Pre-Existing Anti-Inflammatory Immune Conditions Influence Early Antibody Avidity and Isotype Profile Following Comirnaty® Vaccination in Mice
Abstract
1. Introduction
2. Materials and Methods
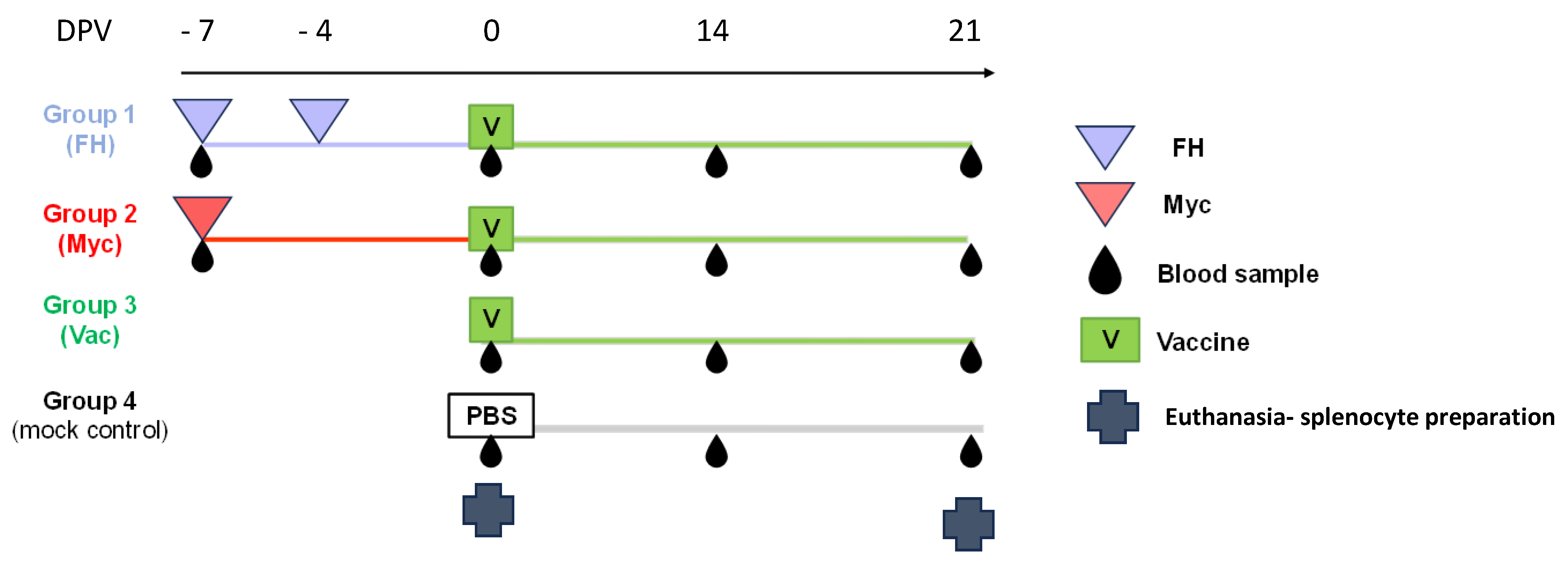
2.1. Animals and Experimental Design
2.2. Preparation of F. hepatica Protein Extract (FH)
2.3. Stimulation of Cryopreserved Splenocytes
2.4. Cytokine Quantification by ELISA
2.5. Humoral Response Against SARS-CoV-2 RBD
2.6. Statistical Analysis
3. Results
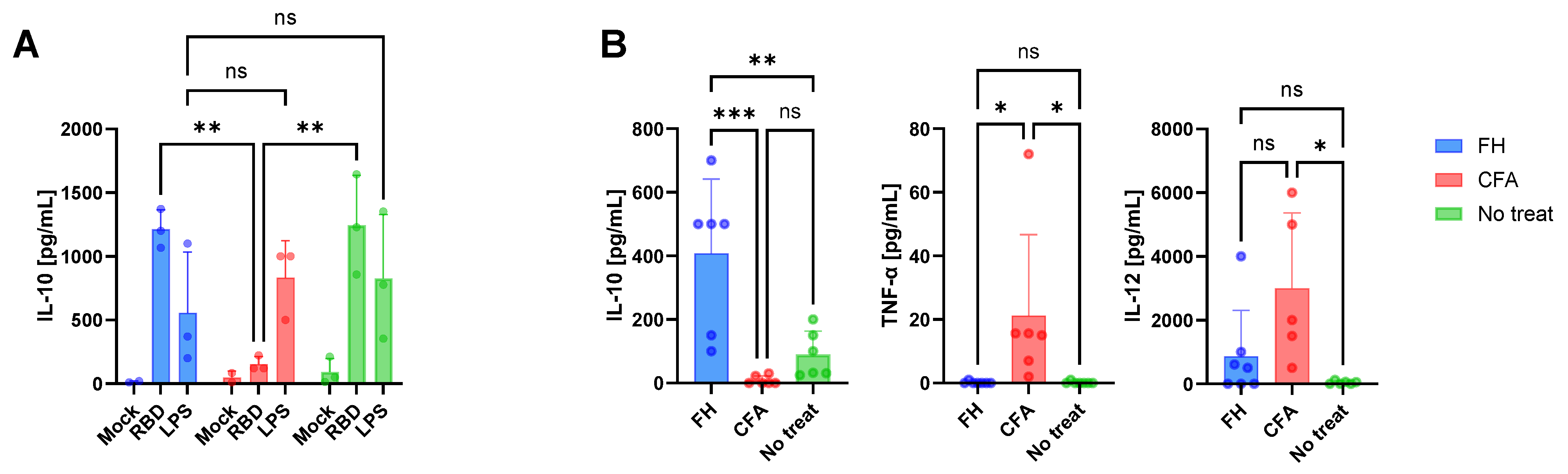
3.1. Immune Conditions at the Time of Vaccination
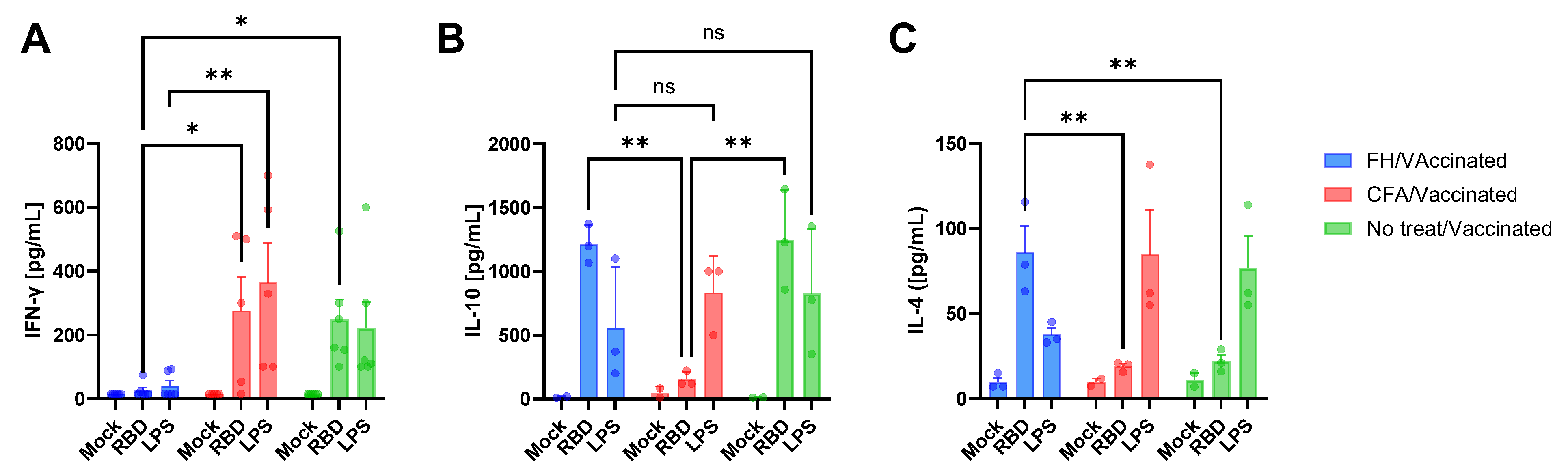
3.2. Vaccine-Induced Humoral and Cytokine Immune Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Netea, M.G.; Quintin, J.; van der Meer, J.W.M. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.M.; Cunningham, K.; Raverdeau, M.; Walsh, R.J.; Curham, L.; Malara, A.; Mills, K.H.G. Anti-inflammatory Trained Immunity Mediated by Helminth Products Attenuates the Induction of T Cell-Mediated Autoimmune Disease. Front. Immunol. 2019, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Andrés, J.; Dos Santos, J.C.; Bekkering, S.; Mulder, W.J.M.; van der Meer, J.W.M.; Riksen, N.P.; Joosten, L.A.B.; Netea, M.G. Trained immunity: Adaptation within innate immune mechanisms. Physiol. Rev. 2023, 103, 313–346. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Zhang, X.-H.; Xing, J.-L.; Zhang, A.-H.; Wang, H.-R.; Zhang, X.-C.; Gao, J.-F.; Wang, C.-R. Global prevalence of liver disease in human and domestic animals caused by Fasciola: A systematic review and meta-analysis. J. Glob. Health 2024, 14, 04223. [Google Scholar] [CrossRef]
- Kaplan, R.M. Fasciola hepatica: A review of the economic impact in cattle and considerations for control. Vet. Ther. Res. Appl. Vet. Med. 2001, 2, 40–50. [Google Scholar]
- Saleha, A.A. Liver fluke disease (fascioliasis): Epidemiology, economic impact and public health significance. Southeast Asian J. Trop. Med. Public Health 1991, 22, 361–364. [Google Scholar]
- Mehmood, K.; Zhang, H.; Sabir, A.J.; Abbas, R.Z.; Ijaz, M.; Durrani, A.Z.; Saleem, M.H.; Rehman, M.U.; Iqbal, M.K.; Wang, Y.; et al. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb. Pathog. 2017, 109, 253–262. [Google Scholar] [CrossRef]
- Ryan, S.; Shiels, J.; Taggart, C.C.; Dalton, J.P.; Weldon, S. Fasciola hepatica-Derived Molecules as Regulators of the Host Immune Response. Front. Immunol. 2020, 11, 2182. [Google Scholar] [CrossRef]
- Flynn, R.J.; Musah-Eroje, M. Evasion of Host Immunity During Fasciola hepatica Infection. In Fasciola hepatica; Cancela, M., Maggioli, G., Eds.; Springer: New York, NY, USA, 2020; Volume 2137, pp. 107–115. [Google Scholar] [CrossRef]
- Aldridge, A.; O’Neill, S.M. Fasciola hepatica tegumental antigens induce anergic-like T cells via dendritic cells in a mannose receptor-dependent manner. Eur. J. Immunol. 2016, 46, 1180–1192. [Google Scholar] [CrossRef]
- Costa, M.; Mansilla, F.; Manuel Sala, J.; Saravia, A.; Ubios, D.; Lores, P.; Capozzo, A.V. Fasciola hepatica infection modifies IgG1 specific immune response to foot-and-mouth disease virus induced by vaccination. Vaccine 2024, 42, 541–547. [Google Scholar] [CrossRef]
- Billiau, A.; Matthys, P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Fragoulis, G.E.; Siebert, S.; McInnes, I.B. Therapeutic Targeting of IL-17 and IL-23 Cytokines in Immune-Mediated Diseases. Annu. Rev. Med. 2016, 67, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, M.; Stanisavljević, S.; Nikolovski, N.; Dimitrijević, M.; Miljković, Đ. Complete Freund’s adjuvant as a confounding factor in multiple sclerosis research. Front. Immunol. 2024, 15, 1353865. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Di Genaro, M.S.; Kalergis, A.M. Trained Immunity Contribution to Autoimmune and Inflammatory Disorders. Front. Immunol. 2022, 13, 868343. [Google Scholar] [CrossRef]
- Gangaplara, A.; Massilamany, C.; Lasrado, N.; Steffen, D.; Reddy, J. Evidence for Anti-Viral Effects of Complete Freund’s Adjuvant in the Mouse Model of Enterovirus Infection. Vaccines 2020, 8, 364. [Google Scholar] [CrossRef]
- Mone, K.; Seravalli, J.; Reddy, J. Metabolic changes induced by complete Freund’s adjuvant resemble the features of trained immunity. J. Immunol. 2023, 210, 160.27. [Google Scholar] [CrossRef]
- Mansilla, F.C.; Quintana, M.E.; Langellotti, C.; Wilda, M.; Martinez, A.; Fonzo, A.; Moore, D.P.; Cardoso, N.; Capozzo, A.V. Immunization with Neospora caninum profilin induces limited protection and a regulatory T-cell response in mice. Exp. Parasitol. 2016, 160, 1–10. [Google Scholar] [CrossRef]
- Costa, M.; da Costa, V.; Frigerio, S.; Festari, M.F.; Landeira, M.; Rodríguez-Zraquia, S.A.; Lores, P.; Carasi, P.; Freire, T. Heme-Oxygenase-1 Attenuates Oxidative Functions of Antigen Presenting Cells and Promotes Regulatory T Cell Differentiation during Fasciola hepatica Infection. Antioxidants 2021, 10, 1938. [Google Scholar] [CrossRef]
- Argentinian AntiCovid Consortium. Structural and functional comparison of SARS-CoV-2-spike receptor binding domain produced in Pichia pastoris and mammalian cells. Sci. Rep. 2020, 10, 21779. [Google Scholar] [CrossRef]
- Cardoso, N.P.; Rivero, C.; Castillo, M.; Mansilla, F.C.; Pastorino, F.; Piccirilli, G.; Alonso, L.; Martínez, G.; Di Lullo, D.; Bentancor, L.V.; et al. Serological screening of SARS-CoV-2 infection in companion animals of Buenos Aires suburbs. Front. Vet. Sci. 2023, 10, 1161820. [Google Scholar] [CrossRef]
- Lavoria, M.Á.; Di-Giacomo, S.; Bucafusco, D.; Franco-Mahecha, O.L.; Pérez-Filgueira, D.M.; Capozzo, A.V. Avidity and subtyping of specific antibodies applied to the indirect assessment of heterologous protection against Foot-and-Mouth Disease Virus in cattle. Vaccine 2012, 30, 6845–6850. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.M.; Haralambieva, I.H.; Warner, N.D.; Grill, D.E.; Quach, H.Q.; Kennedy, R.B. Longitudinal antibody titer, avidity, and neutralizing responses after SARS-CoV-2 infection. Heliyon 2022, 8, e11676. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Vobornikova, I.; Fusek, J. Freund´s complete adjuvant effect on BALB/c mice: An insight into inflammation and oxidative stress after immunity challenge. Bratisl. Lek. Listy. 2016, 117, 268–271. [Google Scholar] [CrossRef]
- Robinson, M.W.; Alvarado, R.; To, J.; Hutchinson, A.T.; Dowdell, S.N.; Lund, M.; Turnbull, L.; Whitchurch, C.B.; O’Brien, B.A.; Dalton, J.P.; et al. A helminth cathelicidin-like protein suppresses antigen processing and presentation in macrophages via inhibition of lysosomal vATPase. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 4614–4627. [Google Scholar] [CrossRef]
- Alvarado, R.; To, J.; Lund, M.E.; Pinar, A.; Mansell, A.; Robinson, M.W.; O’Brien, B.A.; Dalton, J.P.; Donnelly, S. The immune modulatory peptide FhHDM-1 secreted by the helminth Fasciola hepatica prevents NLRP3 inflammasome activation by inhibiting endolysosomal acidification in macrophages. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 85–95. [Google Scholar] [CrossRef]
- Dalton, J.P.; Robinson, M.W.; Mulcahy, G.; O’Neill, S.M.; Donnelly, S. Immunomodulatory molecules of Fasciola hepatica: Candidates for both vaccine and immunotherapeutic development. Vet. Parasitol. 2013, 195, 272–285. [Google Scholar] [CrossRef]
- Flores-Velázquez, L.M.; Ruiz-Campillo, M.T.; Herrera-Torres, G.; Martínez-Moreno, Á.; Martínez-Moreno, F.J.; Zafra, R.; Buffoni, L.; Rufino-Moya, P.J.; Molina-Hernández, V.; Pérez, J. Fasciolosis: Pathogenesis, host-parasite interactions, and implication in vaccine development. Front. Vet. Sci. 2023, 10, 1270064. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Carasi, P.; Rodríguez, E.; da Costa, V.; Frigerio, S.; Brossard, N.; Noya, V.; Robello, C.; Anegón, J.; Freire, T. Heme-Oxygenase-1 Expression Contributes to the Immunoregulation Induced by Fasciola hepatica and Promotes Infection. Front. Immunol. 2017, 8, 883. [Google Scholar] [CrossRef]
- Vijayan, V.; Wagener, F.A.D.T.G.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.J.; Allen, J.E. The expanding world of tissue-resident macrophages. Eur. J. Immunol. 2021, 51, 1882–1896. [Google Scholar] [CrossRef] [PubMed]
- Tzima, S.; Victoratos, P.; Kranidioti, K.; Alexiou, M.; Kollias, G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J. Exp. Med. 2009, 206, 1167–1179. [Google Scholar] [CrossRef]
- Greil, J.; Verga-Falzacappa, M.V.; Echner, N.E.; Behnisch, W.; Bandapalli, O.R.; Pechanska, P.; Immenschuh, S.; Vijayan, V.; Balla, J.; Tsukahara, H.; et al. Mutating heme oxygenase-1 into a peroxidase causes a defect in bilirubin synthesis associated with microcytic anemia and severe hyperinflammation. Haematologica 2016, 101, e436–e439. [Google Scholar] [CrossRef]
- Nelson, C.E.; Foreman, T.W.; Kauffman, K.D.; Sakai, S.; Gould, S.T.; Fleegle, J.D.; Gomez, F.; NIAID/DIR Tuberculosis Imaging Program; Nouën, C.L.; Liu, X.; et al. IL-10 suppresses T cell expansion while promoting tissue-resident memory cell formation during SARS-CoV-2 infection in rhesus macaques. PLoS Pathog. 2024, 20, e1012339. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Zhou, J.-J.; Zhu, H.-P.; Xu, F.; Zhao, W.-B.; Lu, H.-J.; Wang, Z.; Chen, S.-Q.; Yao, P.-P.; Jiang, J.-M.; et al. Development of a Recombinant RBD Subunit Vaccine for SARS-CoV-2. Viruses 2021, 13, 1936. [Google Scholar] [CrossRef]
- Helfgott, S.M.; Kieval, R.I.; Breedveld, F.C.; Brahn, E.; Young, C.T.; Dynesius-Trentham, R.; Trentham, D.E. Detection of arthritogenic factor in adjuvant arthritis. J. Immunol. Baltim. Md. 1950 1988, 140, 1838–1843. [Google Scholar] [CrossRef]
- Mone, K.; Garcia, E.J.T.; Abdullatif, F.; Rasquinha, M.T.; Sur, M.; Hanafy, M.; Zinniel, D.K.; Singh, S.; Thomas, R.; Barletta, R.G.; et al. Metabolic Reprogramming in Response to Freund’s Adjuvants: Insights from Serum Metabolomics. Microorganisms 2025, 13, 492. [Google Scholar] [CrossRef]
- Moura, A.D.; da Costa, H.H.M.; Correa, V.A.; de SLima, A.K.; Lindoso, J.A.L.; De Gaspari, E.; Hong, M.A.; Cunha-Junior, J.P.; Prudencio, C.R. Assessment of avidity related to IgG subclasses in SARS-CoV-2 Brazilian infected patients. Sci. Rep. 2021, 11, 17642. [Google Scholar] [CrossRef]
- Bauer, G. High avidity of vaccine-induced immunoglobulin G against SARS-CoV-2: Potential relevance for protective humoral immunity. Explor. Immunol. 2022, 133–156. [Google Scholar] [CrossRef]
- Flynn, R.J.; Mulcahy, G. The roles of IL-10 and TGF-beta in controlling IL-4 and IFN-gamma production during experimental Fasciola hepatica infection. Int. J. Parasitol. 2008, 38, 1673–1680. [Google Scholar] [CrossRef]
- Brady, M.T.; O’Neill, S.M.; Dalton, J.P.; Mills, K.H. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect. Immun. 1999, 67, 5372–5378. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Carasi, P.; Frigerio, S.; da Costa, V.; van Vliet, S.; Noya, V.; Brossard, N.; van Kooyk, Y.; García-Vallejo, J.J.; Freire, T. Fasciola hepatica Immune Regulates CD11c+ Cells by Interacting with the Macrophage Gal/GalNAc Lectin. Front. Immunol. 2017, 8, 264. [Google Scholar] [CrossRef]
- Costa, M.; Saravia, A.; Ubios, D.; Paolazzi, C.; Capozzo, A.; Freire, T. Impact of Fasciola hepatica Infection and Triclabendazole Treatment on Humoral Immune Response in Cattle. Parasite Immunol. 2024, 46, e13079. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Alameh, M.-G.; Tombácz, I.; Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Chacín-Bonilla, L. Effects of helminth co-infections on COVID-19 outcome. J. Allergy Infect. Dis. 2024, 5, 29–34. [Google Scholar] [CrossRef]
- Bradbury, R.S.; Piedrafita, D.; Greenhill, A.; Mahanty, S. Will helminth co-infection modulate COVID-19 severity in endemic regions? Nat. Rev. Immunol. 2020, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.; Pierce, D.; Giacomin, P.; Loukas, A.; Bourke, P.; McDermott, R. Helminth coinfection and COVID-19: An alternate hypothesis. PLoS Negl. Trop. Dis. 2020, 14, e0008628. [Google Scholar] [CrossRef]
- Chacon, N.; Chacin-Bonilla, L.; Cesari, M.I. Implications of helminth immunomodulation on COVID-19 co-infections. Life Res. 2021, 4, 27. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; González-Miguel, J.; Geller, R.; Sanjuan, R.; Pérez-Arévalo, J.; Martínez-Moreno, Á. Potential Influence of Helminth Molecules on COVID-19 Pathology. Trends Parasitol. 2021, 37, 11–14. [Google Scholar] [CrossRef]

| Parameter | AUC | Group 1 (FH/ Vaccinated) | Group 2 (Myc/ Vaccinated) | Group 3 (No Treatment/ Vaccinated) | Group 4 (Not Vaccinated) |
|---|---|---|---|---|---|
| Total IgG | Total Area | 27.50 | 29.37 | 27.95 | 2.24 |
| 95% Confidence Interval | 25.74 to 29.25 | 27.82 to 30.92 | 27.82 to 30.92 | 0.4 to 1.66 | |
| IgG avidity | Total Area | 1060 | 1176 | 1356 * | 2.10 |
| 95% Confidence Interval | 901.6 to 1202 | 1000 to 1352 | 1213 to 1509 | 0.33 to 1.45 | |
| IgG1 | Total Area | 7.69 | 7.11 | 8.25 | 2.25 |
| 95% Confidence Interval | 6.71 to 8.66 | 5.08 to 9.14 | 7.11 to 9.38 | 1.44 to 3.05 | |
| IgG2 | Total Area | 6.60 | 8.97 | 9.35 * | 1.89 |
| 95% Confidence Interval | 4.65 to 8.06 | 7.86 to 10.08 | 8.27 to 10.42 | 1.58 to 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, M.; Miraglia, M.C.; Mansilla, F.C.; Randazzo, C.P.; Bentancor, L.V.; Freire, T.; Capozzo, A.V. Pre-Existing Anti-Inflammatory Immune Conditions Influence Early Antibody Avidity and Isotype Profile Following Comirnaty® Vaccination in Mice. Vaccines 2025, 13, 677. https://doi.org/10.3390/vaccines13070677
Castillo M, Miraglia MC, Mansilla FC, Randazzo CP, Bentancor LV, Freire T, Capozzo AV. Pre-Existing Anti-Inflammatory Immune Conditions Influence Early Antibody Avidity and Isotype Profile Following Comirnaty® Vaccination in Mice. Vaccines. 2025; 13(7):677. https://doi.org/10.3390/vaccines13070677
Chicago/Turabian StyleCastillo, Mariangeles, María C. Miraglia, Florencia C. Mansilla, Cecilia P. Randazzo, Leticia V. Bentancor, Teresa Freire, and Alejandra V. Capozzo. 2025. "Pre-Existing Anti-Inflammatory Immune Conditions Influence Early Antibody Avidity and Isotype Profile Following Comirnaty® Vaccination in Mice" Vaccines 13, no. 7: 677. https://doi.org/10.3390/vaccines13070677
APA StyleCastillo, M., Miraglia, M. C., Mansilla, F. C., Randazzo, C. P., Bentancor, L. V., Freire, T., & Capozzo, A. V. (2025). Pre-Existing Anti-Inflammatory Immune Conditions Influence Early Antibody Avidity and Isotype Profile Following Comirnaty® Vaccination in Mice. Vaccines, 13(7), 677. https://doi.org/10.3390/vaccines13070677







