Evaluation of Anti-HPV18 Antibody Titers Preceding an Incident Cervical HPV18/45 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cases and Controls
2.3. HPV Testing
2.4. IgG-Specific Anti-HPV18 L1 VLP-Based Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Serum Samples
2.6. Statistical Analysis
3. Results
3.1. Comparison Between Cases and Controls at the Pre-Infection Visit
3.2. Subgroup Analysis: A Longitudinal Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AYW | Adolescent and Young Adult Women |
| Abs | Antibodies |
| 2vHPV | Bivalent HPV Vaccine |
| ELISA | Enzyme-linked immunosorbent assay |
| HRC | High-Risk Control |
| HPV | Human Papillomavirus |
| IgG | Immunoglobin G |
| MSAHC | Mt. Sinai Adolescent Health Center |
| NAbs | Neutralizing antibodies |
| 4vHPV | Quadrivalent HPV vaccine |
| RC | Random Control |
| SBS | Sexual Risk Behavior Score |
| VLPs | Virus-Like Particles |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 11 July 2024).
- Markowitz, L.E.; Unger, E.R. Human papillomavirus vaccination. N. Engl. J. Med. 2023, 388, 1790–1798. [Google Scholar] [CrossRef]
- Drolet, M.; Benard, E.; Perez, N.; Brisson, M.; Group, H.P.V.V.I.S. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Stanley, M.; Joura, E.; Yen, G.P.; Kothari, S.; Luxembourg, A.; Saah, A.; Walia, A.; Perez, G.; Khoury, H.; Badgley, D.; et al. Systematic literature review of neutralizing antibody immune responses to non-vaccine targeted high-risk HPV types induced by the bivalent and the quadrivalent vaccines. Vaccine 2021, 39, 2214–2223. [Google Scholar] [CrossRef]
- Schiller, J.; Lowy, D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine 2018, 36 Pt A, 4768–4773. [Google Scholar] [CrossRef]
- Orumaa, M.; Lahlum, E.J.; Gulla, M.; Tota, J.E.; Nygård, M.; Nygård, S. Quadrivalent HPV Vaccine Effectiveness Against Cervical Intraepithelial Lesion Grade 2 or Worse in Norway: A Registry-Based Study of 0.9 Million Norwegian Women. J. Infect. Dis. 2024, 230, e1202–e1206. [Google Scholar] [CrossRef]
- Kjaer, S.K.; Nygård, M.; Sundström, K.; Dillner, J.; Tryggvadottir, L.; Munk, C.; Berger, S.; Enerly, E.; Hortlund, M.; Ágústsson, Á.I.; et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four nordic countries. eClinicalMedicine 2020, 23, 10041. [Google Scholar] [CrossRef]
- Li, C.; Hall, T.G.; Hall, J.J.; He, W.Q. Effectiveness of quadrivalent HPV vaccination in reducing vaccine-type and nonvaccine-type high risk HPV infection. Epidemiol. Infect. 2023, 151, e37. [Google Scholar] [CrossRef]
- Garland, S.M.; Kjaer, S.K.; Muñoz, N.; Block, S.L.; Brown, D.R.; DiNubile, M.J.; Lindsay, B.R.; Kuter, B.J.; Perez, G.; Dominiak-Felden, G.; et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Clin. Infect. Dis. 2016, 63, 519–527. [Google Scholar] [CrossRef]
- Lehtinen, M.; van Damme, P.; Beddows, S.; Pinto, L.A.; Mariz, F.; Gray, P.; Dillner, J. Scientific approaches to defining HPV vaccine-induced protective immunity. Int. J. Cancer 2025, 156, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Roden, R.B.S.; Stern, P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 2018, 18, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, S.E. Human papillomavirus (HPV) vaccines in adults: Learnings from long-term follow-up of quadrivalent HPV vaccine clinical trials. Hum. Vaccines Immunother. 2023, 19, 2184760. [Google Scholar] [CrossRef]
- Whitworth, H.S.; Gallagher, K.E.; Howard, N.; Mounier-Jack, S.; Mbwanji, G.; Kreimer, A.R.; Basu, P.; Kelly, H.; Drolet, M.; Brisson, M.; et al. Efficacy and immunogenicity of a single dose of human papillomavirus vaccine compared to no vaccination or standard three and two-dose vaccination regimens: A systematic review of evidence from clinical trials. Vaccine 2020, 38, 1302–1314. [Google Scholar] [CrossRef]
- Quang, C.; Chung, A.W.; Frazer, I.H.; Toh, Z.Q.; Licciardi, P.V. Single-dose HPV vaccine immunity: Is there a role for non-neutralizing antibodies? Trends Immunol. 2022, 43, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, F.; Mantelli, B.; Gallerani, E.; Telatin, V.; Bonazzi, I.; Marconi, P.; Gavioli, R.; Gabrielli, L.; Lazzarotto, T.; Barzon, L.; et al. HPV-Specific Systemic Antibody Responses and Memory B Cells are Independently Maintained up to 6 Years and in a Vaccine-Specific Manner Following Immunization with Cervarix and Gardasil in Adolescent and Young Adult Women in Vaccination Programs in Italy. Vaccines 2020, 8, 26. [Google Scholar] [CrossRef]
- Gradissimo, A.; Shankar, V.; Wiek, F.; St Peter, L.; Studentsov, Y.; Nucci-Sack, A.; Diaz, A.; Pickering, S.; Schlecht, N.F.; Burk, R.D. Anti-HPV16 Antibody Titers Prior to an Incident Cervical HPV16/31 Infection. Viruses 2021, 13, 1548. [Google Scholar] [CrossRef]
- Schlecht, N.F.; Diaz, A.; Nucci-Sack, A.; Shyhalla, K.; Shankar, V.; Guillot, M.; Hollman, D.; Strickler, H.D.; Burk, R.D. Incidence and Types of Human Papillomavirus Infections in Adolescent Girls and Young Women Immunized with the Human Papillomavirus Vaccine. JAMA Netw. Open 2021, 4, e2121893. [Google Scholar] [CrossRef]
- Schlecht, N.F.; Burk, R.D.; Nucci-Sack, A.; Shankar, V.; Peake, K.; Lorde-Rollins, E.; Porter, R.; Linares, L.O.; Rojas, M.; Strickler, H.D.; et al. Cervical, anal and oral HPV in an adolescent inner-city health clinic providing free vaccinations. PLoS ONE 2012, 7, e37419. [Google Scholar] [CrossRef]
- Braun-Courville, D.K.; Schlecht, N.F.; Burk, R.D.; Strickler, H.D.; Rojas, M.; Lorde-Rollins, E.; Nucci-Sack, A.; Hollman, D.; Linares, L.O.; Diaz, A. Strategies for conducting adolescent health research in the clinical setting: The Mount Sinai Adolescent Health Center HPV experience. J. Pediatr. Adolesc. Gynecol. 2014, 27, e103–e108. [Google Scholar] [CrossRef]
- Schlecht, N.F.; Diaz, A.; Shankar, V.; Szporn, A.H.; Wu, M.; Nucci-Sack, A.; Peake, K.; Strickler, H.D.; Burk, R.D. Risk of Delayed Human Papillomavirus Vaccination in Inner-City Adolescent Women. J. Infect. Dis. 2016, 214, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.H.; Basu, P.; Bender, N.; Herrero, R.; Kemp, T.J.; Kreimer, A.R.; Muller, M.; Panicker, G.; Pawlita, M.; Pinto, L.A.; et al. Evaluation of serological assays to monitor antibody responses to single-dose HPV vaccines. Vaccine 2020, 38, 5997–6006. [Google Scholar] [CrossRef]
- Buck, C.B.; Thompson, C.D. Production of papillomavirus-based gene transfer vectors. Curr. Protoc. Cell Biol. 2007, 37, 26.1.1–26.1.19, Unit 26.21. [Google Scholar] [CrossRef]
- Herrin, D.M.; Coates, E.E.; Costner, P.J.; Kemp, T.J.; Nason, M.C.; Saharia, K.K.; Yuanji, P.; Sarwar, U.N.; Lasonji, H.; Galina, Y.; et al. Comparison of adaptive and innate immune responses induced by licensed vaccines for human papillomavirus. Hum. Vaccines Immunother. 2014, 10, 3446–3454. [Google Scholar] [CrossRef]
- Studentsov, Y.Y.; Ho, G.Y.; Marks, M.A.; Bierman, R.; Burk, R.D. Polymer-based enzyme-linked immunosorbent assay using human papillomavirus type 16 (HPV16) virus-like particles detects HPV16 clade-specific serologic responses. J. Clin. Microbiol. 2003, 41, 2827–2834. [Google Scholar] [CrossRef]
- Chen, Z.; DeSalle, R.; Schiffman, M.; Herrero, R.; Burk, R.D. Evolutionary dynamics of variant genomes of human papillomavirus types 18, 45, and 97. J. Virol. 2009, 83, 1443–1455. [Google Scholar] [CrossRef]
- Pinheiro, M.; Wentzensen, N.; Dean, M.; Yeager, M.; Chen, Z.; Shastry, A.; Boland, J.F.; Bass, S.; Burdett, L.; Lorey, T.; et al. Somatic mutations in 3929 HPV positive cervical cells associated with infection outcome and HPV type. Nat. Commun. 2024, 15, 7895. [Google Scholar] [CrossRef]
- Smith, J.F.; Michelle, B.; Martha, B.; Rose, K.; Esser, M.T.; Wanda, R.; Eliav, B.; Brown, D.R.; Bryan, J.T. Antibodies from Women Immunized with Gardasil ® Cross-Neutralize HPV 45 Pseudovirions. Hum. Vaccines 2007, 3, 109–115. [Google Scholar] [CrossRef]
- Pinto, L.A.; Dillner, J.; Beddows, S.; Unger, E.R. Immunogenicity of HPV prophylactic vaccines: Serology assays and their use in HPV vaccine evaluation and development. Vaccine 2018, 36 Pt A, 4792–4799. [Google Scholar] [CrossRef]
- Brown, D.R.; Joura, E.A.; Yen, G.P.; Kothari, S.; Luxembourg, A.; Saah, A.; Walia, A.; Perez, G.; Khoury, H.; Badgley, D.; et al. Systematic literature review of cross-protective effect of HPV vaccines based on data from randomized clinical trials and real-world evidence. Vaccine 2021, 39, 2224–2236. [Google Scholar] [CrossRef]
- Mariz, F.C.; Bender, N.; Anantharaman, D.; Basu, P.; Bhatla, N.; Pillai, M.R.; Prabhu, P.R.; Sankaranarayanan, R.; Eriksson, T.; Pawlita, M.; et al. Peak neutralizing and cross-neutralizing antibody levels to human papillomavirus types 6/16/18/31/33/45/52/58 induced by bivalent and quadrivalent HPV vaccines. NPJ Vaccines 2020, 5, 14. [Google Scholar] [CrossRef]
- Mariz, F.C.; Gray, P.; Bender, N.; Eriksson, T.; Kann, H.; Apter, D.; Paavonen, J.; Pajunen, E.; Prager, K.M.; Sehr, P.; et al. Sustainability of neutralising antibodies induced by bivalent or quadrivalent HPV vaccines and correlation with efficacy: A combined follow-up analysis of data from two randomised, double-blind, multicentre, phase 3 trials. Lancet Infect. Dis. 2021, 21, 1458–1468. [Google Scholar] [CrossRef]
- Safaeian, M.; Kemp, T.J.; Pan, D.Y.; Porras, C.; Rodriguez, A.C.; Schiffman, M.; Cortes, B.; Katki, H.; Wacholder, S.; Schiller, J.T.; et al. Cross-protective vaccine efficacy of the bivalent HPV vaccine against HPV31 is associated with humoral immune responses: Results from the Costa Rica Vaccine Trial. Hum. Vaccin. Immunother. 2013, 9, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Jung, W.; Linde, C.; Coates, E.; Ledgerwood, J.; Costner, P.; Yamshchikov, G.; Streeck, H.; Juelg, B.; Lauffenburger, D.A.; et al. Differences in HPV-specific antibody Fc-effector functions following Gardasil® and Cervarix® vaccination. npj Vaccines 2023, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.; Bissett, S.L.; Howell-Jones, R.; Edwards, D.; Munslow, G.; Soldan, K.; Beddows, S. Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera. Vaccine 2011, 29, 8585–8590. [Google Scholar] [CrossRef]
- Longet, S.; Schiller, J.T.; Bobst, M.; Jichlinski, P.; Nardelli-Haefliger, D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J. Virol. 2011, 85, 13253–13259. [Google Scholar] [CrossRef] [PubMed]
- Hoes, J.; Pasmans, H.; Knol, M.J.; Donken, R.; van Marm-Wattimena, N.; Schepp, R.M.; King, A.J.; van der Klis, F.R.M.; de Melker, H.E. Persisting Antibody Response 9 Years After Bivalent Human Papillomavirus (HPV) Vaccination in a Cohort of Dutch Women: Immune Response and the Relation to Genital HPV Infections. J. Infect. Dis. 2020, 221, 1884–1894. [Google Scholar] [CrossRef]
- Ryser, M.D.; Rositch, A.; Gravitt, P.E. Modeling of US Human Papillomavirus (HPV) Seroprevalence by Age and Sexual Behavior Indicates an Increasing Trend of HPV Infection Following the Sexual Revolution. J. Infect. Dis. 2017, 216, 604–611. [Google Scholar] [CrossRef]
- Del Pino, M.; Vorsters, A.; Joura, E.A.; Doorbar, J.; Haniszewski, M.; Gudina, I.A.; Kodjamanova, P.; Velicer, C.; Drury, R. Risk factors for human papillomavirus infection and disease: A targeted literature summary. J. Med. Virol. 2024, 96, e29420. [Google Scholar] [CrossRef]
- Pauli, S.; Kops, N.L.; Bessel, M.; Lina Villa, L.; Moreno Alves Souza, F.; Mendes Pereira, G.F.; Neves Hugo, F.; Comerlato, J.; Bandeira, I.; Fernandes, B.; et al. Sexual practices and HPV infection in unvaccinated young adults. Sci. Rep. 2022, 12, 12385. [Google Scholar] [CrossRef]
- Garland, S.M.; Brotherton, J.M.L.; Moscicki, A.B.; Kaufmann, A.M.; Stanley, M.; Bhatla, N.; Sankaranarayanan, R.; de Sanjosé, S.; Palefsky, J.M. HPV vaccination of immunocompromised hosts. Papillomavirus Res. 2017, 4, 35–38. [Google Scholar] [CrossRef]
- Waheed, D.-e.-N.; Burdier, F.R.; Eklund, C.; Baussano, I.; Mariz, F.C.; Téblick, L.; Mugo, N.; Watson-Jones, D.; Stanley, M.; Baay, M.; et al. An update on one-dose HPV vaccine studies, immunobridging and humoral immune responses—A meeting report. Prev. Med. Rep. 2023, 35, 102368. [Google Scholar] [CrossRef]
- De Toni, L.; Muscianisi, F.; Corsini, C.; Ghezzi, M.; Di Nisio, A.; Foresta, C.; Garolla, A. Serum Anti-HPV Antibody Titer as a Marker of Vaccine Effectiveness in Males with Genital Infection. Vaccines 2020, 8, 743. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.M.; Cornall, A.M.; Brotherton, J.M.L.; Wark, J.D.; Malloy, M.J.; Tabrizi, S.N.; on Behalf of the VACCINE Study Group. Final analysis of a study assessing genital human papillomavirus genoprevalence in young Australian women, following eight years of a national vaccination program. Vaccine 2018, 36, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Saccucci, M.; Franco, E.L.; Ding, L.; Bernstein, D.I.; Brown, D.; Kahn, J.A. Non-Vaccine-Type Human Papillomavirus Prevalence After Vaccine Introduction: No Evidence for Type Replacement but Evidence for Cross-Protection. Sex. Transm. Dis. 2018, 45, 260–265. [Google Scholar] [CrossRef] [PubMed]
| Variables | Cases (n = 37) | Random Controls (n = 37) | High-Risk Controls (n = 37) | p-Value |
|---|---|---|---|---|
| Age at Study Enrollment Mean (years ± SD) | 17.76 ± 1.56 | 17.94 ± 1.30 | 17.79 ± 1.54 | 0.8550 |
| Age at Coitarche Mean (years ± SD) | 14.57 ± 1.21 | 15.05 ± 1.51 | 13.97 ± 1.83 | 0.0124 |
| Age at First 4vHPV Dose Mean (years ± SD) | 14.18 ± 2.23 | 14.70 ± 2.23 | 14.65 ± 2.10 | 0.9648 |
| Race n (%) | ||||
| Hispanic | 19 (51.35) | 22 (59.46) | 21 (56.76) | 0.6264 |
| African American | 16 (43.24) | 13 (35.14) | 16 (43.24) | |
| Other | 2 (5.41) | 2 (5.41) | 0 (0) | |
| Lifetime Number of Partners n (%) | ||||
| 1 | 0 (0) | 3 (8.11) | 0 (0) | 0.0027 |
| 2 | 4 (10.81) | 7 (18.92) | 0 (0) | |
| 3 | 12 (32.43) | 8 (21.62) | 6 (16.22) | |
| 4+ | 21 (56.76) | 19 (51.35) | 31 (83.78) | |
| Number of Past Partners (6 months) n (%) | ||||
| 0 | 2 (5.41) | 1 (2.70) | 0 (0) | 0.0254 |
| 1 | 23 (62.16) | 23 (62.16) | 15 (40.54) | |
| 2+ | 12 (32.43) | 13 (35.14) | 22 (59.46) | |
| Chlamydia Trachomatis n (%) | ||||
| Yes | 21 (56.76) | 20 (54.05) | 32 (86.49) | 0.8073 |
| Any Pregnancy n (%) | ||||
| Yes | 17 (45.95) | 12 (32.43) | 17 (45.95) | 0.3986 |
| Emergency Contraception Ever n (%) | ||||
| Yes | 30 (81.08) | 26 (70.27) | 37 (100) | 0.0022 |
| Anal Sex Ever n (%) | ||||
| Yes | 18 (48.65) | 15 (40.54) | 35 (94.59) | <0.0001 |
| Lifetime Number of Anal Sex Partners n (%) | ||||
| 0 | 19 (51.35) | 22 (59.46) | 2 (5.41) | <0.0001 |
| 1 | 12 (32.43) | 6 (16.22) | 13 (35.14) | |
| 2+ | 6 (16.22) | 9 (24.32) | 22 (59.46) | |
| Sexual Risk Behavior Score Mean ± SD | 7.70 ± 2.31 | 7.11 ± 2.60 | 10.30 ± 1.10 | <0.0001 |
| First Vaccine Dose Before Coitarche n (%) | ||||
| Yes | 19 (51.35) | 17 (45.95) | 7 (18.92) | 0.0094 |
| Follow-up Time (years) | ||||
| Median (Min-Max) | 2.86 (0.60–8.28) | 6.26 (1.71–10.00) | 6.16 (0.84–8.87) | 0.0017 |
| Time since last 4vHPV Dose to pre-infection visit Mean (years ± SD) | 3.6 ± 2.5 | 4.4 ± 2.5 | 4.5 ± 3.5 | 0.3111 |
| Anti-HPV18 Antibody Titer at pre-infection visit, Median | 1144.74 | 1793.35 | 2729.56 | |
| (Min-Max) | (102.09–26,039.36) | (48.82–19,952.51) | (207.58–34,589.68) | <0.0001 |
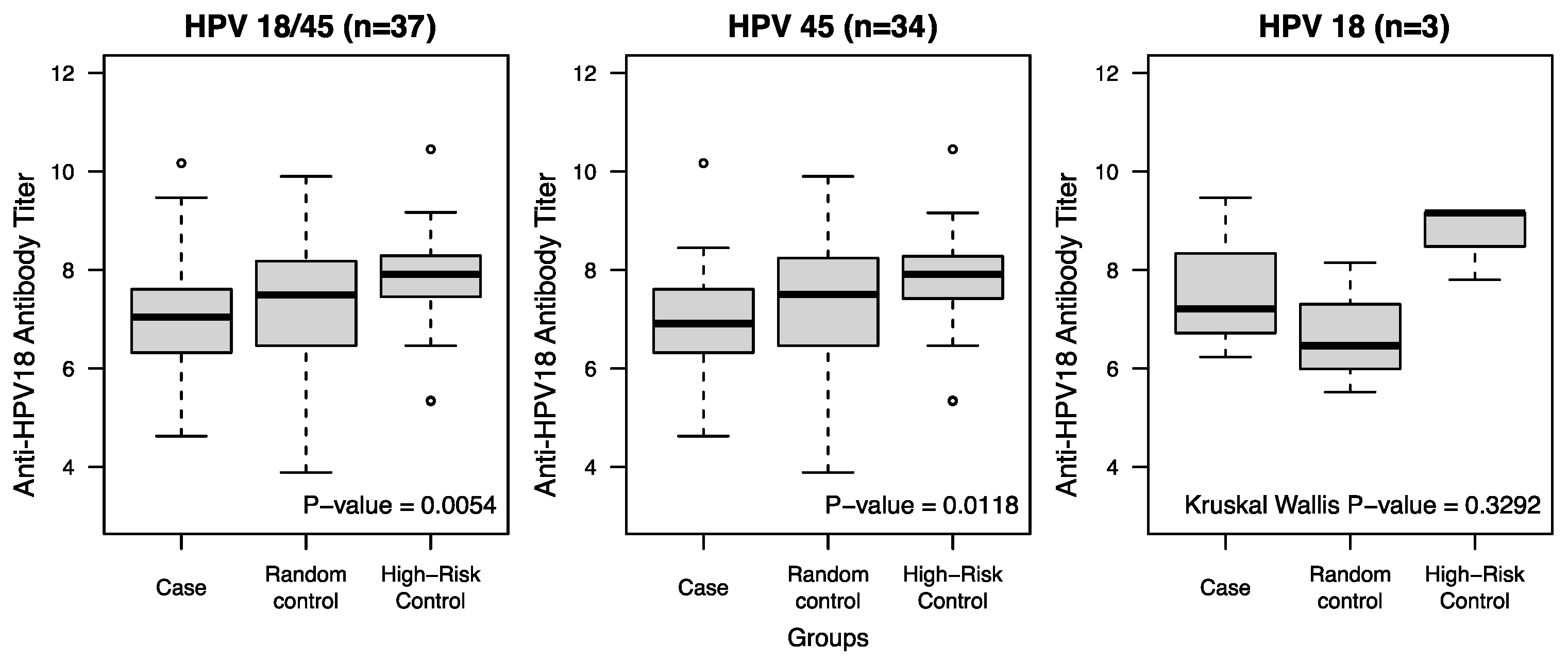
| Log Anti-HPV18 Antibody Titer at pre-infection visit Mean ± SD | 7.01 ± 1.14 | 7.24 ± 1.38 | 7.88 ± 0.98 | 0.0054 |
| Log Anti-HPV18 Antibody Titer at pre-infection visit Mean ± SD | ||||
| Case vs. Random Control | 7.01 ± 1.14 | 7.24 ± 1.38 | - | 0.2487 |
| Log Anti-HPV18 Antibody Titer at pre-infection visit Mean ± SD | ||||
| Case vs. High-Risk Control | 7.01 ± 1.14 | - | 7.88 ± 0.98 | 0.0007 |
| HPV18/45 (n = 37) | HPV45 Only (n = 34) | |||||
|---|---|---|---|---|---|---|
| OR (95% CI) § | p-Value | FDR p-Value | OR (95% CI) § | p-Value | FDR p-Value | |
| Controls (RC + HRC) vs. Cases | 1.37 (0.93–2.02) | 0.1086 | 0.1629 | 1.52 (0.98–2.38) | 0.0647 | 0.1362 |
| RC vs. Cases | 1.18 (0.40–4.17) | 0.4459 | 0.4454 | 1.33 (0.82–2.16) | 0.2453 | 0.2453 |
| HRC vs. Cases | 1.66 (0.96–2.85) | 0.0673 | 0.1629 | 1.60 (0.93–2.77) | 0.0908 | 0.1362 |
| HPV16/18/31/45 (n = 80) | HPV16/18 Only (n = 29) | HPV 31/45 Only (n = 51) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) § | p-Value | FDR p-Value | OR (95% CI) § | p-Value | FDR p-Value | OR (95% CI) § | p-Value | FDR p-Value | |
| Controls (RC + HRC) vs. Cases | 1.40 (1.09, 1.79) | 0.0090 | 0.0135 | 1.18 (0.81, 1.72) | 0.3782 | 0.5673 | 1.59 (1.12, 2.24) | 0.0089 | 0.0206 |
| RC vs. Cases | 1.22 (0.93, 1.60) | 0.1468 | 0.1468 | 1.02 (0.67, 1.54) | 0.9348 | 0.9348 | 1.39 (0.96, 2.01) | 0.0838 | 0.0838 |
| HRC vs. Cases | 1.73 (1.34, 2.52) | 0.0039 | 0.0117 | 1.71 (0.87, 3.38) | 0.1205 | 0.3615 | 1.78 (1.13, 2.80) | 0.0137 | 0.0206 |
| Variables | Pre-Infection (n = 37) Mean ± SD | Peri-Infection (n = 37) * Mean ± SD | Post-Infection (n = 25) Mean ± SD |
|---|---|---|---|
| Log Anti-HPV Antibody Titers | 7.01 ± 1.14 | 7.00 ± 1.30 | 7.02 ± 1.15 |
| Duration of Time from Last Dose (years) | 3.57 ± 2.54 | 4.82 ± 2.66 | 6.00 ± 2.59 |
| Serum Collection Time Relative to Infection (years) | −1.25 ± 0.78 | 0 ± 0 | 1.53 ± 0.67 |
| Variables | β (SE) | p-Value |
|---|---|---|
| Intercept (mean log anti-HPV18 antibody titers at pre-infection) | 7.10 (0.20) | <0.0001 |
| Time (change from pre- to peri-infection) | 0.095 (0.11) | 0.3796 |
| Time+ (additional change peri- to post-infection) | −0.14 (0.18) | 0.4450 |
| Random Effects | ||
| Variance (random intercept) | 0.92 (0.27) | 0.0003 |
| Variance (residual) | 0.38 (0.09) | <0.0001 |
| SE = standard error | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiek, F.; Shankar, V.; Gradissimo, A.; Diaz, A.; Pinto, L.A.; Schlecht, N.F.; Burk, R.D. Evaluation of Anti-HPV18 Antibody Titers Preceding an Incident Cervical HPV18/45 Infection. Vaccines 2025, 13, 722. https://doi.org/10.3390/vaccines13070722
Wiek F, Shankar V, Gradissimo A, Diaz A, Pinto LA, Schlecht NF, Burk RD. Evaluation of Anti-HPV18 Antibody Titers Preceding an Incident Cervical HPV18/45 Infection. Vaccines. 2025; 13(7):722. https://doi.org/10.3390/vaccines13070722
Chicago/Turabian StyleWiek, Fanua, Viswanathan Shankar, Ana Gradissimo, Angela Diaz, Ligia A. Pinto, Nicolas F. Schlecht, and Robert D. Burk. 2025. "Evaluation of Anti-HPV18 Antibody Titers Preceding an Incident Cervical HPV18/45 Infection" Vaccines 13, no. 7: 722. https://doi.org/10.3390/vaccines13070722
APA StyleWiek, F., Shankar, V., Gradissimo, A., Diaz, A., Pinto, L. A., Schlecht, N. F., & Burk, R. D. (2025). Evaluation of Anti-HPV18 Antibody Titers Preceding an Incident Cervical HPV18/45 Infection. Vaccines, 13(7), 722. https://doi.org/10.3390/vaccines13070722







