus–Acellular Pertussis, Haemophilus influenzae Type b, and ACYW135 Meningococcal Conjugate Vaccine in Murine and Rat Models. Vaccines 2025, 13, 724. https://doi.org/10.3390/vaccines13070724
Sui X, Shao Z, Ji Y, Wang H, Xu Q, Wei B, Duan Z, Wang C, Yang Y, Zhao J, et al. An Evaluation of the Safety, Immunogenicity, and Protective Efficacy of a Combined Diphtheria–Tetanus–Acellular Pertussis, Haemophilus influenzae Type b, and ACYW135 Meningococcal Conjugate Vaccine in Murine and Rat Models. Vaccines. 2025; 13(7):724. https://doi.org/10.3390/vaccines13070724
Chicago/Turabian StyleSui, Xiuwen, Zhujun Shao, Yuanyuan Ji, Hairui Wang, Qingfu Xu, Bochao Wei, Zhuojun Duan, Chang Wang, Ying Yang, Jiayu Zhao, and et al. 2025. "An Evaluation of the Safety, Immunogenicity, and Protective Efficacy of a Combined Diphtheria–Tetanus–Acellular Pertussis, Haemophilus influenzae Type b, and ACYW135 Meningococcal Conjugate Vaccine in Murine and Rat Models" Vaccines 13, no. 7: 724. https://doi.org/10.3390/vaccines13070724
APA StyleSui, X., Shao, Z., Ji, Y., Wang, H., Xu, Q., Wei, B., Duan, Z., Wang, C., Yang, Y., Zhao, J., & Zhu, T. (2025). An Evaluation of the Safety, Immunogenicity, and Protective Efficacy of a Combined Diphtheria–Tetanus–Acellular Pertussis, Haemophilus influenzae Type b, and ACYW135 Meningococcal Conjugate Vaccine in Murine and Rat Models. Vaccines, 13(7), 724. https://doi.org/10.3390/vaccines13070724





