Rapid Progression of Cutaneous Lymphoma Following mRNA COVID-19 Vaccination: A Case Report and Pathogenetic Insights
Abstract
1. Introduction
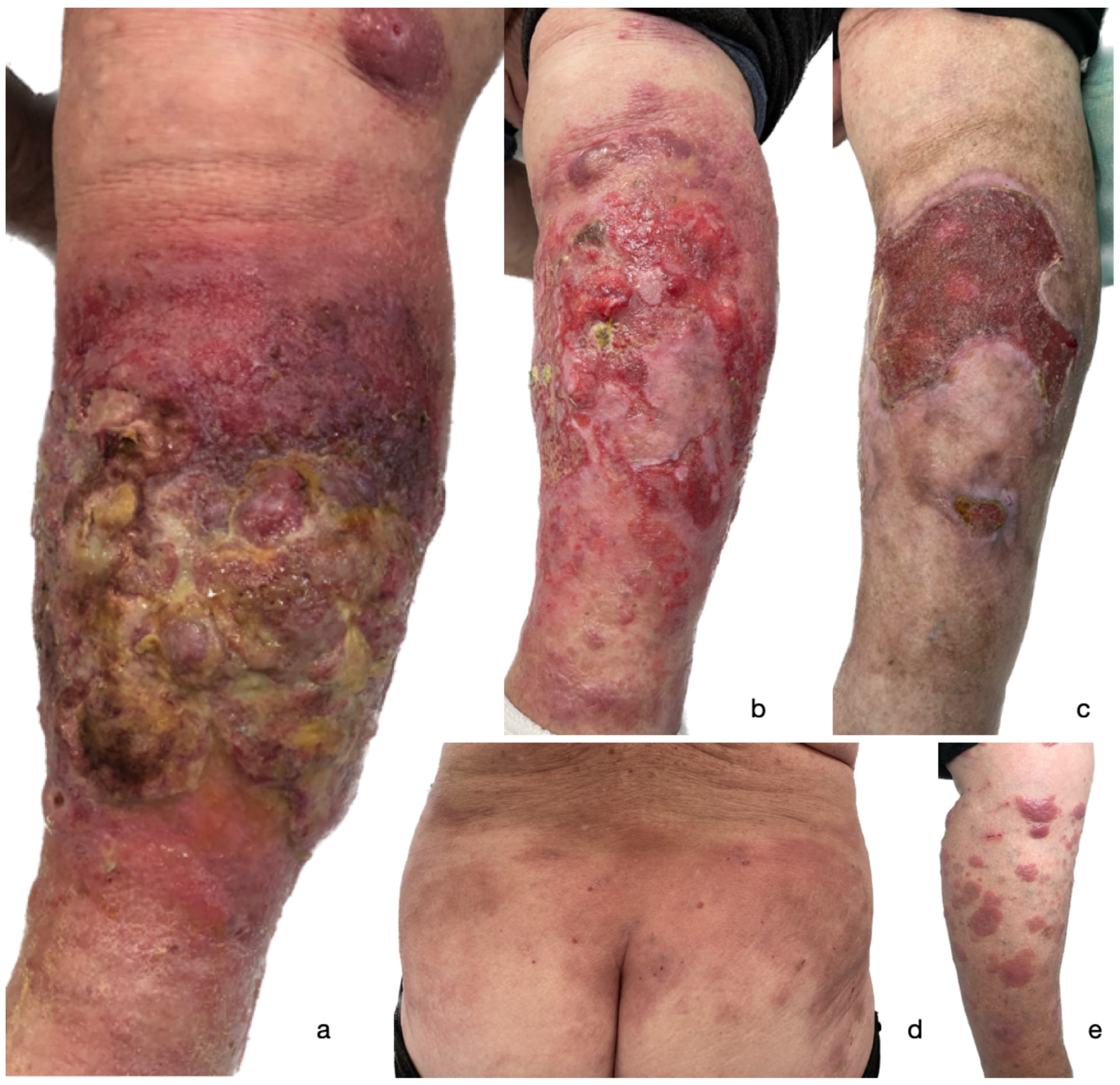
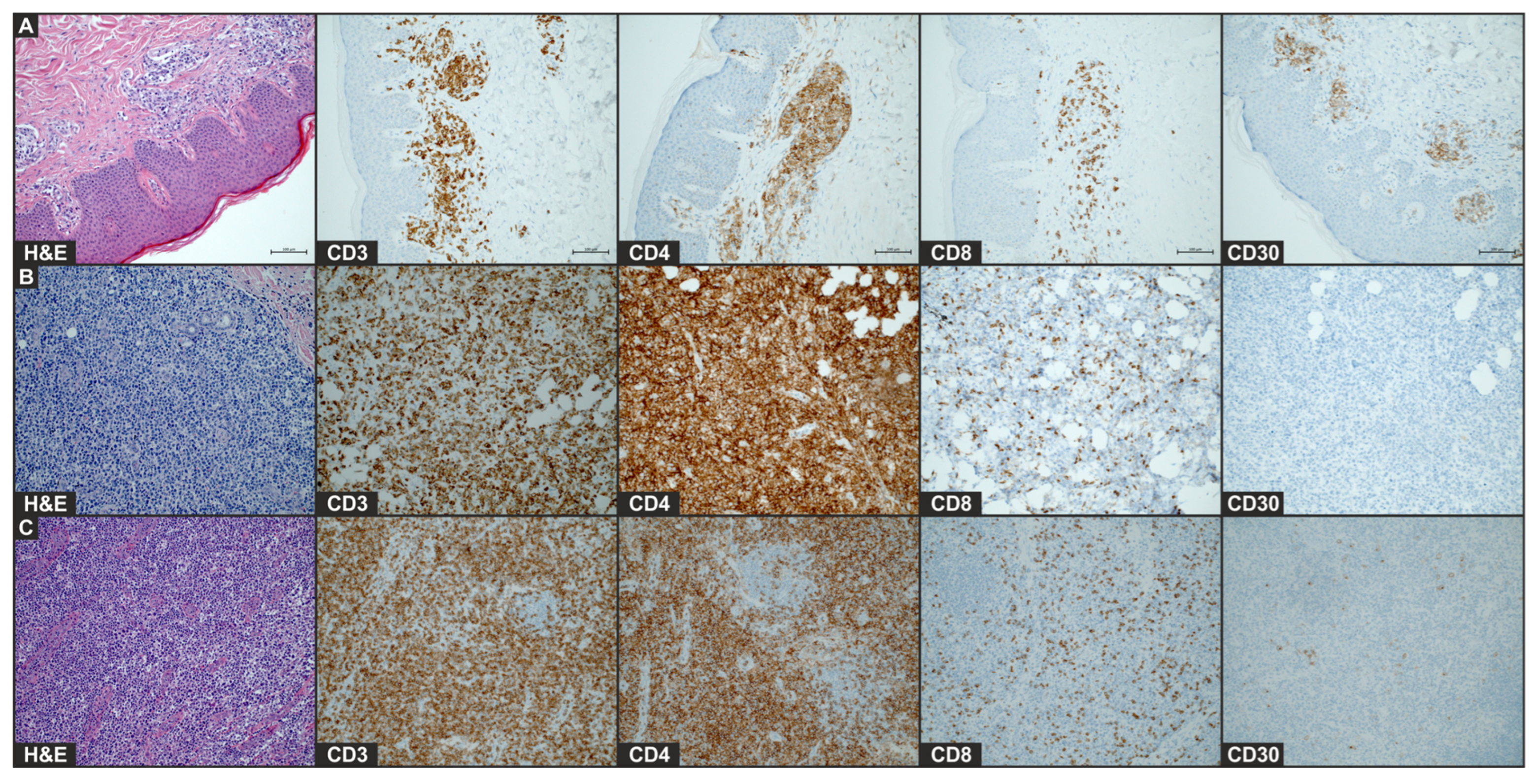
2. Case Presentation
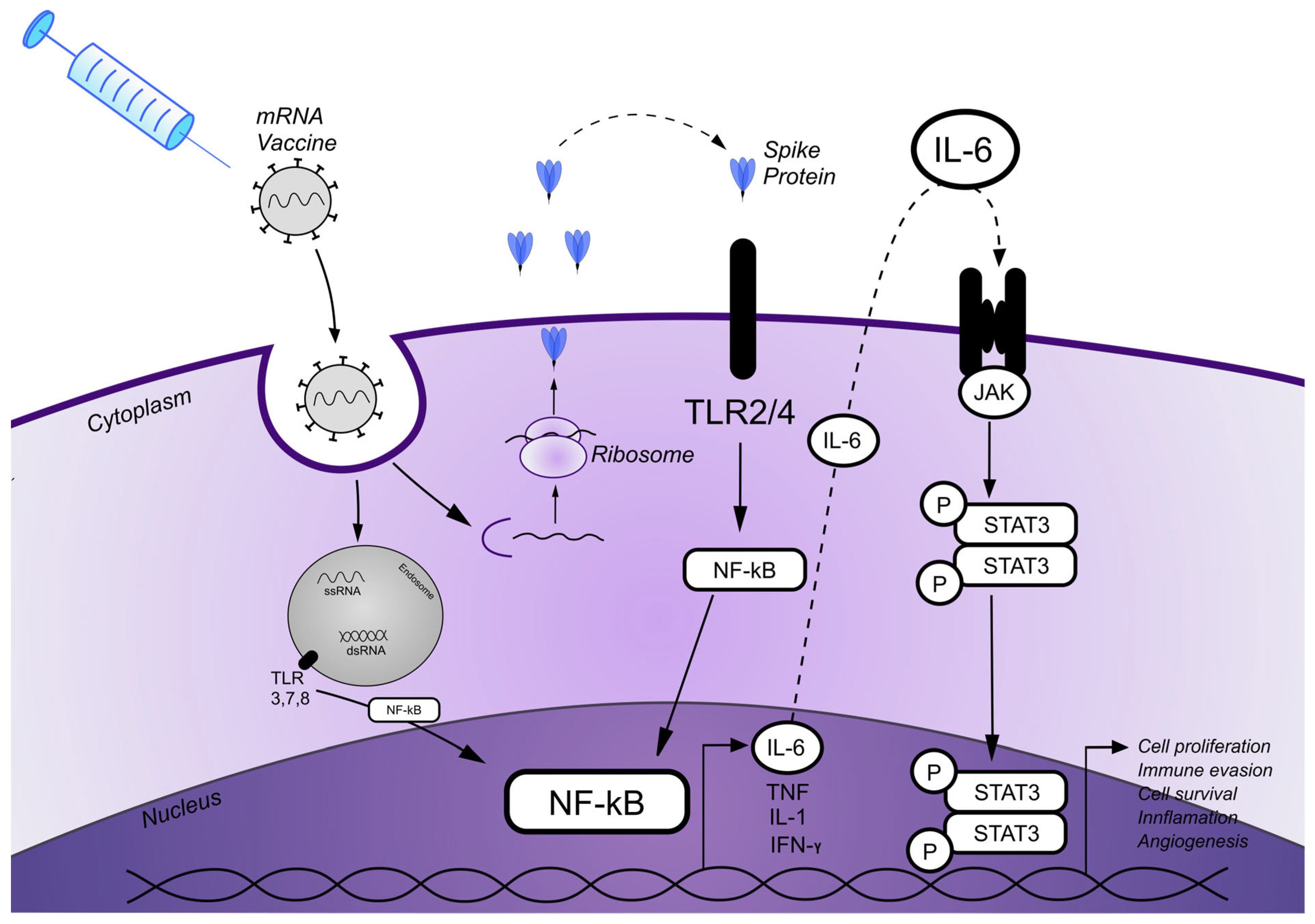
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEP | Acute generalized exanthematous pustulosis |
| AEs | Adverse events |
| CLs | Cutaneous lymphomas |
| CRT-P | Cardiac resynchronization therapy pacemaker |
| CTCLs | Cutaneous T-cell lymphomas |
| dsRNA | Low e-stranded RNA |
| LNPs | Pipid nanoparticles |
| LyP | Lyphomatoid papulosis |
| MF | Mycosis fungoides |
| MTX | Methotrexate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| S | Spike |
| SS | Sézary syndrome |
| ssRNA | Single-stranded RNA |
| STAT3 | Signal transducer and activator of transcription 3 |
| TLRs | Toll-like receptors |
References
- Annabi, E.; Dupin, N.; Sohier, P.; Garel, B.; Franck, N.; Aractingi, S.; Guégan, S.; Oulès, B. Rare cutaneous adverse effects of COVID-19 vaccines: A case series and review of the literature. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e847–e850. [Google Scholar] [CrossRef] [PubMed]
- Brumfiel, C.M.; Patel, M.H.; Di Caudo, D.J.; Rosenthal, A.C.; Pittelkow, M.R.; Mangold, A.R. Recurrence of primary cutaneous CD30-positive lymphoproliferative disorder following COVID-19 vaccination. Leuk. Lymphoma 2021, 62, 2554–2555. [Google Scholar] [CrossRef] [PubMed]
- Panou, E.; Nikolaou, V.; Marinos, L.; Kallambou, S.; Sidiropoulou, P.; Gerochristou, M.; Stratigos, A. Recurrence of cutaneous T-cell lymphoma post viral vector COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e91–e93. [Google Scholar] [CrossRef] [PubMed]
- Avallone, G.; Maronese, C.A.; Conforti, C.; Fava, P.; Gargiulo, L.; Marzano, A.V.; Massone, C.; Mastorino, L.; Paradisi, A.; Pileri, A.; et al. Real-world data on primary cutaneous lymphoproliferative disorders following SARS-CoV-2 vaccination: A multicentre experience from tertiary referral hospitals. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e451–e455. [Google Scholar] [CrossRef]
- Li, H.O.; Lipson, J. New mycosis fungoides-like lymphomatoid reaction following COVID-19 vaccination: A case report. SAGE Open Med. Case Rep. 2022, 10, 2050313X221131859. [Google Scholar] [CrossRef]
- Sallusto, F.; Mackay, C.R.; Lanzavecchia, A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 2000, 18, 593–620. [Google Scholar] [CrossRef]
- Patil, K.; Kuttikrishnan, S.; Khan, A.Q.; Ahmad, F.; Alam, M.; Buddenkotte, J.; Ahmad, A.; Steinhoff, M.; Uddin, S. Molecular pathogenesis of Cutaneous T cell Lymphoma: Role of chemokines, cytokines, and dysregulated signaling pathways. Semin. Cancer Biol. 2022, 86 Pt 3, 382–399. [Google Scholar] [CrossRef]
- Ellis, T.M.; Simms, P.E.; Slivnick, D.J.; Jäck, H.M.; Fisher, R.I. CD30 is a signal-transducing molecule that defines a subset of human activated CD45RO+ T cells. J. Immunol. 1993, 151, 2380–2389. [Google Scholar] [CrossRef]
- Benitez Fuentes, J.D.; Mohamed Mohamed, K.; de Luna Aguilar, A.; García, C.J.; Guevara-Hoyer, K.; Fernandez-Arquero, M.; de la Peña, M.A.R.; Bravo, L.G.; Ortega, A.F.J.; Navarro, P.F.; et al. Evidence of exhausted lymphocytes after the third anti-SARS-CoV-2 vaccine dose in cancer patients. Front. Oncol. 2022, 12, 975980. [Google Scholar] [CrossRef]
- Bellamkonda, N.; Lambe, U.P.; Sawant, S.; Nandi, S.S.; Chakraborty, C.; Shukla, D. Immune Response to SARS-CoV-2 Vaccines. Biomedicines 2022, 10, 1464. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lonez, C.; Vandenbranden, M.; Ruysschaert, J.M. Cationic lipids activate intracellular signaling pathways. Adv. Drug Deliv. Rev. 2012, 64, 1749–1758. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Knol, A.C.; Ehst, B.D.; Dompmartin, A.; Quéreux, G.; Nguyen, J.; Comoz, F.; Renaut, J.; Khammari, A.; Vonderheid, E.; Dréno, B. Toll-like receptor 2, 4, 7 and 9 expression in primary cutaneous CD30+ T-cell lymphoma. Br. J. Dermatol. 2009, 161, 1414–1416. [Google Scholar] [CrossRef]
- Jarrousse, V.; Quereux, G.; Marques-Briand, S.; Knol, A.C.; Khammari, A.; Dreno, B. Toll-like receptors 2, 4 and 9 expression in cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Eur. J. Dermatol. 2006, 16, 636–641. [Google Scholar]
- Hirsiger, J.R.; Tzankov, A.; Alborelli, I.; Recher, M.; Daikeler, T.; Parmentier, S.; Berger, C.T. Case Report: mRNA vaccination-mediated STAT3 overactivation with agranulocytosis and clonal T-LGL expansion. Front. Immunol. 2023, 14, 1087502. [Google Scholar] [CrossRef]
- Netchiporouk, E.; Litvinov, I.V.; Moreau, L.; Gilbert, M.; Sasseville, D.; Duvic, M. Deregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progression. Cell Cycle 2014, 13, 3331–3335. [Google Scholar] [CrossRef]
- Sommer, V.H.; Clemmensen, O.J.; Nielsen, O.; Wasik, M.; Lovato, P.; Brender, C.; Eriksen, K.W.; Woetmann, A.; Kaestel, C.G.; Nissen, M.H.; et al. In vivo activation of STAT3 in cutaneous T-cell lymphoma. Evidence for an antiapoptotic function of STAT3. Leukemia 2004, 18, 1288–1295. [Google Scholar] [CrossRef]
- Pérez, C.; Mondéjar, R.; García-Díaz, N.; Cereceda, L.; León, A.; Montes, S.; Vian, C.D.; Paredes, M.P.; González-Morán, A.; De Miguel, V.A.; et al. Advanced-stage mycosis fungoides: Role of the signal transducer and activator of transcription 3, nuclear factor-κB and nuclear factor of activated T cells pathways. Br. J. Dermatol. 2020, 182, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Zhang, L.; Bhushan, A.; Swanson, B.; Zhang, L.; Mamede, J.I.; Voigt, R.M.; Shaikh, M.; Engen, P.A.; Keshavarzian, A. The SARS-CoV-2 S1 Spike Protein Promotes MAPK and NF-kB Activation in Human Lung Cells and Inflammatory Cytokine Production in Human Lung and Intestinal Epithelial Cells. Microorganisms 2022, 10, 1996. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife 2021, 10, e68563. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Nikolaienko, S.I.; Dibrova, V.A.; Dibrova, Y.V.; Vasylyk, V.M.; Novikov, M.Y.; Shults, N.V.; Gychka, S.G. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. Vasc. Pharmacol. 2021, 137, 106823. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Gychka, S.G. SARS-CoV-2 Spike Protein Elicits Cell Signaling in Human Host Cells: Implications for Possible Consequences of COVID-19 Vaccines. Vaccines 2021, 9, 36. [Google Scholar] [CrossRef]
- McFarland, B.C.; Hong, S.W.; Rajbhandari, R.; Twitty, G.B., Jr.; Gray, G.K.; Yu, H.; Benveniste, E.N.; Nozell, S.E. NF-κB-induced IL-6 ensures STAT3 activation and tumor aggressiveness in glioblastoma. PLoS ONE 2013, 8, e78728. [Google Scholar] [CrossRef]
- Carpenter, R.L.; Lo, H.W. STAT3 Target Genes Relevant to Human Cancers. Cancers 2014, 6, 897–925. [Google Scholar] [CrossRef]
- Al Zaid Siddiquee, K.; Turkson, J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008, 18, 254–267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska, B.; Zaryczańska, A.; Bieńkowski, M.; Nowicki, R.J.; Sokołowska-Wojdyło, M. Rapid Progression of Cutaneous Lymphoma Following mRNA COVID-19 Vaccination: A Case Report and Pathogenetic Insights. Vaccines 2025, 13, 678. https://doi.org/10.3390/vaccines13070678
Olszewska B, Zaryczańska A, Bieńkowski M, Nowicki RJ, Sokołowska-Wojdyło M. Rapid Progression of Cutaneous Lymphoma Following mRNA COVID-19 Vaccination: A Case Report and Pathogenetic Insights. Vaccines. 2025; 13(7):678. https://doi.org/10.3390/vaccines13070678
Chicago/Turabian StyleOlszewska, Berenika, Anna Zaryczańska, Michał Bieńkowski, Roman J. Nowicki, and Małgorzata Sokołowska-Wojdyło. 2025. "Rapid Progression of Cutaneous Lymphoma Following mRNA COVID-19 Vaccination: A Case Report and Pathogenetic Insights" Vaccines 13, no. 7: 678. https://doi.org/10.3390/vaccines13070678
APA StyleOlszewska, B., Zaryczańska, A., Bieńkowski, M., Nowicki, R. J., & Sokołowska-Wojdyło, M. (2025). Rapid Progression of Cutaneous Lymphoma Following mRNA COVID-19 Vaccination: A Case Report and Pathogenetic Insights. Vaccines, 13(7), 678. https://doi.org/10.3390/vaccines13070678







