Stopping Tuberculosis at the Gate: The Role of M. tuberculosis Adhesins in Infection and Intervention
Abstract
1. Introduction
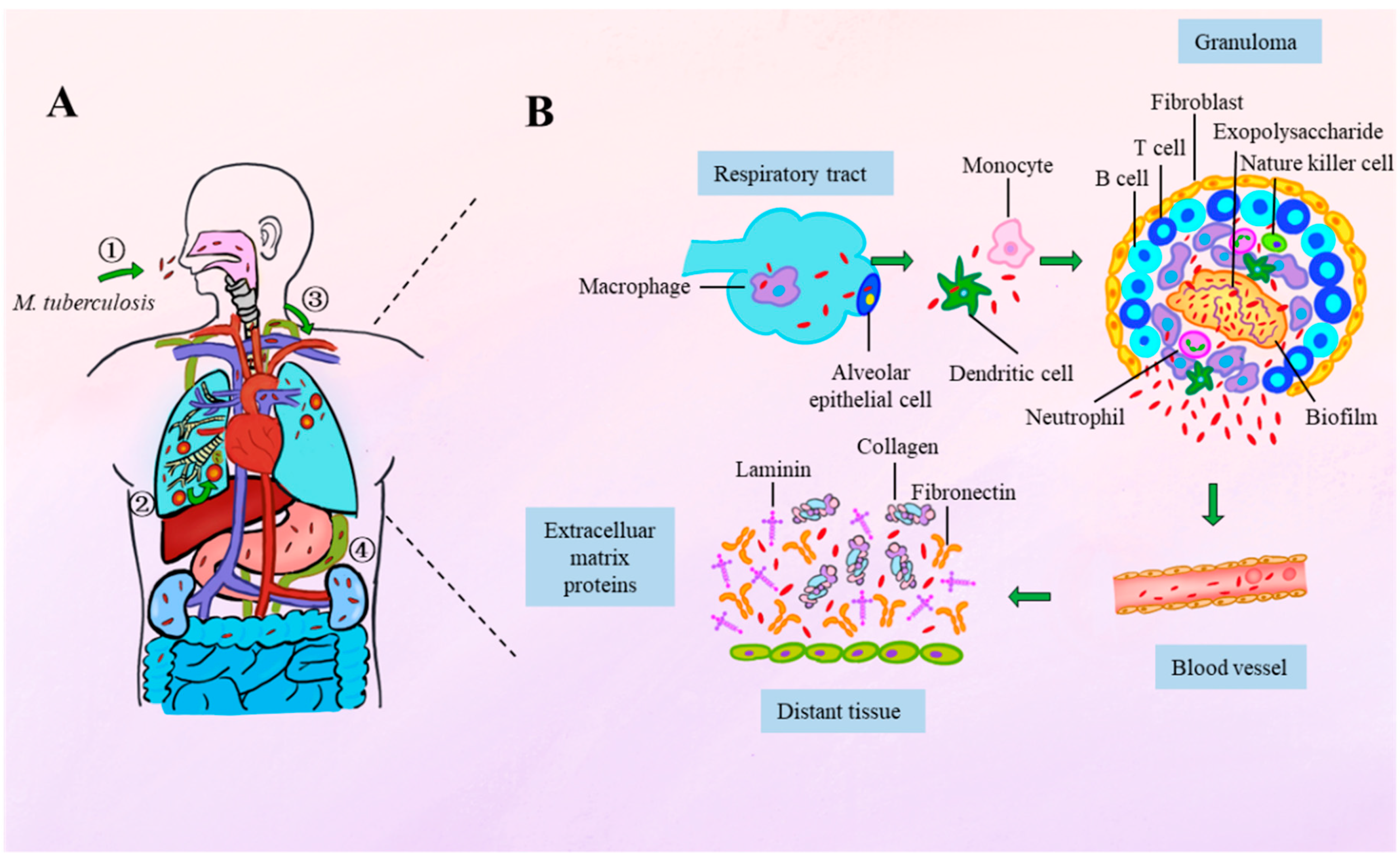
2. Spatiotemporal Dynamics of Mycobacterial Adhesion During Infection
3. The M. tuberculosis Capsule: A Reservoir for Adhesins
3.1. Cytoplasmic Membrane
3.2. Peptidoglycan (PG)
3.3. Arabinogalactan (AG)
3.4. Mycolic Acids (MAs), Trehalose Monomycolate (TMM), and Trehalose 6,6′-Dimycolate (TDM)
3.5. Phthiocerol Dimycocerosate (PDIM)
4. M. tuberculosis Adhesins
4.1. Non-Protein Adhesins in the Mycobacterial Cell Wall and Their Host Interactions
4.1.1. Mannose-Capped Lipoarabinomannan (ManLAM) and PIMs
4.1.2. MAs
4.1.3. TDM
4.1.4. α-Glucan
4.2. Protein Adhesins in the Mycobacterial Cell Wall and Their Host Interactions
4.2.1. Early Secretory Antigenic Target 6 kDa (ESAT-6) and Culture Filtrate Protein 10 kDa (CFP-10)
4.2.2. Antigen 85 (Ag85) Complex
4.2.3. HBHA
4.2.4. Mycobacterium Tuberculosis Curli Pili (MTP)
4.2.5. 19-kDa Antigen
4.2.6. Alanine and Proline-Rich Protein (Apa)
4.2.7. Mycobacterial Mammalian Cell Entry Protein 1A (Mce1A)
4.2.8. PE_PGRS33 (Rv1818c)
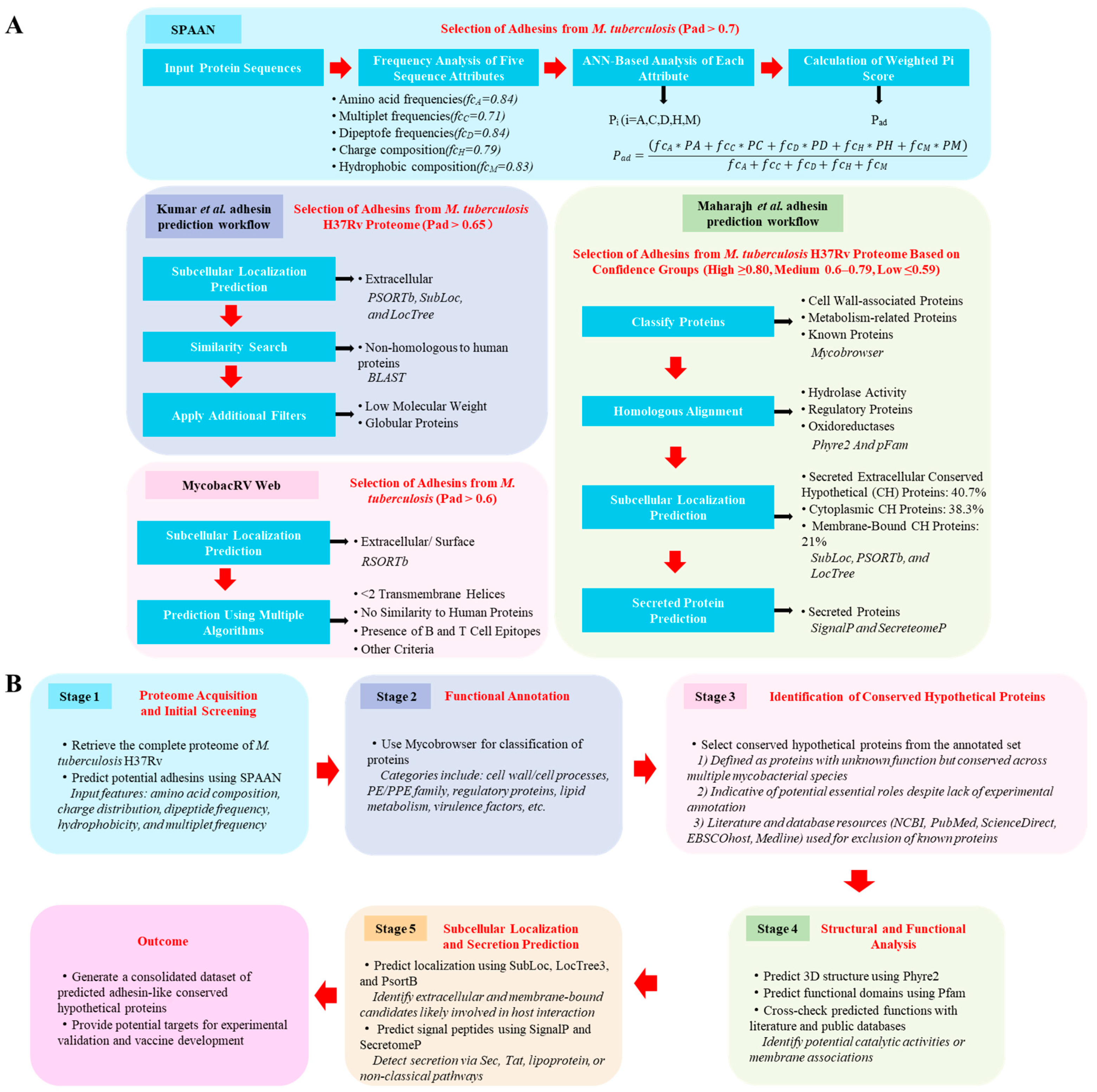
5. Advances in In Silico Prediction of M. tuberculosis Adhesins
6. Challenges and Controversies in Adhesin-Targeted Strategies
6.1. Functional Complexity and Contradictory Evidence
6.2. Translational and Practical Challenges
6.3. Strategic Solutions and Future Directions
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Glossary | Full Term |
| AEC | Alveolar epithelial cells |
| AG | Arabinogalactan |
| AG–PG | Arabinogalactan–peptidoglycan |
| AM | Alveolar macrophages |
| Antigen 85 | Ag85 |
| ANN | Artificial neural network |
| Apa | Alanine and proline-rich protein |
| BLF | Bovine lactoferrin |
| CD1⁻ DCs | CD1-negative dendritic cells |
| CFP-10 | Culture filtrate protein 10 kDa |
| CH | Conserved Hypothetica |
| COVID-19 | Coronavirus disease |
| DC-SIGN | Dendritic cell-specific ICAM-3-grabbing non-integrin |
| ECM | Extracellular matrix |
| ESAT-6 | Early secretory antigenic target 6 kDa |
| Fn | Fibronectin |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GlcNAc | N-acetylglucosamine |
| GlcB | Malate synthase |
| HBHA | Heparin-binding hemagglutinin adhesin |
| HMVEC | Human microvascular endothelial cells |
| H. pylori | Helicobacter pylori |
| LAMs | Lipoarabinomannans |
| LMs | Lipomannans |
| MARCO | Macrophage receptor with collagenous structure |
| MAs | Mycolic acids |
| ManLAM | Mannose-capped lipoarabinomannan |
| M. bovis BCG | Mycobacterium bovis Bacillus Calmette-Guérin |
| Mce1A | Mycobacterial mammalian cell entry protein 1A |
| MDP | Muramyl dipeptide |
| Mincle | Macrophage-inducible C-type lectin |
| MR | Macrophage mannose receptor |
| MTP | Mycobacterium tuberculosis curli pili |
| M. tuberculosis | Mycobacterium tuberculosis |
| TB | Tuberculosis |
| TDM | Trehalose 6,6′-dimycolate |
| TLR2 | Toll-like receptor 2 |
| TLRs | Toll-like receptors |
| TMM | Trehalose monomycolate |
| UN | United Nation |
| UPEC | Uropathogenic Escherichia coli |
| Pad | Protein being an adhesin |
| PAMPs | Pathogen-associated molecular patterns |
| PDIM | Phenolic glycolipid |
| PG | Peptidoglycan |
| PIMs | Phosphatidylinositol mannosides |
| Plg | Plasminogen |
| PRRs | Pattern recognition receptors |
| PSP-A | Human pulmonary surfactant protein A |
| SPAAN | Software Program for Prediction of Adhesins and Adhesin-like Proteins using Neural Networks |
| WHO | World Health Organization |
References
- Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024; Licence: CC BY-NC-SA 3.0 IGO.
- Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023; Licence: CC BY-NC-SA 3.0 IGO.
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Cossart, P. Bacterial adhesion and entry into host cells. Cell 2006, 124, 715–727. [Google Scholar] [CrossRef]
- Stones, D.H.; Krachler, A.M. Against the tide: The role of bacterial adhesion in host colonization. Biochem. Soc. Trans. 2016, 44, 1571–1580. [Google Scholar] [CrossRef]
- Viljoen, A.; Dufrêne, Y.F.; Nigou, J. Mycobacterial Adhesion: From Hydrophobic to Receptor-Ligand Interactions. Microorganisms 2022, 10, 454. [Google Scholar] [CrossRef]
- Pecoraro, C.; Carbone, D.; Parrino, B.; Cascioferro, S.; Diana, P. Recent Developments in the Inhibition of Bacterial Adhesion as Promising Anti-Virulence Strategy. Int. J. Mol. Sci. 2023, 24, 4872. [Google Scholar] [CrossRef]
- Asadi, A.; Razavi, S.; Talebi, M.; Gholami, M. A review on anti-adhesion therapies of bacterial diseases. Infection 2019, 47, 13–23. [Google Scholar] [CrossRef]
- Wang, N.; Cai, T.; Liu, X.; Zhu, W. Bovine lactoferrin inhibits resistant Helicobacter pylori in vitro and protects gastric mucosal injury in vivo. Int. Dairy. J. 2024, 147, 105770. [Google Scholar] [CrossRef]
- Hablass, F.H.; Lashen, S.A.; Alsayed, E.A. Efficacy of Lactoferrin with Standard Triple Therapy or Sequential Therapy for Helicobacter pylori Eradication: A Randomized Controlled Trial. Turk. J. Gastroenterol. 2021, 32, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Imoto, I.; Yasuma, T.; D’Alessandro-Gabazza, C.N.; Oka, S.; Misaki, M.; Horiki, N.; Gabazza, E.C. Antimicrobial Effects of Lactoferrin against Helicobacter pylori Infection. Pathogens 2023, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Dong, J.; Yu, X.F. Meta-analysis: The effect of supplementation with lactoferrin on eradication rates and adverse events during Helicobacter pylori eradication therapy. Helicobacter 2009, 14, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and Anti-Adhesive Therapeutics: A Disarming Strategy Against Uropathogens. Antibiotics 2020, 9, 397. [Google Scholar] [CrossRef]
- Shin, A.R.; Lee, K.S.; Lee, J.S.; Kim, S.Y.; Song, C.H.; Jung, S.B.; Yang, C.S.; Jo, E.K.; Park, J.K.; Paik, T.H.; et al. Mycobacterium tuberculosis HBHA protein reacts strongly with the serum immunoglobulin M of tuberculosis patients. Clin. Vaccine Immunol. 2006, 13, 869–875. [Google Scholar] [CrossRef]
- Cambier, C.J.; Falkow, S.; Ramakrishnan, L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell 2014, 159, 1497–1509. [Google Scholar] [CrossRef]
- Bisht, D.; Meena, L.S. Adhesion molecules facilitate host-pathogen interaction & mediate Mycobacterium tuberculosis pathogenesis. Indian. J. Med. Res. 2019, 150, 23–32. [Google Scholar] [CrossRef]
- Russell, D.G. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 2007, 5, 39–47. [Google Scholar] [CrossRef]
- Ishikawa, E.; Mori, D.; Yamasaki, S. Recognition of Mycobacterial Lipids by Immune Receptors. Trends Immunol. 2017, 38, 66–76. [Google Scholar] [CrossRef]
- Neyrolles, O.; Guilhot, C. Recent advances in deciphering the contribution of Mycobacterium tuberculosis lipids to pathogenesis. Tuberculosis 2011, 91, 187–195. [Google Scholar] [CrossRef]
- Pethe, K.; Alonso, S.; Biet, F.; Delogu, G.; Brennan, M.J.; Locht, C.; Menozzi, F.D. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 2001, 412, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Pieters, J. The Trojan horse: Survival tactics of pathogenic mycobacteria in macrophages. Trends Cell Biol. 2005, 15, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lei, X.; Chai, S.; Su, G.; Du, L. From pathogenesis to antigens: The key to shaping the future of TB vaccines. Front. Immunol. 2024, 15, 1440935. [Google Scholar] [CrossRef] [PubMed]
- Vidal Pessolani, M.C.; Marques, M.A.; Reddy, V.M.; Locht, C.; Menozzi, F.D. Systemic dissemination in tuberculosis and leprosy: Do mycobacterial adhesins play a role? Microbes Infect. 2003, 5, 677–684. [Google Scholar] [CrossRef]
- Franklin, A.; Salgueiro, V.C.; Layton, A.J.; Sullivan, R.; Mize, T.; Vázquez-Iniesta, L.; Benedict, S.T.; Gurcha, S.S.; Anso, I.; Besra, G.S.; et al. The mycobacterial glycoside hydrolase LamH enables capsular arabinomannan release and stimulates growth. Nat. Commun. 2024, 15, 5740. [Google Scholar] [CrossRef]
- Kitzmiller, C.E.; Cheng, T.Y.; Prandi, J.; Sparks, I.L.; Moody, D.B.; Morita, Y.S. Detergent-induced quantitatively limited formation of diacyl phosphatidylinositol dimannoside in Mycobacterium smegmatis. J. Lipid Res. 2024, 65, 100533. [Google Scholar] [CrossRef]
- Gonzales-Huerta, L.E.; Williams, T.J.; Aljohani, R.; Robertson, B.; Evans, C.A.; Armstrong-James, D. Mycobacterial lipoarabinomannan negatively interferes with macrophage responses to Aspergillus fumigatus in-vitro. bioRxiv, 2024; preprint. [Google Scholar] [CrossRef]
- Chiang, C.Y.; West, N.P. The fall of the mycobacterial cell wall: Interrogating peptidoglycan synthesis for novel anti-TB agents. PeerJ 2024, 12, e18404. [Google Scholar] [CrossRef]
- Behr, M.A.; Divangahi, M. Freund’s adjuvant, NOD2 and mycobacteria. Curr. Opin. Microbiol. 2015, 23, 126–132. [Google Scholar] [CrossRef]
- Dulberger, C.L.; Rubin, E.J.; Boutte, C.C. The mycobacterial cell envelope—A moving target. Nat. Rev. Microbiol. 2020, 18, 47–59. [Google Scholar] [CrossRef]
- Qin, L.; Xu, J.; Chen, J.; Wang, S.; Zheng, R.; Cui, Z.; Liu, Z.; Wu, X.; Wang, J.; Huang, X.; et al. Cell-autonomous targeting of arabinogalactan by host immune factors inhibits mycobacterial growth. eLife 2024, 13, RP92737. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Y.; Zheng, R.; Tang, F.; Qin, L.; Lai, D.; Zhang, L.; Chen, L.; Yan, B.; Yang, H.; et al. Sensing of mycobacterial arabinogalactan by galectin-9 exacerbates mycobacterial infection. EMBO Rep. 2021, 22, e51678. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Klenotic, P.A.; Cui, M.; Lyu, M.; Morgan, C.E.; Yu, E.W. Structures of the mycobacterial membrane protein MmpL3 reveal its mechanism of lipid transport. PLoS Biol. 2021, 19, e3001370. [Google Scholar] [CrossRef]
- Iizasa, E.; Chuma, Y.; Uematsu, T.; Kubota, M.; Kawaguchi, H.; Umemura, M.; Toyonaga, K.; Kiyohara, H.; Yano, I.; Colonna, M.; et al. TREM2 is a receptor for non-glycosylated mycolic acids of mycobacteria that limits anti-mycobacterial macrophage activation. Nat. Commun. 2021, 12, 2299. [Google Scholar] [CrossRef]
- Cambier, C.J.; Banik, S.M.; Buonomo, J.A.; Bertozzi, C.R. Spreading of a mycobacterial cell-surface lipid into host epithelial membranes promotes infectivity. eLife 2020, 9, e60648. [Google Scholar] [CrossRef]
- Mittal, E.; Roth, A.T.; Seth, A.; Singamaneni, S.; Beatty, W.; Philips, J.A. Single cell preparations of Mycobacterium tuberculosis damage the mycobacterial envelope and disrupt macrophage interactions. eLife 2023, 12, e85416. [Google Scholar] [CrossRef]
- Driessen, N.N.; Ummels, R.; Maaskant, J.J.; Gurcha, S.S.; Besra, G.S.; Ainge, G.D.; Larsen, D.S.; Painter, G.F.; Vandenbroucke-Grauls, C.M.; Geurtsen, J.; et al. Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect. Immun. 2009, 77, 4538–4547. [Google Scholar] [CrossRef]
- Beatty, W.L.; Rhoades, E.R.; Hsu, D.K.; Liu, F.T.; Russell, D.G. Association of a macrophage galactoside-binding protein with Mycobacterium-containing phagosomes. Cell Microbiol. 2002, 4, 167–176. [Google Scholar] [CrossRef]
- Appelmelk, B.J.; den Dunnen, J.; Driessen, N.N.; Ummels, R.; Pak, M.; Nigou, J.; Larrouy-Maumus, G.; Gurcha, S.S.; Movahedzadeh, F.; Geurtsen, J.; et al. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell Microbiol. 2008, 10, 930–944. [Google Scholar] [CrossRef]
- Yonekawa, A.; Saijo, S.; Hoshino, Y.; Miyake, Y.; Ishikawa, E.; Suzukawa, M.; Inoue, H.; Tanaka, M.; Yoneyama, M.; Oh-Hora, M.; et al. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity 2014, 41, 402–413. [Google Scholar] [CrossRef]
- Decout, A.; Silva-Gomes, S.; Drocourt, D.; Blattes, E.; Rivière, M.; Prandi, J.; Larrouy-Maumus, G.; Caminade, A.M.; Hamasur, B.; Källenius, G.; et al. Deciphering the molecular basis of mycobacteria and lipoglycan recognition by the C-type lectin Dectin-2. Sci. Rep. 2018, 8, 16840. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, L.S.; Hull, S.R.; Kaufman, T.M. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J. Immunol. 1994, 152, 4070–4079. [Google Scholar] [CrossRef] [PubMed]
- Barboni, E.; Coade, S.; Fiori, A. The binding of mycolic acids to galectin-3: A novel interaction between a host soluble lectin and trafficking mycobacterial lipids? FEBS Lett. 2005, 579, 6749–6755. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Tomiyasu, N.; Torigoe, S.; Mizuno, S.; Fukano, H.; Ishikawa, E.; Katano, H.; Hoshino, Y.; Matsuo, K.; Takahashi, M.; et al. Mycobacterial mycolic acids trigger inhibitory receptor Clec12A to suppress host immune responses. Tuberculosis 2023, 138, 102294. [Google Scholar] [CrossRef]
- Ishikawa, E.; Ishikawa, T.; Morita, Y.S.; Toyonaga, K.; Yamada, H.; Takeuchi, O.; Kinoshita, T.; Akira, S.; Yoshikai, Y.; Yamasaki, S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 2009, 206, 2879–2888. [Google Scholar] [CrossRef]
- Schoenen, H.; Bodendorfer, B.; Hitchens, K.; Manzanero, S.; Werninghaus, K.; Nimmerjahn, F.; Agger, E.M.; Stenger, S.; Andersen, P.; Ruland, J.; et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J. Immunol. 2010, 184, 2756–2760. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhu, L.L.; Chang, Q.; Jiang, C.; You, Y.; Luo, T.; Jia, X.M.; Lin, X. C-type lectin receptor dectin-3 mediates trehalose 6,6'-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-κB activation. J. Biol. Chem. 2014, 289, 30052–30062. [Google Scholar] [CrossRef]
- Bowdish, D.M.; Sakamoto, K.; Kim, M.J.; Kroos, M.; Mukhopadhyay, S.; Leifer, C.A.; Tryggvason, K.; Gordon, S.; Russell, D.G. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009, 5, e1000474. [Google Scholar] [CrossRef]
- Martinez, N.; Ketheesan, N.; West, K.; Vallerskog, T.; Kornfeld, H. Impaired Recognition of Mycobacterium tuberculosis by Alveolar Macrophages From Diabetic Mice. J. Infect. Dis. 2016, 214, 1629–1637. [Google Scholar] [CrossRef]
- Geurtsen, J.; Chedammi, S.; Mesters, J.; Cot, M.; Driessen, N.N.; Sambou, T.; Kakutani, R.; Ummels, R.; Maaskant, J.; Takata, H.; et al. Identification of mycobacterial alpha-glucan as a novel ligand for DC-SIGN: Involvement of mycobacterial capsular polysaccharides in host immune modulation. J. Immunol. 2009, 183, 5221–5231. [Google Scholar] [CrossRef]
- Gagliardi, M.C.; Lemassu, A.; Teloni, R.; Mariotti, S.; Sargentini, V.; Pardini, M.; Daffé, M.; Nisini, R. Cell wall-associated alpha-glucan is instrumental for Mycobacterium tuberculosis to block CD1 molecule expression and disable the function of dendritic cell derived from infected monocyte. Cell Microbiol. 2007, 9, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, P.S.; Lightbody, K.L.; Veverka, V.; Muskett, F.W.; Kelly, G.; Frenkiel, T.A.; Gordon, S.V.; Hewinson, R.G.; Burke, B.; Norman, J.; et al. Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. Embo J. 2005, 24, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Kinhikar, A.G.; Verma, I.; Chandra, D.; Singh, K.K.; Weldingh, K.; Andersen, P.; Hsu, T.; Jacobs, W.R., Jr.; Laal, S. Potential role for ESAT6 in dissemination of M. tuberculosis via human lung epithelial cells. Mol. Microbiol. 2010, 75, 92–106. [Google Scholar] [CrossRef]
- Viljoen, A.; Alsteens, D.; Dufrêne, Y. Mechanical Forces between Mycobacterial Antigen 85 Complex and Fibronectin. Cells 2020, 9, 716. [Google Scholar] [CrossRef]
- Xolalpa, W.; Vallecillo, A.J.; Lara, M.; Mendoza-Hernandez, G.; Comini, M.; Spallek, R.; Singh, M.; Espitia, C. Identification of novel bacterial plasminogen-binding proteins in the human pathogen Mycobacterium tuberculosis. Proteomics 2007, 7, 3332–3341. [Google Scholar] [CrossRef]
- Lebrun, P.; Raze, D.; Fritzinger, B.; Wieruszeski, J.M.; Biet, F.; Dose, A.; Carpentier, M.; Schwarzer, D.; Allain, F.; Lippens, G.; et al. Differential contribution of the repeats to heparin binding of HBHA, a major adhesin of Mycobacterium tuberculosis. PLoS ONE 2012, 7, e32421. [Google Scholar] [CrossRef]
- Silva, C.A.; Danelishvili, L.; McNamara, M.; Berredo-Pinho, M.; Bildfell, R.; Biet, F.; Rodrigues, L.S.; Oliveira, A.V.; Bermudez, L.E.; Pessolani, M.C. Interaction of Mycobacterium leprae with human airway epithelial cells: Adherence, entry, survival, and identification of potential adhesins by surface proteome analysis. Infect. Immun. 2013, 81, 2645–2659. [Google Scholar] [CrossRef]
- Ramsugit, S.; Pillay, B.; Pillay, M. Evaluation of the role of Mycobacterium tuberculosis pili (MTP) as an adhesin, invasin, and cytokine inducer of epithelial cells. Braz. J. Infect. Dis. 2016, 20, 160–165. [Google Scholar] [CrossRef]
- Ramsugit, S.; Pillay, M. Mycobacterium tuberculosis Pili promote adhesion to and invasion of THP-1 macrophages. Jpn. J. Infect. Dis. 2014, 67, 476–478. [Google Scholar] [CrossRef]
- Ramsugit, S.; Guma, S.; Pillay, B.; Jain, P.; Larsen, M.H.; Danaviah, S.; Pillay, M. Pili contribute to biofilm formation in vitro in Mycobacterium tuberculosis. Antonie Van Leeuwenhoek 2013, 104, 725–735. [Google Scholar] [CrossRef]
- Alteri, C.J.; Xicohténcatl-Cortes, J.; Hess, S.; Caballero-Olín, G.; Girón, J.A.; Friedman, R.L. Mycobacterium tuberculosis produces pili during human infection. Proc. Natl. Acad. Sci. USA 2007, 104, 5145–5150. [Google Scholar] [CrossRef] [PubMed]
- Pitarque, S.; Herrmann, J.L.; Duteyrat, J.L.; Jackson, M.; Stewart, G.R.; Lecointe, F.; Payre, B.; Schwartz, O.; Young, D.B.; Marchal, G.; et al. Deciphering the molecular bases of Mycobacterium tuberculosis binding to the lectin DC-SIGN reveals an underestimated complexity. Biochem. J. 2005, 392, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Silvestre, H.; Espinosa-Cueto, P.; Sanchez-Gonzalez, A.; Esparza-Ceron, M.A.; Pereira-Suarez, A.L.; Bernal-Fernandez, G.; Espitia, C.; Mancilla, R. The 19-kDa antigen of Mycobacterium tuberculosis is a major adhesin that binds the mannose receptor of THP-1 monocytic cells and promotes phagocytosis of mycobacteria. Microb. Pathog. 2005, 39, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Kinhikar, A.G.; Vargas, D.; Li, H.; Mahaffey, S.B.; Hinds, L.; Belisle, J.T.; Laal, S. Mycobacterium tuberculosis malate synthase is a laminin-binding adhesin. Mol. Microbiol. 2006, 60, 999–1013. [Google Scholar] [CrossRef]
- Gani, Z.; Boradia, V.M.; Kumar, A.; Patidar, A.; Talukdar, S.; Choudhary, E.; Singh, R.; Agarwal, N.; Raje, M.; Iyengar Raje, C. Mycobacterium tuberculosis glyceraldehyde-3-phosphate dehydrogenase plays a dual role-As an adhesin and as a receptor for plasmin(ogen). Cell Microbiol. 2021, 23, e13311. [Google Scholar] [CrossRef]
- Kuo, C.J.; Gao, J.; Huang, J.W.; Ko, T.P.; Zhai, C.; Ma, L.; Liu, W.; Dai, L.; Chang, Y.F.; Chen, T.H.; et al. Functional and structural investigations of fibronectin-binding protein Apa from Mycobacterium tuberculosis. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1351–1359. [Google Scholar] [CrossRef]
- Ragas, A.; Roussel, L.; Puzo, G.; Rivière, M. The Mycobacterium tuberculosis cell-surface glycoprotein apa as a potential adhesin to colonize target cells via the innate immune system pulmonary C-type lectin surfactant protein A. J. Biol. Chem. 2007, 282, 5133–5142. [Google Scholar] [CrossRef]
- Esparza, M.; Palomares, B.; García, T.; Espinosa, P.; Zenteno, E.; Mancilla, R. PstS-1, the 38-kDa Mycobacterium tuberculosis glycoprotein, is an adhesin, which binds the macrophage mannose receptor and promotes phagocytosis. Scand. J. Immunol. 2015, 81, 46–55. [Google Scholar] [CrossRef]
- Kumar, S.; Puniya, B.L.; Parween, S.; Nahar, P.; Ramachandran, S. Identification of novel adhesins of M. tuberculosis H37Rv using integrated approach of multiple computational algorithms and experimental analysis. PLoS ONE 2013, 8, e69790. [Google Scholar] [CrossRef]
- Idris, I.; Abdurrahman, A.H.; Fatulrachman; Aftitah, V.B.; Fadlitha, V.B.; Sato, N.; Fujimura, T.; Takimoto, H. Invasion of human microvascular endothelial cells by Mycobacterium leprae through Mce1A protein. J. Dermatol. 2019, 46, 853–858. [Google Scholar] [CrossRef]
- Kohwiwattanagun, J.; Kawamura, I.; Fujimura, T.; Mitsuyama, M. Mycobacterial mammalian cell entry protein 1A (Mce1A)-mediated adherence enhances the chemokine production by A549 alveolar epithelial cells. Microbiol. Immunol. 2007, 51, 253–261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blake, R.; Jensen, K.; Mabbott, N.; Hope, J.; Stevens, J. The role of Mce proteins in Mycobacterium avium paratuberculosis infection. Sci. Rep. 2024, 14, 14964. [Google Scholar] [CrossRef] [PubMed]
- Ramsugit, S.; Pillay, M. Identification of Mycobacterium tuberculosis adherence-mediating components: A review of key methods to confirm adhesin function. Iran. J. Basic. Med. Sci. 2016, 19, 579–584. [Google Scholar]
- Stamm, C.E.; Pasko, B.L.; Chaisavaneeyakorn, S.; Franco, L.H.; Nair, V.R.; Weigele, B.A.; Alto, N.M.; Shiloh, M.U. Screening Mycobacterium tuberculosis Secreted Proteins Identifies Mpt64 as a Eukaryotic Membrane-Binding Bacterial Effector. mSphere 2019, 4, e00354-19. [Google Scholar] [CrossRef]
- Kramarska, E.; Squeglia, F.; De Maio, F.; Delogu, G.; Berisio, R. PE_PGRS33, an Important Virulence Factor of Mycobacterium tuberculosis and Potential Target of Host Humoral Immune Response. Cells 2021, 10, 161. [Google Scholar] [CrossRef]
- Palucci, I.; Camassa, S.; Cascioferro, A.; Sali, M.; Anoosheh, S.; Zumbo, A.; Minerva, M.; Iantomasi, R.; De Maio, F.; Di Sante, G.; et al. PE_PGRS33 Contributes to Mycobacterium tuberculosis Entry in Macrophages through Interaction with TLR2. PLoS ONE 2016, 11, e0150800. [Google Scholar] [CrossRef]
- Minerva, M.; De Maio, F.; Camassa, S.; Battah, B.; Ivana, P.; Manganelli, R.; Sanguinetti, M.; Sali, M.; Delogu, G. Evaluation of PE_PGRS33 as a potential surface target for humoral responses against Mycobacterium tuberculosis. Pathog. Dis. 2017, 75, ftx100. [Google Scholar] [CrossRef]
- Espitia, C.; Laclette, J.P.; Mondragón-Palomino, M.; Amador, A.; Campuzano, J.; Martens, A.; Singh, M.; Cicero, R.; Zhang, Y.; Moreno, C. The PE-PGRS glycine-rich proteins of Mycobacterium tuberculosis: A new family of fibronectin-binding proteins? Microbiology 1999, 145 Pt 12, 3487–3495. [Google Scholar] [CrossRef]
- Torrelles, J.B.; Azad, A.K.; Schlesinger, L.S. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J. Immunol. 2006, 177, 1805–1816. [Google Scholar] [CrossRef]
- Ehlers, S. DC-SIGN and mannosylated surface structures of Mycobacterium tuberculosis: A deceptive liaison. Eur. J. Cell Biol. 2010, 89, 95–101. [Google Scholar] [CrossRef]
- Nuzzo, I.; Galdiero, M.; Bentivoglio, C.; Galdiero, R.; Romano Carratelli, C. Apoptosis modulation by mycolic acid, tuberculostearic acid and trehalose 6,6'-dimycolate. J. Infect. 2002, 44, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.M.; Yi, F.S.; Huang, Z.Y.; Peng, P.; Wu, F.Y.; Shi, H.Z.; Zhai, K. T Cell Receptor Repertoire Analysis Reveals Signatures of T Cell Responses to Human Mycobacterium tuberculosis. Front. Microbiol. 2022, 13, 829694. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.A.; Trank-Greene, M.; Nguyenla, X.; Anastas, A.; Gurmessa, S.K.; Merutka, I.R.; Dixon, S.D.; Shumate, A.; Groncki, A.R.; Parson, M.A.H.; et al. ESAT-6 undergoes self-association at phagosomal pH and an ESAT-6-specific nanobody restricts M. tuberculosis growth in macrophages. eLife 2024, 12, RP91930. [Google Scholar] [CrossRef]
- Krishnan, N.; Robertson, B.D.; Thwaites, G. The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis. Tuberculosis 2010, 90, 361–366. [Google Scholar] [CrossRef]
- Passos, B.B.S.; Araújo-Pereira, M.; Vinhaes, C.L.; Amaral, E.P.; Andrade, B.B. The role of ESAT-6 in tuberculosis immunopathology. Front. Immunol. 2024, 15, 1383098. [Google Scholar] [CrossRef]
- Ryndak, M.B.; Chandra, D.; Laal, S. Understanding dissemination of Mycobacterium tuberculosis from the lungs during primary infection. J. Med. Microbiol. 2016, 65, 362–369. [Google Scholar] [CrossRef]
- Tengattini, S.; Bavaro, T.; Rinaldi, F.; Temporini, C.; Pollegioni, L.; Terreni, M.; Piubelli, L. Novel tuberculosis vaccines based on TB10.4 and Ag85B: State-of-art and advocacy for good practices. Vaccine 2025, 53, 126932. [Google Scholar] [CrossRef]
- Rambukkana, A.; Das, P.K.; Burggraaf, J.D.; Yong, S.; Faber, W.R.; Thole, J.E.; Harboe, M. Heterogeneity of monoclonal antibody-reactive epitopes on mycobacterial 30-kilodalton-region proteins and the secreted antigen 85 complex and demonstration of antigen 85B on the Mycobacterium leprae cell wall surface. Infect. Immun. 1992, 60, 5172–5181. [Google Scholar] [CrossRef]
- Kuo, C.J.; Bell, H.; Hsieh, C.L.; Ptak, C.P.; Chang, Y.F. Novel mycobacteria antigen 85 complex binding motif on fibronectin. J. Biol. Chem. 2012, 287, 1892–1902. [Google Scholar] [CrossRef]
- Bentley-Hibbert, S.I.; Quan, X.; Newman, T.; Huygen, K.; Godfrey, H.P. Pathophysiology of antigen 85 in patients with active tuberculosis: Antigen 85 circulates as complexes with fibronectin and immunoglobulin G. Infect. Immun. 1999, 67, 581–588. [Google Scholar] [CrossRef]
- Naito, M.; Ohara, N.; Matsumoto, S.; Yamada, T. The novel fibronectin-binding motif and key residues of mycobacteria. J. Biol. Chem. 1998, 273, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, F.D.; Reddy, V.M.; Cayet, D.; Raze, D.; Debrie, A.S.; Dehouck, M.P.; Cecchelli, R.; Locht, C. Mycobacterium tuberculosis heparin-binding haemagglutinin adhesin (HBHA) triggers receptor-mediated transcytosis without altering the integrity of tight junctions. Microbes Infect. 2006, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ashokcoomar, S.; Reedoy, K.S.; Loots, D.T.; Beukes, D.; van Reenen, M.; Pillay, B.; Pillay, M.M. tuberculosis curli pili (MTP) facilitates a reduction of microbicidal activity of infected THP-1 macrophages during early stages of infection. Comp. Immunol. Microbiol. Infect. Dis. 2022, 90–91, 101907. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ning, Y.; Wang, Y.; Deng, G.; Pace, S.; Barth, S.A.; Menge, C.; Zhang, K.; Dai, Y.; Cai, Y.; et al. Mycobacterium tuberculosis-Induced Upregulation of the COX-2/mPGES-1 Pathway in Human Macrophages Is Abrogated by Sulfasalazine. Front. Immunol. 2022, 13, 849583. [Google Scholar] [CrossRef]
- Yeremeev, V.V.; Lyadova, I.V.; Nikonenko, B.V.; Apt, A.S.; Abou-Zeid, C.; Inwald, J.; Young, D.B. The 19-kD antigen and protective immunity in a murine model of tuberculosis. Clin. Exp. Immunol. 2000, 120, 274–279. [Google Scholar] [CrossRef]
- Yeruva, V.C.; Kulkarni, A.; Khandelwal, R.; Sharma, Y.; Raghunand, T.R. The PE_PGRS Proteins of Mycobacterium tuberculosis Are Ca2+ Binding Mediators of Host-Pathogen Interaction. Biochemistry 2016, 55, 4675–4687. [Google Scholar] [CrossRef]
- Parada, C.; Neri-Badillo, I.C.; Vallecillo, A.J.; Segura, E.; Silva-Miranda, M.; Guzmán-Gutiérrez, S.L.; Ortega, P.A.; Coronado-Aceves, E.W.; Cancino-Villeda, L.; Torres-Larios, A.; et al. New Insights into the Methylation of Mycobacterium tuberculosis Heparin Binding Hemagglutinin Adhesin Expressed in Rhodococcus erythropolis. Pathogens 2021, 10, 1139. [Google Scholar] [CrossRef]
- Kulmanov, M.; Hoehndorf, R. DeepGOPlus: Improved protein function prediction from sequence. Bioinformatics 2020, 36, 422–429. [Google Scholar] [CrossRef]
- Goodswen, S.J.; Kennedy, P.J.; Ellis, J.T. A guide to current methodology and usage of reverse vaccinology towards in silico vaccine discovery. FEMS Microbiol. Rev. 2023, 47, fuad004. [Google Scholar] [CrossRef]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E., 3rd; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef]
- Kapopoulou, A.; Lew, J.M.; Cole, S.T. The MycoBrowser portal: A comprehensive and manually annotated resource for mycobacterial genomes. Tuberculosis 2011, 91, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, G.; Kumar, K.; Jain, P.; Ramachandran, S. SPAAN: A software program for prediction of adhesins and adhesin-like proteins using neural networks. Bioinformatics 2005, 21, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Nwoko, E.Q.A.; Okeke, I.N. Bacteria autoaggregation: How and why bacteria stick together. Biochem. Soc. Trans. 2021, 49, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Lipke, P.N.; Ragonis-Bachar, P. Sticking to the Subject: Multifunctionality in Microbial Adhesins. J. Fungi 2023, 9, 419. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Kulshreshtha, D.; Raghunandanan, M.V.; Ramachandran, S. Integrative immunoinformatics for Mycobacterial diseases in R platform. Syst. Synth. Biol. 2014, 8, 27–39. [Google Scholar] [CrossRef]
- Maharajh, R.; Pillay, M.; Senzani, S. A computational method for the prediction and functional analysis of potential Mycobacterium tuberculosis adhesin-related proteins. Expert. Rev. Proteomics 2023, 20, 483–493. [Google Scholar] [CrossRef]
- Fonseca, K.L.; Lozano, J.J.; Despuig, A.; Habgood-Coote, D.; Sidorova, J.; Aznar, D.; Arias, L.; Del Río-Álvarez, Á.; Carrillo-Reixach, J.; Goff, A.; et al. Unravelling the transcriptome of the human tuberculosis lesion and its clinical implications. Nat. Commun. 2025, 16, 5028. [Google Scholar] [CrossRef]
- Hossain, M.M.; Norazmi, M.N. Pattern recognition receptors and cytokines in Mycobacterium tuberculosis infection--the double-edged sword? BioMed Res. Int. 2013, 2013, 179174. [Google Scholar] [CrossRef]

| No. | Adhesin | Receptor | Phenotype During Infection | Refs. |
|---|---|---|---|---|
| 1 | Phosphatidylinositol-mannosides (PIMs) | Dendritic cell-specific ICAM-3-grabbing non-integrin (DC-SIGN) | PIMs contribute to the interaction between mycobacteria and dendritic cells, although other unknown ligands may be more dominant in this process. | [39] |
| Galectin-3 | Infection of galectin-3-deficient mice with M. tuberculosis reveals a reduced capacity to clear late-stage, but not early-stage, infection. | [40] | ||
| 2 | Mannose-capped lipoarabinomannan (manLAM) | DC-SIGN | ManLAM’s mannose cap is thought to inhibit phagolysosome fusion and promote IL-10 production, but this effect is less important in interactions with living bacteria. | [41] |
| Dectin-2 | Dectin-2 deficiency exacerbates lung pathology during mycobacterial infection in mice. | [42,43,44] | ||
| 3 | Arabinogalactan (AG) | Galectin-9 | Deletion of galectin-9 blocks AG-induced lung pathology, and the AG-galectin-9 axis exacerbates M. tuberculosis infection in mice. | [34] |
| 4 | Mycolic acid (MA) | Galectin-3 | N.A. | [45] |
| Clec12A | Clec12A-deficient mice show enhanced innate and T cell responses after infection, while human Clec12A transgenic mice are more susceptible to M. tuberculosis infection. | [46] | ||
| 5 | Trehalose 6,6′-dimycolate (TDM) | Mincle | In vivo administration of TDM triggers a significant increase in inflammatory cytokines in serum and induces characteristic lung inflammation, including granuloma formation. | [47,48] |
| Dectin-3 | TDM stimulation of Dectin-3 induces Mincle expression, potentially boosting the host’s innate immune response to Mycobacterium infection. | [49] | ||
| Macrophage receptor with collagenous structure (MARCO) | MARCO-expressing macrophages secrete pro-inflammatory cytokines in response to TDM, while macrophages from MARCO(−/−) mice produce significantly lower cytokine levels upon infection with virulent M. tuberculosis. | [50,51] | ||
| 6 | α-glucan | DC-SIGN | Inhibits DC maturation and suppresses DC-mediated immune responses; additionally, induces IL-10 secretion. | [52,53] |
| 7 | Early secretory antigenic target 6 kDa (ESAT-6) *, culture filtrate protein 10 kDa (CFP-10) * | N.A. | The binding of the fluorescently labeled ESAT-6-CFP-10 complex to the surface of macrophages is directly mediated by the protein complex. Moreover, the flexible C-terminal arm of CFP-10 plays a crucial role in this interaction, forming an essential part of the binding site for the cell surface receptor. | [54] |
| 8 | ESAT-6 * | Laminin | ESAT-6 induces cytolysis in both type 1 and type 2 pneumocytes. | [55] |
| 9 | Antigen 85 (Ag85) complex | Fibronectin (Fn) | Fn depletion through siRNA significantly impaired the binding of purified Ag85B to human epithelial cells, highlighting the crucial role of the Ag85B-Fn interaction in epithelial adhesion. | [56] |
| Ag85B | Plasminogen (Plg) | Ag85B binds to human Plg, and promotes its activation to plasmin (Plm), potentially enhancing the extracellular matrix degradation and tissue invasion of M. tuberculosis. | [57] | |
| 10 | Heparin-Binding Hemagglutinin Adhesin (HBHA) | Heparan sulfate glycosaminoglycans | Facilitates bacillary adhesion to host cells and promotes epithelial transcytosis, enabling M. tuberculosis to establish systemic infection. | [58,59] |
| 11 | Mycobacterium tuberculosis curli pili (MTP) | N.A. | An MTP-deficient strain of Mycobacterium tuberculosis exhibits a significant reduction in its ability to adhere to and invade A549 pulmonary epithelial cells, with decreases of 69.39% (p = 0.047) and 56.20% (p = 0.033), respectively. | [60] |
| N.A. | Adhesion to and invasion of macrophages are reduced by 42.16% (p = 0.107) and 69.02% (p = 0.052), respectively, in the pili-deficient Δmtp mutant compared to the wild-type. | [61] | ||
| Laminin | MTP binds to the extracellular matrix protein laminin and contributes to biofilm formation. Isogenic mtp mutants lose the ability to produce MTP in vitro and demonstrate decreased laminin-binding capabilities. | [62,63] | ||
| 12 | 19-kDa antigen | DC-SIGN | N.A. | [64] |
| Mannose receptor | The 19-kDa antigen (Rv3763) binds to THP-1 macrophages through the macrophage mannose receptor (MR), promoting mycobacterial uptake. | [65] | ||
| 13 | Malate synthase (GlcB) | Laminin, Fn | Antibodies targeting the C-terminal laminin/fibronectin-binding domain inhibit the binding of M. tuberculosis to laminin and fibronectin, significantly reducing its adherence to A549 lung epithelial cells. | [66] |
| 14 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Plg, Plm | Enhances bacterial binding to and translocation across lung epithelial cell barriers. | [67] |
| 15 | Alanine and proline-rich protein (Apa) | DC-SIGN | N.A. | [64] |
| Fibronectin | Peptides 177-201 and 269-292 of Apa-A specifically bind fibronectin, with peptide 269-292 additionally inhibiting full-length protein interactions. | [68] | ||
| human pulmonary surfactant protein A (PSP-A) | Apa remains stably associated with the cell wall, providing a platform that facilitates the attachment of PSP-A, thereby enhancing bacterial adhesion to host surfaces. | [69] | ||
| 16 | PstS-1 | Mannose receptor | N.A. | [70] |
| 17 | N-acetylmuramoyl-L-alanine amidase (Rv3717) | Fn, laminin | N.A. | [71] |
| 18 | Mycobacterial mammalian cell entry protein 1A (Mce1A) | N.A. | Mce1A protein is crucial for Mycobacterium leprae invasion into human microvascular endothelial cells (HMVECs), and antibodies targeting Mce1A may inhibit this process. | [72] |
| N.A. | Mce1A enhances bacterial adhesion and intracellular survival, promoting infection in monocytes and 3D bovine enteroids. | [73,74] | ||
| 19 | GlnA1 | Plg, Fn | GlnA1 binds to human Plg and facilitates its activation to Plm, while also interacting with the extracellular matrix protein fibronectin, potentially enhancing M. tuberculosis tissue invasion. | [57] |
| 20 | Mpt64 | N.A. | N.A. | [57,75,76] |
| 21 | PE_PGRS33 (Rv1818c) | Toll-like receptor 2 (TLR2) | PE-PGRS33 binds to TLR2 on macrophages in a calcium-dependent manner, facilitating bacterial adhesion and entry. | [77,78,79] |
| 22 | PE_PGRS81 (Rv1759c) | Fn | PE-PGRS81 binds fibronectin via its C-terminal fragment and shows reactivity with sera from TB patients. | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Ma, Y.; Lei, X.; Chai, S.; Zhang, S.; Su, G.; Li, S.; Du, L. Stopping Tuberculosis at the Gate: The Role of M. tuberculosis Adhesins in Infection and Intervention. Vaccines 2025, 13, 676. https://doi.org/10.3390/vaccines13070676
Yang H, Ma Y, Lei X, Chai S, Zhang S, Su G, Li S, Du L. Stopping Tuberculosis at the Gate: The Role of M. tuberculosis Adhesins in Infection and Intervention. Vaccines. 2025; 13(7):676. https://doi.org/10.3390/vaccines13070676
Chicago/Turabian StyleYang, Haoyan, Yinuo Ma, Xinkui Lei, Siyu Chai, Sigen Zhang, Guimin Su, Songping Li, and Lin Du. 2025. "Stopping Tuberculosis at the Gate: The Role of M. tuberculosis Adhesins in Infection and Intervention" Vaccines 13, no. 7: 676. https://doi.org/10.3390/vaccines13070676
APA StyleYang, H., Ma, Y., Lei, X., Chai, S., Zhang, S., Su, G., Li, S., & Du, L. (2025). Stopping Tuberculosis at the Gate: The Role of M. tuberculosis Adhesins in Infection and Intervention. Vaccines, 13(7), 676. https://doi.org/10.3390/vaccines13070676





