Kidney Involvement in SARS-CoV-2 Infection: Peritoneal Dialysis as the Preferred Modality
Abstract
1. Introduction
2. Immunity
2.1. Cellular Immunity
2.2. Humoral Immunity
3. SARS-CoV-2 Vaccination
3.1. SARS-CoV-2 Vaccine Platforms and Effectiveness
3.2. Booster Doses
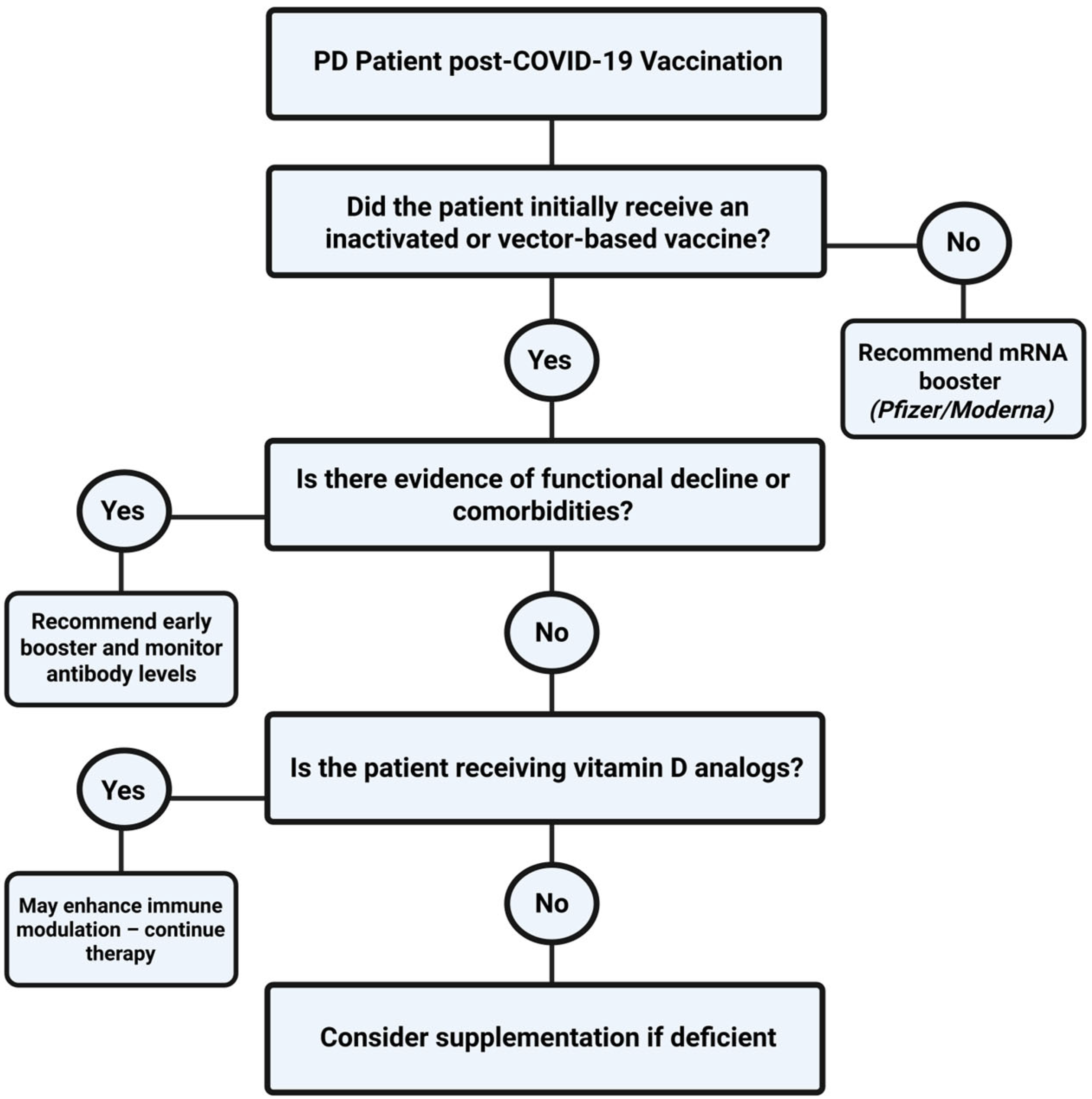
3.3. Vaccination Recommendations for High-Risk Comorbidities
3.4. Complications of SARS-CoV-2 Vaccines
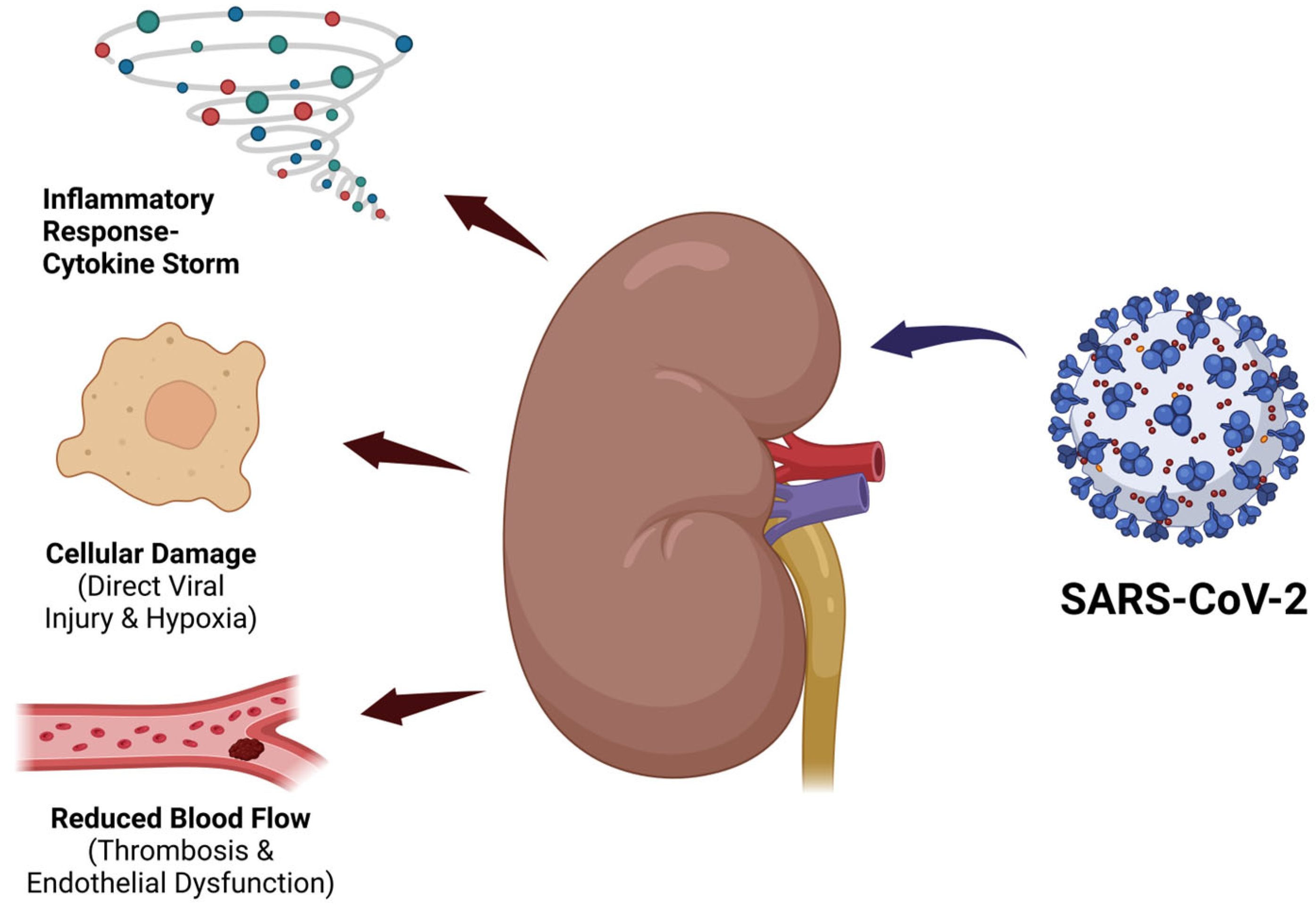
4. Chronic Kidney Disease in SARS-CoV-2 Infection
5. Peritoneal Dialysis
5.1. PD Treatment Modalities
5.2. PD Solutions
6. SARS-CoV-2 Infection in Patients on Peritoneal Dialysis
7. Discussion
8. Public Health and Policy Considerations
9. Limitations of Current Evidence
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mariano, G.; Farthing, R.J.; Lale-Farjat, S.L.M.; Bergeron, J.R.C. Structural Characterization of SARS-CoV-2: Where We Are, and Where We Need to Be. Front. Mol. Biosci. 2020, 7, 605236. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An Overview of Their Replication and Pathogenesis. In Methods in Molecular Biology; Maier, H.J., Bickerton, E., Britton, P., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1282, pp. 1–23. [Google Scholar] [CrossRef]
- Kakavandi, S.; Zare, I.; VaezJalali, M.; Dadashi, M.; Azarian, M.; Akbari, A.; Ramezani Farani, M.; Zalpoor, H.; Hajikhani, B. Structural and Non-Structural Proteins in SARS-CoV-2: Potential Aspects to COVID-19 Treatment or Prevention of Progression of Related Diseases. Cell Commun. Signal. 2023, 21, 110. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of Angiotensin-Converting Enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- Soleimani, M. Acute Kidney Injury in SARS-CoV-2 Infection: Direct Effect of Virus on Kidney Proximal Tubule Cells. Int. J. Mol. Sci. 2020, 21, 3275. [Google Scholar] [CrossRef]
- Kroll, M.-K.; Schloer, S.; Candan, P.; Korthals, N.; Wenzel, C.; Ihle, H.; Gilhaus, K.; Liedtke, K.R.; Schöfbänker, M.; Surmann, B.; et al. Importance of ACE2 for SARS-CoV-2 Infection of Kidney Cells. Biomolecules 2023, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.L.; Laftih, S.; Andresen, J.K.; Pedersen, R.M.; Andersen, T.E.; Madsen, L.W.; Madsen, K.; Hinrichs, G.R.; Zachar, R.; Svenningsen, P.; et al. ACE2 and TMPRSS2 in Human Kidney Tissue and Urine Extracellular Vesicles with Age, Sex, and COVID-19. Pflug. Arch. Eur. J. Physiol. 2024, 477, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.C.; Dejenie, T.A. Protective Roles and Protective Mechanisms of Neutralizing Antibodies Against SARS-CoV-2 Infection and Their Potential Clinical Implications. Front. Immunol. 2023, 14, 1055457. [Google Scholar] [CrossRef]
- Shrotri, M.; van Schalkwyk, M.C.I.; Post, N.; Eddy, D.; Huntley, C.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. T Cell Response to SARS-CoV-2 Infection in Humans: A Systematic Review. PLoS ONE 2021, 16, e0245532. [Google Scholar] [CrossRef]
- Shen, J.; Fan, J.; Zhao, Y.; Jiang, D.; Niu, Z.; Zhang, Z.; Cao, G. Innate and Adaptive Immunity to SARS-CoV-2 and Predisposing Factors. Front. Immunol. 2023, 14, 1159326. [Google Scholar] [CrossRef]
- Samuel, C.E. Interferon at the Crossroads of SARS-CoV-2 Infection and COVID-19 Disease. J. Biol. Chem. 2023, 299, 104960. [Google Scholar] [CrossRef]
- Strannegård, Ö. Interferons and Their Therapeutic Applications. J. Int. Fed. Clin. Chem. Lab. Med. 1999, 11, 52–58. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6351751/ (accessed on 13 April 2025).
- Zhang, J.M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Meidaninikjeh, S.; Sabouni, N.; Marzouni, H.Z.; Bengar, S.; Khalili, A.; Jafari, R. Monocytes and Macrophages in COVID-19: Friends and Foes. Life Sci. 2021, 269, 119010. [Google Scholar] [CrossRef]
- Zhou, Y.; Hann, J.; Schenten, V.; Plançon, S.; Bueb, J.L.; Tolle, F.; Bréchard, S. Role of S100A8/A9 for Cytokine Secretion, Revealed in Neutrophils Derived from ER-Hoxb8 Progenitors. Int. J. Mol. Sci. 2021, 22, 8845. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ye, L.; Ye, L.; Li, B.; Gao, B.; Zeng, Y.; Kong, L.; Fang, X.; Zheng, H.; Wu, Z.; et al. Up-Regulation of IL-6 and TNF-α Induced by SARS-Coronavirus Spike Protein in Murine Macrophages via NF-κB Pathway. Virus Res. 2007, 128, 1–8. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, G.S. Status of Mannose-Binding Lectin (MBL) and Complement System in COVID-19 Patients and Therapeutic Applications of Antiviral Plant MBLs. Mol. Cell. Biochem. 2021, 476, 2917–2942. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An Update on the Regulatory Mechanisms of NLRP3 Inflammasome Activation. Cell Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Xie, Z.; Suleman, M.; Shah, A.; Khan, S.; Luo, S. Roles and Functions of SARS-CoV-2 Proteins in Host Immune Evasion. Front. Immunol. 2022, 13, 940756. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.N.; Tamjidifar, R.; Rahman, H.S.; Adili, A.; Ghoreishizadeh, S.; Saeedi, H.; Thangavelu, L.; Shomali, N.; Aslaminabad, R.; Marofi, F.; et al. A Comprehensive Review About Immune Responses and Exhaustion During Coronavirus Disease (COVID-19). Cell Commun. Signal. 2022, 20, 79. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Asquith, B.; Szydlo, R.; Tregoning, J.S.; Pollock, K.M. Peripheral T Cell Lymphopenia in COVID-19: Potential Mechanisms and Impact. Immunother. Adv. 2021, 1, ltab015. [Google Scholar] [CrossRef]
- Eggenhuizen, P.J.; Ooi, D.J. The Influence of Cross-Reactive T Cells in COVID-19. Biomedicines 2024, 12, 564. [Google Scholar] [CrossRef]
- Singh, L.; Bajaj, S.; Gadewar, M.; Verma, N.; Ansari, M.N.; Saeedan, A.S.; Kaithwas, G.; Singh, M. Modulation of Host Immune Response Is an Alternative Strategy to Combat SARS-CoV-2 Pathogenesis. Front. Immunol. 2021, 12, 660632. [Google Scholar] [CrossRef]
- Torres Acosta, M.A.; Singer, B.D. Pathogenesis of COVID-19-Induced ARDS: Implications for an Ageing Population. Eur. Respir. J. 2020, 56, 2002049. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 Pandemic and Research Gaps: Understanding SARS-CoV-2 Interaction with the ACE2 Receptor and Implications for Therapy. Theranostics 2020, 10, 7448–7464. [Google Scholar] [CrossRef]
- Mansourabadi, A.H.; Aghamajidi, A.; Dorfaki, M.; Keshavarz, F.; Shafeghat, Z.; Moazzeni, A.; Arab, F.L.; Rajabian, A.; Roozbehani, M.; Falak, R.; et al. B Lymphocytes in COVID-19: A Tale of Harmony and Discordance. Arch. Virol. 2023, 168, 148. [Google Scholar] [CrossRef]
- Chen, S.; Guan, F.; Candotti, F.; Benlagha, K.; Camara, N.O.S.; Herrada, A.A.; James, L.K.; Lei, J.; Miller, H.; Kubo, M.; et al. The Role of B Cells in COVID-19 Infection and Vaccination. Front. Immunol. 2022, 13, 988536. [Google Scholar] [CrossRef]
- Abril, A.; Alejandre, J.; Mariscal, A.; Alserawan, L.; Rabella, N.; Roman, E.; Lopez-Contreras, J.; Navarro, F.; Serrano, E.; Nomdedeu, J.F.; et al. Titers of IgG and IgA Against SARS-CoV-2 Proteins and Their Association with Symptoms in Mild COVID-19 Infection. Sci. Rep. 2024, 14, 12725. [Google Scholar] [CrossRef]
- Pitiriga, V.C.; Papamentzelopoulou, M.; Konstantinakou, K.E.; Vasileiou, I.V.; Sakellariou, K.S.; Spyrou, N.I.; Tsakris, A. Persistence of T-Cell Immunity Responses Against SARS-CoV-2 for Over 12 Months Post COVID-19 Infection in Unvaccinated Individuals with No Detectable IgG Antibodies. Vaccines 2023, 11, 1764. [Google Scholar] [CrossRef]
- Pušnik, J.; König, J.; Mai, K.; Richter, E.; Zorn, J.; Proksch, H.; Schulte, B.; Alter, G.; Streeck, H. Persistent Maintenance of Intermediate Memory B Cells Following SARS-CoV-2 Infection and Vaccination Recall Response. J. Virol. 2022, 96, e0076022. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.C.; Dorabawila, V.; León, T.M.; Henry, H.; Johnson, A.G.; Rosenberg, E.; Mansfield, J.A.; Midgley, C.M.; Plumb, I.D.; Aiken, J.; et al. Trends in Laboratory-Confirmed SARS-CoV-2 Reinfections and Associated Hospitalizations and Deaths Among Adults Aged ≥18 Years—18 U.S. Jurisdictions, September 2021–December 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 683–689. [Google Scholar] [CrossRef]
- Shalash, A.O.; Hussein, W.M.; Skwarczynski, M.; Toth, I. Key Considerations for the Development of Safe and Effective SARS-CoV-2 Subunit Vaccine: A Peptide-Based Vaccine Alternative. Adv. Sci. 2021, 8, 2100985. [Google Scholar] [CrossRef]
- Islam, M.A. A Review of SARS-CoV-2 Variants and Vaccines: Viral Properties, Mutations, Vaccine Efficacy, and Safety. Infect. Med. 2023, 2, 247–261. [Google Scholar] [CrossRef]
- Pavel, S.T.I.; Yetiskin, H.; Uygut, M.A.; Aslan, A.F.; Aydın, G.; İnan, Ö.; Kaplan, B.; Ozdarendeli, A. Development of an Inactivated Vaccine Against SARS-CoV-2. Vaccines 2021, 9, 1266. [Google Scholar] [CrossRef]
- Kan, A.K.C.; Li, P.H. Inactivated COVID-19 Vaccines: Potential Concerns of Antibody-Dependent Enhancement and Original Antigenic Sin. Immunol. Lett. 2023, 259, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines 2023, 11, 432. [Google Scholar] [CrossRef]
- Vanaparthy, R.; Mohan, G.; Vasireddy, D.; Atluri, P. Review of COVID-19 Viral Vector-Based Vaccines and COVID-19 Variants. Infez. Med. 2021, 29, 328–338. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Katella, K. Comparing the COVID-19 Vaccines: How Are They Different? Yale Medicine. 2024. Available online: https://www.yalemedicine.org/news/covid-19-vaccine-comparison (accessed on 13 March 2025).
- Zabidi, N.Z.; Liew, H.L.; Farouk, I.A.; Puniyamurti, A.; Yip, A.J.W.; Wijesinghe, V.N.; Low, Z.Y.; Tang, J.W.; Chow, V.T.K.; Lal, S.K. Evolution of SARS-CoV-2 Variants: Implications on Immune Escape, Vaccination, Therapeutic and Diagnostic Strategies. Viruses 2023, 15, 944. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection against COVID-19 by BNT162b2 Booster across Age Groups. N. Engl. J. Med. 2021, 385, 2421–2430. [Google Scholar] [CrossRef]
- AlQahtani, M.; Du, X.; Bhattacharyya, S.; Alawadi, A.; Al Mahmeed, H.; Al Sayed, J.; Justman, J.; El-Sadr, W.M.; Hidary, J.; Mukherjee, S. Post-Vaccination Outcomes in Association with Four COVID-19 Vaccines in the Kingdom of Bahrain. Sci. Rep. 2022, 12, 9236. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhang, H.; Zheng, Z.; Jiang, Y.; Huang, Y.; Lin, S.; Yu, J.; Deng, X.; He, J.; Shen, C.; et al. Serosurvey in SARS-CoV-2 Inactivated Vaccine-Elicited Neutralizing Antibodies Against Authentic SARS-CoV-2 and Its Viral Variants. J. Med. Virol. 2022, 94, 6065–6072. [Google Scholar] [CrossRef]
- Cohn, B.A.; Cirillo, P.M.; Murphy, C.C.; Krigbaum, N.Y.; Wallace, A.W. SARS-CoV-2 Vaccine Protection and Deaths Among US Veterans During 2021. Science 2022, 375, 331–336. [Google Scholar] [CrossRef]
- Siedner, M.J.; Alba, C.; Fitzmaurice, K.P.; Gilbert, R.F.; Scott, J.A.; Shebl, F.M.; Ciaranello, A.; Reddy, K.P.; Freedberg, K.A. Cost-Effectiveness of Coronavirus Disease 2019 Vaccination in Low- and Middle-Income Countries. J. Infect. Dis. 2022, 226, 1887–1896. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-Specific T Cell Immunity is Maintained at 6 Months Following Primary Infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef]
- Iversen, P.L.; Bavari, S. Is There Space for a Three-Dose Vaccine to Fight the Spread of SARS-CoV-2? Lancet Infect. Dis. 2021, 21, 1054–1055. [Google Scholar] [CrossRef]
- To, K.K.; Sridhar, S.; Chiu, K.H.; Hung, D.L.; Li, X.; Hung, I.F.; Tam, A.R.; Chung, T.W.; Chan, J.F.; Zhang, A.J.; et al. Lessons Learned One Year After SARS-CoV-2 Emergence Leading to COVID-19 Pandemic. Emerg. Microbes Infect. 2021, 10, 507–535. [Google Scholar] [CrossRef] [PubMed]
- Costa Clemens, S.A.; Weckx, L.Y.; Clemens, R.; Almeida Mendes, A.V.; Ramos, S.Z.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; Pinto, M.I.d.M.; Gonzalez, I.G.S.; et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): A phase 4, non-inferiority, single-blind, randomised study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a Third Dose of the BNT162b2 mRNA COVID-19 Vaccine for Preventing Severe Outcomes in Israel: An Observational Study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Kim, S.S.; Chung, J.R.; Talbot, H.K.; Grijalva, C.G.; Wernli, K.J.; Kiniry, E.; Martin, E.T.; Monto, A.S.; Belongia, E.A.; McLean, H.Q.; et al. Effectiveness of Two and Three mRNA COVID-19 Vaccine Doses Against Omicron- and Delta-Related Outpatient Illness Among Adults, October 2021–February 2022. Influenza Other Respir. Viruses 2022, 16, 975–985. [Google Scholar] [CrossRef]
- Garg, I.; Sheikh, A.B.; Pal, S.; Shekhar, R. Mix-and-Match COVID-19 Vaccinations (Heterologous Boost): A Review. Infect. Dis. Rep. 2022, 14, 537–546. [Google Scholar] [CrossRef]
- Shaw, R.H.; Stuart, A.; Greenland, M.; Liu, X.; Nguyen Van-Tam, J.S.; Snape, M.D.; Com-COV Study Group. Heterologous Prime-Boost COVID-19 Vaccination: Initial Reactogenicity Data. Lancet 2021, 397, 2043–2046. [Google Scholar] [CrossRef]
- Sonmezer, M.C.; Dizman, G.T.; Erul, E.; Sahin, T.K.; Saricaoglu, T.; Alp, A.; Tanriover, M.D.; Uzun, O.; Unal, S.; Akova, M. Relative Vaccine Effectiveness of the Third Dose of CoronaVac or BNT162b2 Following a Two-Dose CoronaVac Regimen: A Prospective Observational Cohort Study from an Adult Vaccine Center in Turkey. Vaccines 2022, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.J.; Wu, M.; Harvey, R.; Wall, E.C.; Kelly, G.; Hussain, S.; Howell, M.; Kassiotis, G.; Swanton, C.; Gandhi, S.; et al. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet 2021, 398, 1038–1041. [Google Scholar] [CrossRef]
- Zizza, A.; Recchia, V.; Aloisi, A.; Guido, M. Clinical Features of COVID-19 and SARS Epidemics: A Literature Review. J. Prev. Med. Hyg. 2021, 62, E13–E24. [Google Scholar] [CrossRef]
- Tylicki, L.; Biedunkiewicz, B.; Puchalska-Reglińska, E.; Gellert, R.; Burnier, M.; Wolf, J.; Dȩbska-Ślizień, A. COVID-19 Vaccination Reduces Mortality in Patients on Maintenance Hemodialysis. Front. Med. 2022, 9, 937167. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Muecksch, F.; Cho, A.; Agarwal, D.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; et al. COVID-19 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef] [PubMed]
- De Picker, L.J.; Dias, M.C.; Benros, M.E.; Vai, B.; Branchi, I.; Benedetti, F.; Borsini, A.; Dazzan, P.; Dean, K.; Fusar-Poli, P.; et al. Severe mental illness and European COVID-19 vaccination strategies. Lancet Psychiatry 2021, 8, 356–359. [Google Scholar] [CrossRef]
- Cai, X.; Wu, G.; Zhang, J.; Yang, L. Risk Factors for Acute Kidney Injury in Adult Patients with COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 719472. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, H.; Shajahan, S.; Gulati, A.; Synn, S.; Khurana, S.; Nazar, N.; Shrestha, S.; Kerstein, J. COVID-19 mRNA Vaccine-Associated Myocarditis. Cureus 2022, 14, e21009. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef]
- Park, D.Y.; An, S.; Kaur, A.; Malhotra, S.; Vij, A. Myocarditis After COVID-19 mRNA Vaccination: A Systematic Review of Case Reports and Case Series. Clin. Cardiol. 2022, 45, 691–700. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia After ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Vaccine Adverse Event Reporting System (VAERS). Available online: https://vaers.hhs.gov (accessed on 28 June 2025).
- European Medicines Agency. Safety of COVID-19 Vaccines. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines/safety-covid-19-vaccines (accessed on 28 June 2025).
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- McNeil, M.M.; DeStefano, F. Vaccine-Associated Hypersensitivity. J. Allergy Clin. Immunol. 2018, 141, 463–472. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N.; et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines 2021, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Shay, D.K.; Gee, J.; Su, J.R.; Myers, T.R.; Marquez, P.; Liu, R.; Zhang, B.; Licata, C.; Clark, T.A.; Shimabukuro, T.T. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 680–684. [Google Scholar] [CrossRef]
- Sellaturay, P.; Nasser, S.; Islam, S.; Gurugama, P.; Ewan, P.W. Polyethylene Glycol (PEG) is a Cause of Anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 Vaccine. Clin. Exp. Allergy 2021, 51, 861–863. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A.; Robinson, L.B.; Long, A.A.; Blumenthal, K.G. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Hardenberg, J.B.; Luft, F.C. COVID-19, ACE2 and the Kidney. Acta Physiol. 2020, 230, e13539. [Google Scholar] [CrossRef]
- He, W.; Liu, X.; Hu, B.; Li, D.; Chen, L.; Li, Y.; Tu, Y.; Xiong, S.; Wang, G.; Deng, J.; et al. Mechanisms of SARS-CoV-2 Infection-Induced Kidney Injury: A Literature Review. Front. Cell Infect. Microbiol. 2022, 12, 838213. [Google Scholar] [CrossRef]
- Lu, J.Y.; Lu, J.Y.; Wang, S.; Zhang, Y.; Liu, M.; Chen, L.; Rivera, M.; Patel, V.; Gupta, A.; Thompson, R.; et al. Long Term Outcomes of Patients with Chronic Kidney Disease after COVID-19 in an Urban Population in the Bronx. Sci. Rep. 2025, 15, 6119. [Google Scholar] [CrossRef] [PubMed]
- Parise, R.S.; Govindarajulu, M.; Ramesh, S.; Thomas, T.; Moore, T.; Dhanasekaran, M. COVID-19 Induced Renal Injury Differs from That in Other Viral Infections. Emerg. Crit. Care Med. 2022, 2, 23–31. [Google Scholar] [CrossRef]
- Aguilar-Bretones, M.; den Hartog, Y.; van Dijk, L.L.A.; van Baarle, D.; van Gageldonk-Lafeber, A.B.; Reinders, M.E.J.; van Zuilen, A.D.; Mulder, M.; Baas, M.C.; van den Dorpel, M.A.; et al. SARS-CoV-2-Specific Immune Responses Converge in Kidney Disease Patients and Controls with Hybrid Immunity. npj Vaccines 2024, 9, 93. [Google Scholar] [CrossRef]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-Associated Acute Kidney Injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef]
- Mahalingasivam, V.; Faucon, A.L.; Sjölander, A.; Bosi, A.; González-Ortiz, A.; Lando, S.; Fu, E.L.; Nitsch, D.; Bruchfeld, A.; Evans, M.; et al. Kidney Function Decline After COVID-19 Infection. JAMA Netw. Open 2024, 7, e2450014. [Google Scholar] [CrossRef] [PubMed]
- Hoilat, G.J.; Das, G.; Shahnawaz, M.; Shanley, P.; Bukhari, S.H. COVID-19 Induced Collapsing Glomerulopathy and Role of APOL1. QJM Int. J. Med. 2021, 114, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Blake, P.; Cordy, P.; Garg, A.X. Global Trends in Rates of Peritoneal Dialysis. J. Am. Soc. Nephrol. 2012, 23, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Yu, Z.; Fang, W.; Lin, A.; Ni, Z.; Qian, J.; Woodrow, G.; Jenkins, S.B.; Wilkie, M.E.; Davies, S.J. Longitudinal Bioimpedance Vector Plots Add Little Value to Fluid Management of Peritoneal Dialysis Patients. Kidney Int. 2016, 89, 487–497. [Google Scholar] [CrossRef]
- Davies, S.J.; Caskey, F.J.; Coyle, D.; Lindley, E.; Macdonald, J.; Mitra, S.; Wilkie, M.; Davenport, A.; Farrington, K.; Dasgupta, I.; et al. Rationale and Design of BISTRO: A Randomized Controlled Trial to Determine Whether Bioimpedance Spectroscopy-Guided Fluid Management Maintains Residual Kidney Function in Incident Haemodialysis Patients. BMC Nephrol. 2017, 18, 138. [Google Scholar] [CrossRef]
- Rippe, B. A Three-Pore Model of Peritoneal Transport. Perit. Dial. Int. 1993, 13 (Suppl. 2), S35–S38. [Google Scholar] [CrossRef]
- Sam, R. Hemodialysis: Diffusion and Ultrafiltration. Austin J. Nephrol. Hypertens. 2014, 1, 1010. [Google Scholar]
- Tomino, Y. Mechanisms and Interventions in Peritoneal Fibrosis. Clin. Exp. Nephrol. 2012, 16, 109–114. [Google Scholar] [CrossRef]
- Yu, Z.; Lambie, M.; Davies, S.J. Longitudinal Study of Small Solute Transport and Peritoneal Protein Clearance in Peritoneal Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 326–334. [Google Scholar] [CrossRef]
- Qayyum, A.; Yang, L.; Fan, S.L. Optimizing Peritoneal Dialysis Catheter Placement by Lateral Abdomen X-Ray. Perit. Dial. Int. 2015, 35, 760–762. [Google Scholar] [CrossRef]
- Ito, Y.; Sun, T.; Tawada, M.; Kinashi, H.; Yamaguchi, M.; Katsuno, T.; Kim, H.; Mizuno, M.; Ishimoto, T. Pathophysiological Mechanisms of Peritoneal Fibrosis and Peritoneal Membrane Dysfunction in Peritoneal Dialysis. Int. J. Mol. Sci. 2024, 25, 8607. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Johnson, D.W.; Vesey, D.A.; Hawley, C.M.; Pascoe, E.M.; Clarke, M.; Topley, N.; balANZ Trial Investigators. Higher Dialysate Matrix Metalloproteinase-2 Levels Are Associated with Peritoneal Membrane Dysfunction. Perit. Dial. Int. 2016, 36, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Scalzotto, E.; Giavarina, D.; Rodighiero, M.P.; Crepaldi, C.; Day, S.; Ronco, C. The Role of NGAL in Peritoneal Dialysis Effluent in Early Diagnosis of Peritonitis: Case-Control Study in Peritoneal Dialysis Patients. Perit. Dial. Int. 2015, 35, 559–565. [Google Scholar] [CrossRef]
- Lee, S.M.; Min, Y.S.; Son, Y.K.; Kim, S.E.; An, W.S. Comparison of Clinical Outcome Between Incremental Peritoneal Dialysis and Conventional Peritoneal Dialysis: A Propensity Score Matching Study. Ren. Fail. 2021, 43, 1222–1228. [Google Scholar] [CrossRef]
- Unal, A.; Sipahioglu, M.H.; Kocyigit, I.; Tunca, O.; Tokgoz, B.; Oymak, O. Risk Factor(s) Related to High Membrane Permeability in Peritoneal Dialysis. Ren. Fail. 2016, 38, 238–241. [Google Scholar] [CrossRef]
- Andreoli, M.C.C.; Totoli, C. Peritoneal Dialysis. Rev. Assoc. Med. Bras. 2020, 66 (Suppl. 1), s37–s44. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Bai, E.; Ge, C.; Winograd, J.; Shah, A.D. Peritoneal Equilibration Testing: Your Questions Answered. Perit. Dial. Int. 2023, 43, 361–373. [Google Scholar] [CrossRef]
- Domenici, A.; Giuliani, A. Automated Peritoneal Dialysis: Patient Perspectives and Outcomes. Int. J. Nephrol. Renovasc. Dis. 2021, 14, 385–392. [Google Scholar] [CrossRef]
- Shi, X.; Du, H.; Zhang, Z.; Zhou, Y. Clinical Outcomes of Automated Versus Continuous Ambulatory Peritoneal Dialysis for End-Stage Kidney Disease: Protocol of a Systematic Review and Meta-Analysis. BMJ Open 2022, 12, e065795. [Google Scholar] [CrossRef]
- Luo, P.T.; Li, W.; Li, X.Y.; Zhang, Y.; Du, B.; Cui, W.-P. Impact of Peritoneal Dialysis Modality on Patient and PD Survival: A Systematic Review. Perit. Dial. Int. 2023, 43, 128–138. [Google Scholar] [CrossRef]
- Murashima, M.; Hamano, T.; Abe, M.; Masakane, I. Combination of Once-Weekly Haemodialysis with Peritoneal Dialysis Is Associated with Lower Mortality Compared with Peritoneal Dialysis Alone: A Longitudinal Study. Clin. Kidney J. 2020, 14, 1610–1617. [Google Scholar] [CrossRef]
- Fernandes, A.; Matias, P.; Branco, P. Incremental Peritoneal Dialysis—Definition, Prescription, and Clinical Outcomes. Kidney360 2023, 4, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Htay, H.; Johnson, D.W.; Wiggins, K.J.; Badve, S.V.; Craig, J.C.; Strippoli, G.F.; Cho, Y. Biocompatible Dialysis Fluids for Peritoneal Dialysis. Cochrane Database Syst. Rev. 2018, 10, CD007554. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, M.; Masola, V.; Procino, G.; Zammit, V.; Divino-Filho, J.C.; Arduini, A.; Gambaro, G. How to Improve the Biocompatibility of Peritoneal Dialysis Solutions (without Jeopardizing the Patient’s Health). Int. J. Mol. Sci. 2021, 22, 7955. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.N.; Lam, M.F.; Leung, J.C.; Chan, L.Y.; Lam, C.W.; Chan, I.H.; Chan, H.W.; Li, C.S.; Wong, S.S.; Ho, Y.W.; et al. A Study of the Clinical and Biochemical Profile of Peritoneal Dialysis Fluid Low in Glucose Degradation Products. Perit. Dial. Int. 2012, 32, 280–291. [Google Scholar] [CrossRef]
- Chen, J.H.C.; Johnson, D.W.; Cho, Y.; Cheetham, M.; Sud, K.; Hayat, A.; Stallard, B.; Clayton, P.; Davies, C.E.; Borlace, M.; et al. Associations of Neutral pH, Low-GDP Peritoneal Dialysis Solutions with Patient Survival, Transfer to Haemodialysis and Peritonitis. Nephrol. Dial. Transplant. 2024, 39, 222–232. [Google Scholar] [CrossRef]
- Masola, V.; Bonomini, M.; Onisto, M.; Ferraro, P.M.; Arduini, A.; Gambaro, G. Biological Effects of XyloCore, a Glucose-Sparing PD Solution, on Mesothelial Cells: Focus on Mesothelial-Mesenchymal Transition, Inflammation, and Angiogenesis. Nutrients 2021, 13, 2282. [Google Scholar] [CrossRef]
- Bonomini, M.; Davies, S.; Kleophas, W.; Lambie, M.; Reboldi, G.; Di Liberato, L.; Divino-Filho, J.C.; Heimburger, O.; Ortiz, A.; Povlsen, J.; et al. Rationale and Design of ELIXIR, a Randomized, Controlled Trial to Evaluate Efficacy and Safety of XyloCore, a Glucose-Sparing Solution for Peritoneal Dialysis. Perit. Dial. Int. 2025, 45, 17–25. [Google Scholar] [CrossRef]
- Bartosova, M.; Schmitt, C.P. Biocompatible Peritoneal Dialysis: The Target Is Still Way Off. Front. Physiol. 2019, 9, 1853. [Google Scholar] [CrossRef]
- Candellier, A.; Scohy, A.; Gillet, N.; Muylkens, B.; Morelle, J.; Belkhir, L.; Coupeau, D.; Jadoul, M.; Goffin, É. Absence of SARS-CoV-2 in the Effluent of Peritoneal Dialysis Patients. Perit. Dial. Int. 2020, 40, 499–503. [Google Scholar] [CrossRef]
- Yavuz, D.; Özen, D.S.K.; Demirağ, M.D. COVID-19: Mortality Rates of Patients on Hemodialysis and Peritoneal Dialysis. Int. Urol. Nephrol. 2022, 54, 2713–2718. [Google Scholar] [CrossRef] [PubMed]
- Badrouchi, S.; Barbouch, S.; Bettaieb, A.; Sellami, N.; Hajji, M.; Ben Abdallah, T.; Ben Hamida, F.; Harzallah, A.; Abderrahim, E. Peritoneal Dialysis in the Era of COVID-19: Experience of a Tunisian Center. J. Nephrol. 2022, 35, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, A.C.; Noordzij, M.; Goffin, E.; Sanchez, J.E.; Franssen, C.F.; Vart, P.; Jager, K.J.; van Agteren, M.; Covic, A.; Mitra, S.; et al. Outcomes of COVID-19 in Peritoneal Dialysis Patients: A Report by the European Renal Association COVID-19 Database. Perit. Dial. Int. 2023, 43, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Kazancıoğlu, R.; Öztürk, Ş.; Turgutalp, K.; Gürsu, M.; Arıcı, M.; Oruç, A.; Ahbap, E.; Bek, S.G.; Şengül, E.; Öğütmen, M.B.; et al. COVID-19 Infection in Peritoneal Dialysis Patients: A Comparative Outcome Study with Patients on Hemodialysis and Patients without Kidney Disease. Turk. J. Nephrol. 2022, 31, 33–42. [Google Scholar] [CrossRef]
- Painter, D.F.; Vogt, B.; Lokhande, A.; Berreta, R.S.; Shah, A.D. Impact of COVID-19 on Maintenance Peritoneal Dialysis Patients and Providers: A Review. Ther. Apher. Dial. 2023, 27, 607–620. [Google Scholar] [CrossRef]
- Baralić, M.; Robajac, D.; Penezić, A.; Brković, V.; Gligorijević, N.; Bontić, A.; Pavlović, J.; Nikolić, J.; Miljuš, G.; Dobrijević, Z.; et al. Significance of 1,25-Dihydroxyvitamin D3 on Overall Mortality in Peritoneal Dialysis Patients with COVID-19. Nutrients 2023, 15, 2050. [Google Scholar] [CrossRef]
- Chuengsaman, P.; Boongird, S.; Dandecha, P.; Hemachudha, T.; Nopsopon, T.; Kanjanabuch, T.; Sritippayawan, S.; Kantachuvesiri, S. Fatality Rate, Risk Factors, and Functional Decline in Peritoneal Dialysis Patients with Coronavirus Disease 2019: A Nationwide Cohort Study. Front. Med. 2022, 9, 1051448. [Google Scholar] [CrossRef]
- Polanco, E.; Aquey, M.; Collado, J.; Campos, E.; Guzman, J.; Cuevas-Budhart, M.A.; Divino-Filho, J.C.; Ramos-Sanchez, A. A COVID-19 Pandemic-Specific, Structured Care Process for Peritoneal Dialysis Patients Facilitated by Telemedicine: Therapy Continuity, Prevention, and Complications Management. Ther. Apher. Dial. 2021, 25, 970–978. [Google Scholar] [CrossRef]
- Baralić, M.; Laušević, M.; Ćujić, D.; Bontić, A.; Pavlović, J.; Brković, V.; Kezić, A.; Mihajlovski, K.; Hadži Tanović, L.; Assi Milošević, I.; et al. The Importance of Natural and Acquired Immunity to SARS-CoV-2 Infection in Patients on Peritoneal Dialysis. Vaccines 2024, 12, 135. [Google Scholar] [CrossRef]
- Alfano, G.; Fontana, F.; Giovanella, S.; Ferrari, A.; Perrone, R.; Mori, G.; Ligabue, G.; Cappelli, G. Prevalence, Clinical Course and Outcomes of COVID-19 in Peritoneal Dialysis (PD) Patients: A Single-Center Experience. Clin. Exp. Nephrol. 2023, 27, 171–178. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, M.; Cheng, Y.; Liu, J.; Feng, Z.; Ye, L. Antibody Response and Safety of COVID-19 Vaccine in Peritoneal Dialysis Patients. J. Infect. 2022, 85, e167–e171. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.F.; Bemelman, F.J.; Messchendorp, A.L.; Baan, C.C.; van Baarle, D.; van Binnendijk, R.; Diavatopoulos, D.A.; Frölke, S.C.; Geers, D.; GeurtsvanKessel, C.H.; et al. The RECOVAC Immune-Response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients with Chronic Kidney Disease, on Dialysis, or Living with a Kidney Transplant. Transplantation 2022, 106, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, N.; Bausch-Jurken, M. COVID-19 Vaccination Among Patients Receiving Maintenance Renal Replacement Therapy: Immune Response, Real-World Effectiveness, and Implications for the Future. J. Infect. Dis. 2023, 228 (Suppl. 1), S46–S54. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Abolhassani, H.; Du, L.; Piralla, A.; Bertoglio, F.; de Campos-Mata, L.; Wan, H.; Schubert, M.; Cassaniti, I.; Wang, Y.; et al. Heterologous Immunization with Inactivated Vaccine Followed by mRNA-Booster Elicits Strong Immunity against SARS-CoV-2 Omicron Variant. Nat. Commun. 2022, 13, 2670. [Google Scholar] [CrossRef]
- Babel, N.; Hugo, C.; Westhoff, T.H. Vaccination in Patients with Kidney Failure: Lessons from COVID-19. Nat. Rev. Nephrol. 2022, 18, 708–723. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). COVID-19 Vaccination Coverage and Vaccine Confidence by Dialysis Modality Among Patients Receiving In-Center Hemodialysis, Home Hemodialysis, and Peritoneal Dialysis—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 873–878. [Google Scholar] [CrossRef]
- Kotton, C. Immunization after kidney transplantation—What is necessary and what is safe? Nat. Rev. Nephrol. 2014, 10, 555–562. [Google Scholar] [CrossRef]

| Study/Author | Sample | Main Findings | Key Risk Factors | Vaccine Status | Outcome | Limitations |
|---|---|---|---|---|---|---|
| Candellier et al. (2020) [114] | 10 PD patients | No SARS-CoV-2 RNA detected in PD effluent | Not applicable | Not reported | PD not a likely route of viral transmission | Very small sample; early pandemic stage |
| Badrouchi et al. (2022) [116] | 127 unvaccinated PD patients | Incidence: 8.4 per 1000 pt-months. No significant differences in peritonitis, technique failure, or mortality | Male sex, diabetic nephropathy, glomerulonephritis | Unvaccinated | PD is safe and feasible during COVID-19 | Single-center; small sample size; unvaccinated cohort limits generalizability |
| Abrahams et al. (2023) [117] | 216 PD patients with COVID-19 | 3-month mortality: 40% (hospitalized), 37% (ICU); higher mortality than HD. 78% of survivors recovered fully | Hospitalization, ICU admission | Not reported | PD patients had worse outcomes than HD but good functional recovery in survivors | Observational; lack of detailed vaccine stratification |
| Kazancıoğlu et al. (2022) [118] | 18 PD patients (compared to 18 HD and 18 controls) | Mortality 22.2% in PD; composite outcome (ICU/death) higher in PD (33.3%) than HD and controls | Older age, elevated CRP | Not reported | PD group had worse short-term outcomes than HD and control group | Small sample; retrospective design; limited generalizability |
| Painter et al. (2023) [119] | Systematic review | PD reduces healthcare contact; feasible with telemedicine | Not specified | Varied | PD effective and manageable with telemedicine | Heterogeneity of data; inclusion of case reports/series may introduce bias |
| Baralić et al. (2023) [120] | PD patients with COVID-19 (unspecified N) | Vitamin D linked to better survival. Calcitriol had immunomodulatory benefits | Diabetes, altered fibrinogen glycosylation | Not reported | Vitamin D may improve outcomes | No precise sample size; observational findings; no control group |
| Chuengsaman et al. (2022) [121] | Multicenter registry | 28-day mortality = 13%. Vaccination lowered mortality by ~30% per dose | Functional impairment, Delta variant infection | Vaccinated/ unvaccinated compared | Vaccination improves survival | Registry-based; limited data on vaccination timing or comorbidity control |
| Baralić et al. (2024) [123] | 28 PD patients | Anti-SARS-CoV-2 IgG detected in peritoneal effluent; higher levels in vaccinated | None reported | Sinopharm ± mRNA booster | No reinfection in vaccinated; mild reinfections in unvaccinated | Small sample; single center; short follow-up |
| Alfano et al. (2023) [124] | 146 PD patients | COVID-19 incidence: 0.16/patient-year. Mortality: 22.2% (unvacc.), 0% (vacc.) | Vaccination status | Vaccinated vs. unvaccinated | Vaccination reduces severity and mortality | Single-center; retrospective; no randomized control group |
| Zheng et al. (2022) [125] | PD patients (unspecified N) | Vaccinated PD patients showed adequate antibody responses and fewer complications | Immunocompromised status | Vaccinated | Vaccines are effective in PD population | Sample size not specified; immune response only serologically assessed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baralić, M.; Stojanović, N.; Gajić, S.; Sič, A.; Manzar, A.; Bontić, A.; Pavlović, J.; Bojić, M.N.; Kezić, A. Kidney Involvement in SARS-CoV-2 Infection: Peritoneal Dialysis as the Preferred Modality. Vaccines 2025, 13, 723. https://doi.org/10.3390/vaccines13070723
Baralić M, Stojanović N, Gajić S, Sič A, Manzar A, Bontić A, Pavlović J, Bojić MN, Kezić A. Kidney Involvement in SARS-CoV-2 Infection: Peritoneal Dialysis as the Preferred Modality. Vaccines. 2025; 13(7):723. https://doi.org/10.3390/vaccines13070723
Chicago/Turabian StyleBaralić, Marko, Nikola Stojanović, Selena Gajić, Aleksandar Sič, Aarish Manzar, Ana Bontić, Jelena Pavlović, Mateja N. Bojić, and Aleksandra Kezić. 2025. "Kidney Involvement in SARS-CoV-2 Infection: Peritoneal Dialysis as the Preferred Modality" Vaccines 13, no. 7: 723. https://doi.org/10.3390/vaccines13070723
APA StyleBaralić, M., Stojanović, N., Gajić, S., Sič, A., Manzar, A., Bontić, A., Pavlović, J., Bojić, M. N., & Kezić, A. (2025). Kidney Involvement in SARS-CoV-2 Infection: Peritoneal Dialysis as the Preferred Modality. Vaccines, 13(7), 723. https://doi.org/10.3390/vaccines13070723










