Adverse Events and Associated Economic Burden of COVID-19 Vaccination in Queensland, Australia: Findings from the Cross-Sectional QoVAX-Statewide Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Participants
2.2. Data Collection and Outcomes
2.3. Data Analysis
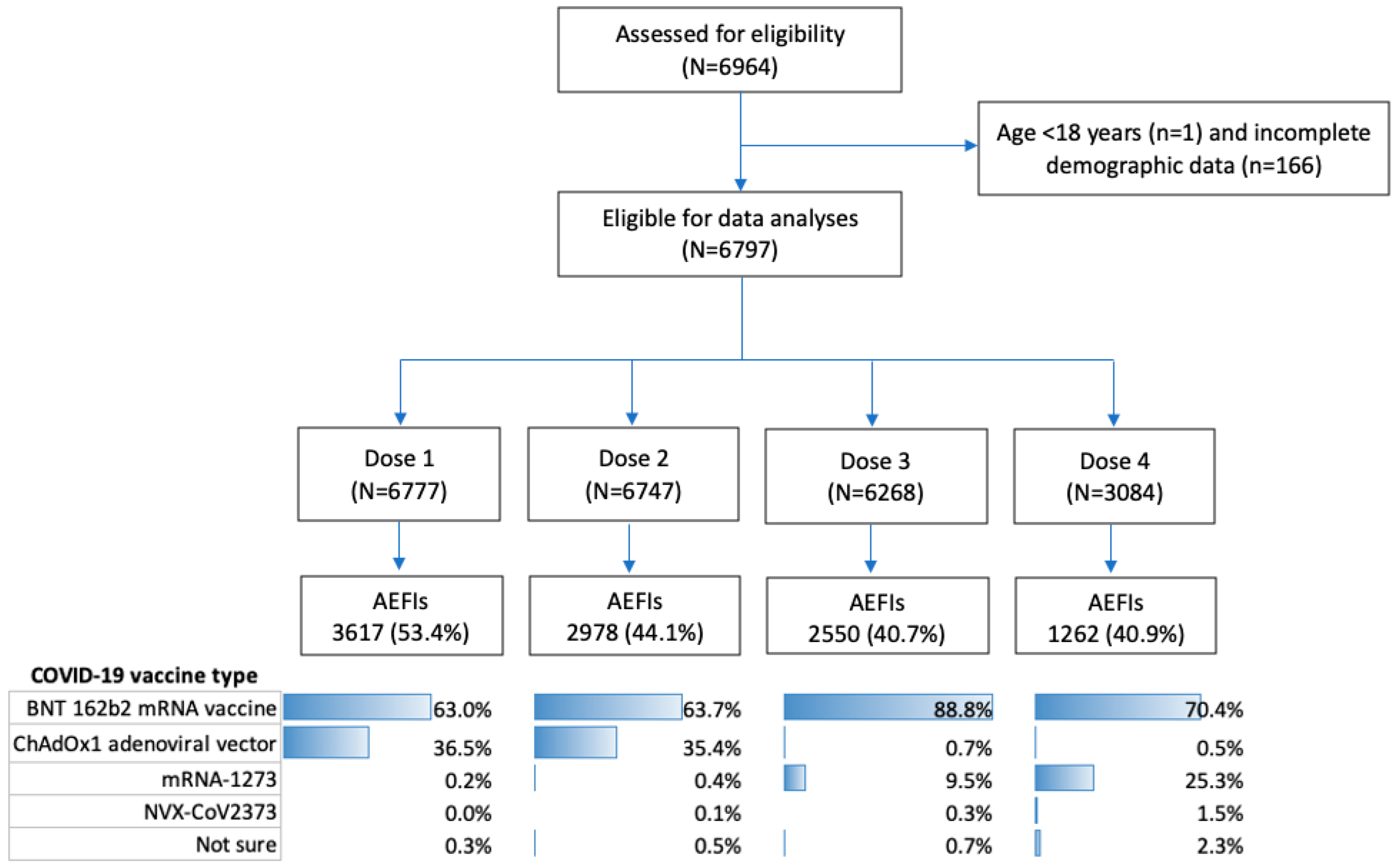
3. Results
3.1. Participant Demographics and Characteristics
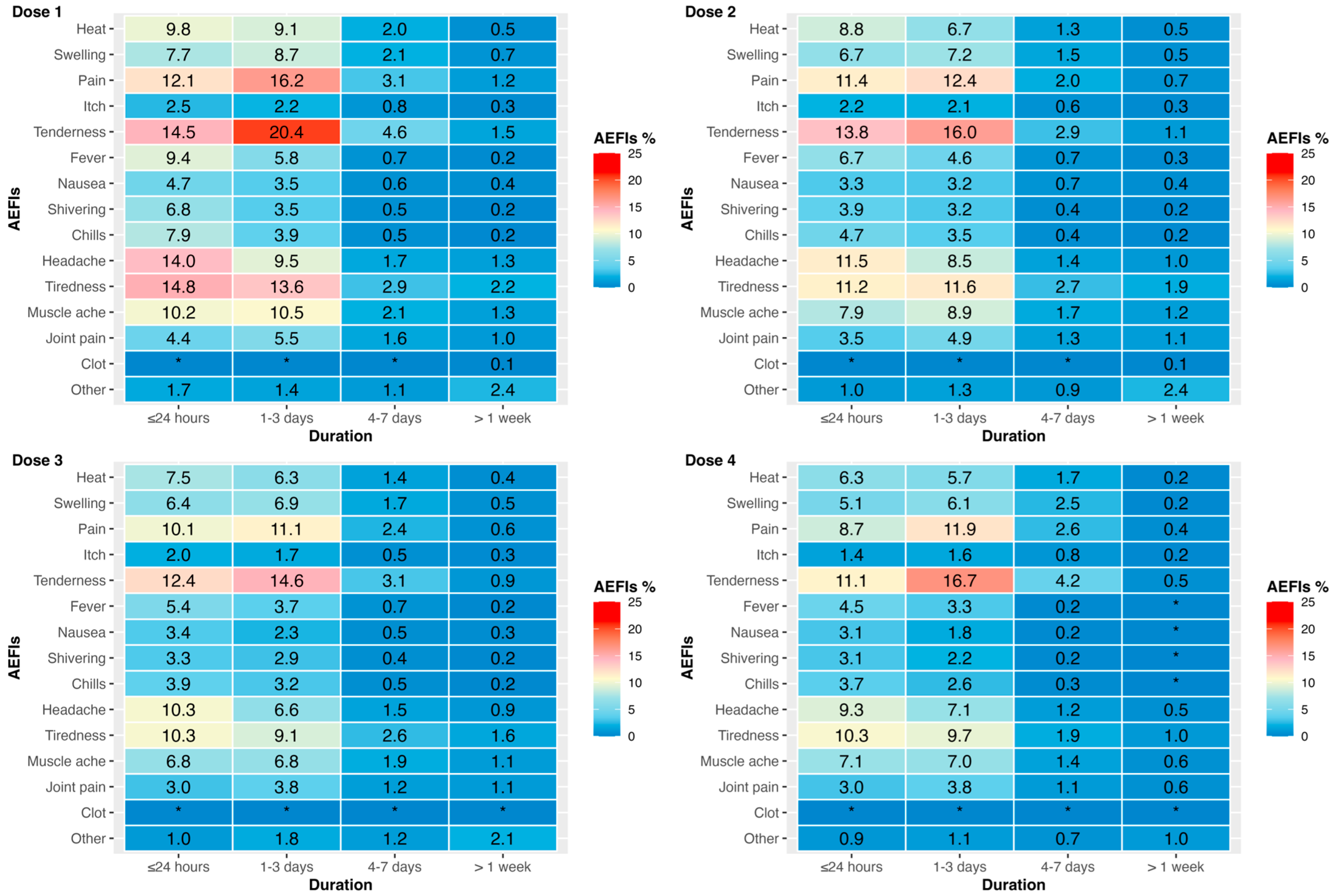
3.2. Vaccine Type and AEFIs by Dose
3.3. Costs of AEFIs
4. Discussion
4.1. Overall Summary of Findings
4.2. Integration with Existing Literature
4.3. Implications for Policy/Practice
4.4. Future Directions
4.5. Strengths/Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Australian National Audit Office (ANAO). Australia’s COVID-19 Vaccine Rollout. Available online: https://www.anao.gov.au/work/performance-audit/australia-covid-19-vaccine-rollout (accessed on 6 June 2024).
- Bhandari, B.; Rayamajhi, G.; Lamichhane, P.; Shenoy, A.K. Adverse Events following Immunization with COVID-19 Vaccines: A Narrative Review. Biomed Res. Int. 2022, 2022, 2911333. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Glover, C.; Deng, L.; Larter, C.; Brogan, C.; Richardson, O.; Huang, Y.; Kay, E.; Macartney, K.; Wood, N. Surveillance of adverse effects following immunisation in Australia, COVID-19 Vaccines, 2021. Commun. Dis. Intell. 2024, 48, 38926650. [Google Scholar] [CrossRef]
- Piechotta, V.; Siemens, W.; Thielemann, I.; Toews, M.; Koch, J.; Vygen-Bonnet, S.; Kothari, K.; Grummich, K.; Braun, C.; Kapp, P.; et al. Safety and effectiveness of vaccines against COVID-19 in children aged 5–11 years: A systematic review and meta-analysis. Lancet Child Adolesc. Health 2023, 7, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Beladiya, J.; Kumar, A.; Vasava, Y.; Parmar, K.; Patel, D.; Patel, S.; Dholakia, S.; Sheth, D.; Boddu, S.H.S.; Patel, C. Safety and efficacy of COVID-19 vaccines: A systematic review and meta-analysis of controlled and randomized clinical trials. Rev. Med. Virol. 2024, 34, e2507. [Google Scholar] [CrossRef] [PubMed]
- Nohl, A.; Brune, B.; Weichert, V.; Standl, F.; Stang, A.; Dudda, M. COVID-19: Vaccination Side Effects and Sick Leave in Frontline Healthcare-Workers-A Web-Based Survey in Germany. Vaccines 2022, 10, 411. [Google Scholar] [CrossRef]
- Reusch, J.; Wagenhäuser, I.; Gabel, A.; Höhn, A.; Lâm, T.T.; Krone, L.B.; Frey, A.; Schubert-Unkmeir, A.; Dölken, L.; Frantz, S.; et al. Inability to work following COVID-19 vaccination-a relevant aspect for future booster vaccinations. Public Health 2023, 222, 186–195. [Google Scholar] [CrossRef]
- Bauernfeind, S.; Huppertz, G.; Mueller, K.; Hitzenbichler, F.; Hardmann, L.; Pemmerl, S.; Hollnberger, H.; Sieber, W.; Wettstein, M.; Seeliger, S.; et al. Health Care Workers’ Sick Leave due to COVID-19 Vaccination in Context with SARS-CoV-2 Infection and Quarantine-A Multicenter Cross-Sectional Survey. Open Forum Infect. Dis. 2022, 9, ofac203. [Google Scholar] [CrossRef]
- Schianchi, A.; Ughi, N.; Cassano, G.; Del Gaudio, F.; Dicuonzo, A.; Scaglione, F.; Alberti, P.M.; Rossetti, C.; Micheloni, G.; Zoppini, L.; et al. Sick leave request following anti-COVID-19 vaccine administration is low among healthcare workers: Results from a retrospective cross-sectional monocentric study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7218–7222. [Google Scholar] [CrossRef]
- Deng, L.; Glover, C.; Dymock, M.; Pillsbury, A.; Marsh, J.A.; Quinn, H.E.; Leeb, A.; Cashman, P.; Snelling, T.L.; Wood, N.; et al. The short term safety of COVID-19 vaccines in Australia: AusVaxSafety active surveillance, February–August 2021. Med. J. Aust. 2022, 217, 195–202. [Google Scholar] [CrossRef]
- Hamilton, E.; Oversby, S.; Kitchener, S.; Ratsch, A. Post COVID-19 vaccination: AusVaxSafety survey participation and adverse events—A community-based regional Queensland study. Aust. N. Z. J. Public Health 2022, 46, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Davies, J. QoVAX Statewide: Long-Term COVID-19 Study in Adults Aged 18 Years and Older Living in Queensland, Australia; Australian New Zealand Clinical Trials Registry (ANZCTR); 2021. Available online: https://www.anzctr.org.au/ (accessed on 15 June 2024).
- Davies, J.P.; Gregory, R.; Hung, J.; Choy, B. QoVAX Statewide: Long-Term COVID-19 Study in Adults Aged 18 Years and Older Living in Queensland, Australia. 2023. Available online: https://dataverse.qcif.edu.au/dataset.xhtml?persistentId=doi:10.60540/XYC4ZN (accessed on 15 June 2024).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Nissen, M. QoVAX SET Statewide Long-Term COVID-19 Study in Adults Aged 18 Years and Older Living in Queensland, Australia (Trial ID: ACTRN12622000020785). Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=383334&isReview=true (accessed on 10 April 2024).
- WHO Headquarters (HQ). Population-Based Age-Stratified Seroepidemiological Investigation Protocol for Coronavirus 2019 (COVID-19) Infection. Version: 2.0. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Seroepidemiology-2020.2 (accessed on 1 February 2024).
- McCaffrey, N.; Kaambwa, B.; Currow, D.C.; Ratcliffe, J. Health-related quality of life measured using the EQ-5D–5L: South Australian population norms. Health Qual. Life Outcomes 2016, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.; Mulhern, B.; Lancsar, E.; Lorgelly, P.; Ratcliffe, J.; Street, D.; Viney, R. The Use of a Discrete Choice Experiment Including Both Duration and Dead for the Development of an EQ-5D-5L Value Set for Australia. Pharmacoeconomics 2023, 41, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Australian Government. MBS Online. Available online: https://www9.health.gov.au/mbs/search.cfm (accessed on 1 June 2024).
- Independent Health and Aged Care Pricing Authority. National Efficient Price Determination 2022–2023. Available online: https://www.ihacpa.gov.au/resources/national-efficient-price-determination-2022-23 (accessed on 1 September 2023).
- Queensland Government. Queensland Ambulance Service. 2022. Available online: https://www.ambulance.qld.gov.au/__data/assets/pdf_file/0028/234487/2021-22-q4-ppi.pdf (accessed on 16 August 2023).
- Queensland Government. Wage Rates—Nursing Stream. Available online: https://www.health.qld.gov.au/hrpolicies/wage-rates/nursing#2022 (accessed on 16 April 2024).
- Krol, M.; Brouwer, W.; Rutten, F. Productivity costs in economic evaluations: Past, present, future. Pharmacoeconomics 2013, 31, 537–549. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Average Weekly Earnings, Australia—Estimates of Weekly Earnings Classified by Industry, Sector, State and Territory. Available online: https://www.abs.gov.au/statistics/labour/earnings-and-working-conditions/average-weekly-earnings-australia/nov-2022 (accessed on 16 August 2023).
- Australian Bureau of Statistics. National, State and Territory Population. Statistics About the Population and Components of Change (Births, Deaths, Migration) for Australia and Its States and Territories. Available online: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release#states-and-territories (accessed on 25 April 2024).
- Kaur, R.J.; Dutta, S.; Bhardwaj, P.; Charan, J.; Dhingra, S.; Mitra, P.; Singh, K.; Yadav, D.; Sharma, P.; Misra, S. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J. Clin. Biochem. 2021, 36, 427–439. [Google Scholar] [CrossRef]
- Kouhpayeh, H.; Ansari, H. Adverse events following COVID-19 vaccination: A systematic review and meta-analysis. Int. Immunopharmacol. 2022, 109, 108906. [Google Scholar] [CrossRef]
- Chen, J.; Cai, Y.; Chen, Y.; Williams, A.P.; Gao, Y.; Zeng, J. Nervous and Muscular Adverse Events after COVID-19 Vaccination: A Systematic Review and Meta-Analysis of Clinical Trials. Vaccines 2021, 9, 939. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.H.; Turner, D.L.; Turner, R.J. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef]
- Xu, W.; Ren, W.; Wu, T.; Wang, Q.; Luo, M.; Yi, Y.; Li, J. Real-World Safety of COVID-19 mRNA Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2023, 11, 1118. [Google Scholar] [CrossRef] [PubMed]
- Soiza, R.L.; Scicluna, C.; Thomson, E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 2021, 50, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Smoll, N.R.; Al Imam, M.H.; Schulz, C.; Booy, R.; Khandaker, G. The effectiveness of vaccination for preventing hospitalisation with COVID-19 in regional Queensland: A data linkage study. Med. J. Aust. 2023, 219, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Australian National Accounts: State Accounts. Available online: https://www.abs.gov.au/statistics/economy/national-accounts/australian-national-accounts-state-accounts/2022-23-financial-year (accessed on 15 July 2023).
- Queensland Government. Expenditure—The Greatest Proportion of Spending in the Health Expenditure Was for Hospitals. Available online: https://www.choreport.health.qld.gov.au/our-investment/expenditure#:~:text=In%202022%E2%80%9323%2C%20%2452.463%20billion,with%20%249%2C597%20per%20person%20nationally (accessed on 15 July 2023).
- Australian Technical Advisory Group on Immunisation (ATAGI). ATAGI Targeted Review 2023: Vaccination for Prevention of Influenza in Australia; ATAGI: Canberra ACT, Australia, 2024. [Google Scholar]
- Fifer, S.; Toh, L.; Yu, D.; Young, K.; Menche, J. Australian preferences for influenza vaccine attributes and cost: A discrete choice experiment. Hum. Vaccin. Immunother 2025, 21, 2440164. [Google Scholar] [CrossRef]
- Avelino-Silva, V.I.; Ferreira-Silva, S.N.; Soares, M.E.M.; Vasconcelos, R.; Fujita, L.; Medeiros, T.; Barbieri, C.L.A.; Couto, M.T. Say it right: Measuring the impact of different communication strategies on the decision to get vaccinated. BMC Public Health 2023, 23, 1162. [Google Scholar] [CrossRef]
- Healthworks. Tips for Boosting Flu Vaccination Rates at Work. Available online: https://www.healthworks.com.au/promoting-flu-vaccinations-in-the-workplace/ (accessed on 15 July 2023).
- Loreto, B.B.L.; de Azevedo, S.C.; da Silva, A.G.; Malloy-Diniz, L.F.; Ornel, F.; Trés, L.; Kessler, F.H.P.; de Castro, M.N. Well-being at work, productivity, and coping with stress during the COVID-19 pandemic. Trends Psychiatry Psychother. 2022, 44, e20210250. [Google Scholar] [CrossRef]
- Gallo, A.T.; Scanlon, L.; Clifford, J.; Patten-Williams, L.; Tweedie, L.; Li, D.; Salter, S.M. Immediate Adverse Events Following COVID-19 Vaccination in Australian Pharmacies: A Retrospective Review. Vaccines 2022, 10, 2041. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Population Clock and Pyramid. Available online: https://www.abs.gov.au/statistics/people/population/population-clock-pyramid (accessed on 15 July 2023).
| Overall Cohort (N = 6797) | ||
|---|---|---|
| Variable | Value | % |
| Age, years | ||
| Mean (SD) | 53.1 (14.5) | NA |
| Median | 54.2 (42.2, 64.0) | NA |
| Min, Max | [18.0, 96.5] | NA |
| Gender | ||
| Male | 2183 | 32.1% |
| Female | 4606 | 67.8% |
| Missing | 8 | 0.1% |
| Aboriginal and/or Torres Strait Islander | ||
| Yes | 149 | 2.2% |
| No | 6531 | 96.1% |
| Missing | 117 | 1.7% |
| BMI, years | ||
| Mean (SD) | 28.0 (6.3) | NA |
| Median | 26.9 (23.7, 31.2) | NA |
| Min, Max | [15.4, 94.3] | NA |
| Obesity | ||
| Yes (BMI > 30 kg/m2) | 2057 | 30.3% |
| No | 4584 | 67.4% |
| Missing | 156 | 2.3% |
| Education | ||
| High school or below | 1206 | 17.7% |
| Certificate/diploma degree | 1881 | 27.7% |
| Bachelor degree | 1645 | 24.2% |
| Postgraduates or above | 739 | 10.9% |
| Missing | 1326 | 19.5% |
| Labour force status # | ||
| Full time | 3027 | 44.5% |
| Part time | 1207 | 17.8% |
| Casual | 423 | 6.2% |
| Unemployed | 161 | 2.4% |
| Not in labour force (this includes carers, volunteers, students, retirees, and home duties) | 185 | 2.7% |
| Other | 1698 | 25.0% |
| Missing | 96 | 1.4% |
| Chronic conditions | ||
| 0 | 4063 | 59.8% |
| 1 | 1595 | 23.5% |
| >1 | 1139 | 16.7% |
| EQ-5D-5L * | ||
| Mean (SD) | 0.94 (0.11) | NA |
| Median | 0.96 (0.92, 1.00) | NA |
| Dose 1 | Dose 2 | Dose 3 | Dose 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Number of Individuals | Average Number of Times | Number of Individuals | Average Number of Times | Number of Individuals | Average Number of Times | Number of Individuals | Average Number of Times | |
| 13 Health # | 31 (12.3%) | 1.26 ± 0.82 | 20 (9.2%) | 2.00 ± 2.75 | 12 (9.3%) | 1.17 ± 0.39 | 2 (8.3%) | 1 ± 0 |
| Ambulance | 14 (5.6%) | 1.43 ± 0.94 | 18 (8.3%) | 1.28 ± 0.67 | 6 (4.7%) | 1.83 ± 0.98 | 1 (4.2%) | NA |
| Community clinic nurse or indigenous health worker | 6 (2.4%) | 1.00 ± 0.00 | 5 (2.3%) | 1.60 ± 0.89 | 2 (1.6%) | 1.50 ± 0.71 | 0 (0.0%) | NA |
| General practitioner (including after-hours/home visit/telephone consult) | 135 (53.6%) | 2.48 ± 3.24 | 120 (55.0%) | 4.50 ± 10.44 | 86 (66.7%) | 2.86 ± 3.36 | 20 (83.3%) | 1.70 ± 1.30 |
| Emergency department | 62 (24.6%) | 1.29 ± 0.66 | 47 (21.6%) | 1.68 ± 1.02 | 21 (16.3%) | 1.38 ± 0.74 | 1 (4.2%) | NA |
| Admitted to public hospital | 4 (1.6%) | 1.09 ± 0.30 | 8 (3.7%) | 1.23 ± 0.69 | 2 (1.6%) | 1.29 ± 0.49 | 0 (0.0%) | NA |
| Total number of individuals reported healthcare use | 252 | 218 | 129 | 24 | ||||
| % among participants reporting AEFIs | 7.0% (252/3617) | 7.3% (218/2978) | 5.1% (129/2550) | 1.9% (24/1262) | ||||
| % among participants vaccinated | 3.7% (252/6777) | 3.2% (218/6747) | 2.1% (129/6268) | 0.8% (24/3084) | ||||
| Overall healthcare use costs * | $87,032 | $261,125 | $57,064 | $5304 | ||||
| Healthcare cost per person reporting AEFIs | $24 | $88 | $22 | $4 | ||||
| Healthcare cost per person vaccinated (overall cohort) | $13 | $39 | $9 | $2 | ||||
| Dose 1 | Dose 2 | Dose 3 | Dose 4 | |
|---|---|---|---|---|
| Number of individuals who reported absenteeism | 597 | 542 | 388 | 141 |
| % among participants reporting AEFIs | 16.5% (597/3617) | 18.2% (542/2978) | 15.2% (388/2550) | 11.2% (141/1262) |
| % among participants vaccinated (overall cohort) | 8.8% (597/6777) | 8.0% (542/6747) | 6.2% (388/6268) | 4.6% (141/3084) |
| Absenteeism, days | ||||
| Mean (SD) | 4.7 ± 23.7 | 7.4 ± 37.7 | 3.6 ± 12.3 | 2.1 ± 6.7 |
| Median (IQR) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) |
| [Min, Max] | [0.2, 365.0] | [0.3, 365.0] | [0.5, 180.0] | [0.1, 80.0] |
| Overall absenteeism cost, AUD | $880,233 | $1,277,451 | $432,663 | $95,222 |
| Absenteeism cost per person reporting AEFI * | $1494 | $2388 | $1136 | $690 |
| Absenteeism cost per person vaccinated (overall cohort) # | $130 | $189 | $69 | $31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Q.; O’Grady, K.-A.F.; Vardon, P.; Ward, S.; Gregory, R.; Davies, J.; Carter, H.E. Adverse Events and Associated Economic Burden of COVID-19 Vaccination in Queensland, Australia: Findings from the Cross-Sectional QoVAX-Statewide Study. Vaccines 2025, 13, 712. https://doi.org/10.3390/vaccines13070712
Xia Q, O’Grady K-AF, Vardon P, Ward S, Gregory R, Davies J, Carter HE. Adverse Events and Associated Economic Burden of COVID-19 Vaccination in Queensland, Australia: Findings from the Cross-Sectional QoVAX-Statewide Study. Vaccines. 2025; 13(7):712. https://doi.org/10.3390/vaccines13070712
Chicago/Turabian StyleXia, Qing, Kerry-Ann F. O’Grady, Peter Vardon, Selina Ward, Rebecca Gregory, Janet Davies, and Hannah E. Carter. 2025. "Adverse Events and Associated Economic Burden of COVID-19 Vaccination in Queensland, Australia: Findings from the Cross-Sectional QoVAX-Statewide Study" Vaccines 13, no. 7: 712. https://doi.org/10.3390/vaccines13070712
APA StyleXia, Q., O’Grady, K.-A. F., Vardon, P., Ward, S., Gregory, R., Davies, J., & Carter, H. E. (2025). Adverse Events and Associated Economic Burden of COVID-19 Vaccination in Queensland, Australia: Findings from the Cross-Sectional QoVAX-Statewide Study. Vaccines, 13(7), 712. https://doi.org/10.3390/vaccines13070712






