A Single Dose of Yellow Fever Vaccine Provides Long-Term Immunity in Japanese Travelers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Trial Registration
2.2. Participants
2.3. Study Procedures
2.4. Measurement of Anti-YFV-Neutralizing Antibody Titers
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Characteristics
3.2. Protection and GMT After a Single Dose of YF Vaccine
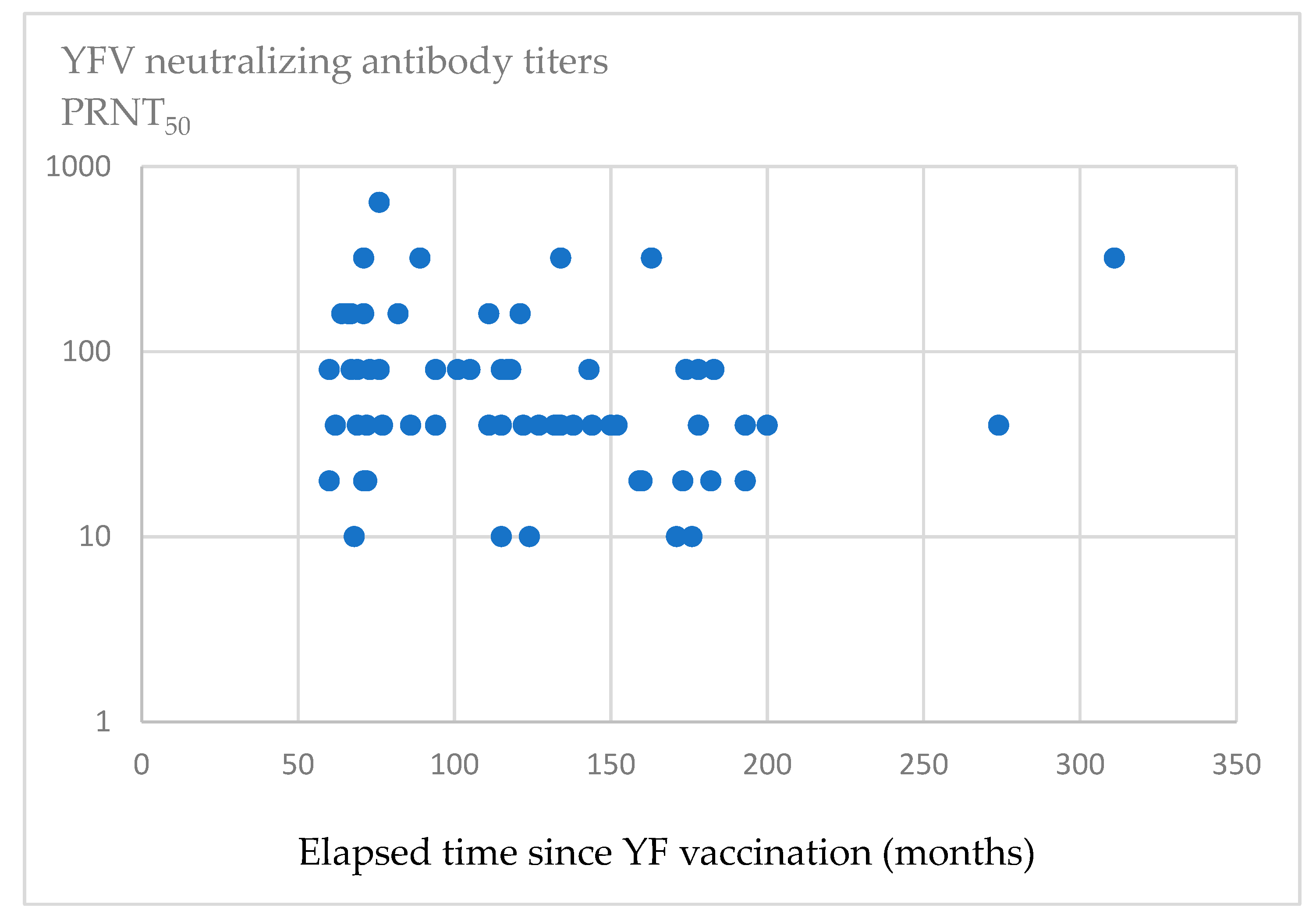
3.3. Relationship Between Antibody Titers and Time Elapsed Since YF Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| YF | Yellow fever |
| YFV | Yellow fever virus |
| WHO | World Health Organization |
| PRNT50 | 50% plaque reduction neutralizing antibody test |
| IQR | Interquartile range |
| GMT | Geometric mean titer |
| CI | Confidence interval |
| PFU | Plaque-forming unit |
References
- WHO. Fact Sheet: Yellow Fever, WHO. 31 May 2023. Available online: http://www.who.int/mediacentre/factsheets/fs100/en/ (accessed on 17 May 2025).
- WHO. Countries with Risk of Yellow Fever Transmission and Countries Requiring Yellow Fever Vaccination (November 2022). Available online: https://www.who.int/publications/m/item/countries-with-risk-of-yellow-fever-transmission-and-countries-requiring-yellow-fever-vaccination-(november-2022) (accessed on 17 May 2025).
- Servadio, J.L.; Muñoz-Zanzi, C.; Convertino, M. Estimating case fatality risk of severe Yellow Fever cases: Systematic literature review and meta-analysis. BMC Infect. Dis. 2021, 21, 819. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.E.; Gershman, M.; Fischer, M. Yellow fever vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2010, 59, 1–27. [Google Scholar]
- WHO. Vaccines and vaccination against yellow fever. WHO position paper—June 2013. Wkly. Epidemiol. Rec. 2013, 88, 269–283. [Google Scholar]
- WHO. Q&A on the Extension to Life for Yellow Fever Vaccination. Available online: https://www.who.int/publications/m/item/q-a-on-the-extension-to-life-for-yellow-fever-vaccination (accessed on 17 May 2025).
- Gotuzzo, E.; Yactayo, S.; Cordova, E. Efficacy and duration of immunity after yellow fever vaccination: Systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 2013, 89, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.E.; Bocchini, J.A., Jr.; Rubin, L.; Fischer, M. Yellow fever vaccine booster doses: Recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 647–650. [Google Scholar]
- Amanna, I.J.; Slifka, M.K. Questions regarding the safety and duration of immunity following live yellow fever vaccination. Expert. Rev. Vaccines 2016, 15, 1519–1533. [Google Scholar] [CrossRef] [PubMed]
- Campi-Azevedo, A.C.; Costa-Pereira, C.; Antonelli, L.R.; Fonseca, C.T.; Teixeira-Carvalho, A.; Villela-Rezende, G.; Santos, R.A.; Batista, M.A.; Campos, F.M.; Pacheco-Porto, L.; et al. Booster dose after 10 years is recommended following 17DD-YF primary vaccination. Hum. Vaccin. Immunother. 2016, 12, 491–502. [Google Scholar] [CrossRef]
- Plotkin, S.A. Ten yearly yellow fever booster vaccinations may still be justified. J. Travel. Med. 2018, 25, tay130. [Google Scholar] [CrossRef]
- De Santis, R.; Faggioni, G.; Amoroso, A.; Ciammaruconi, A.; Pomponi, A.; Stella Lia, M.; Amatore, D.; Molinari, F.; Petralito, G.; Stefanelli, P.; et al. Durability of neutralizing antibodies against yellow fever virus after vaccination in healthy adults. Vaccine 2023, 41, 2761–2763. [Google Scholar] [CrossRef]
- Vianna, C.M.; Noronha, T.G.; Camacho, L.A.B.; Andrade, R.C.; de Souza Brum, R.C.; Dos Santos, E.M.; Aguiar, D.F.; Dos Santos, M.L.B.; de Souza Cruz, R.L.; de Lima, S.M.B.; et al. Duration of post-vaccination immunity to yellow fever in volunteers ten years after a dose-response study—A complementary study. Vaccine 2024, 42, 126083. [Google Scholar] [CrossRef]
- Taga, K.; Imura, S.; Hayashi, A.; Kamakura, K.; Hashimoto, S.; Takasaki, T.; Kurane, I.; Uchida, Y. Antibody responses in Japanese volunteers after immunization with yellow fever vaccine. Kansenshogaku Zasshi J. Jpn. Assoc. Infect. Dis. 2002, 76, 738–746. [Google Scholar] [CrossRef][Green Version]
- Camacho, L.A.; Freire Mda, S.; Leal Mda, L.; Aguiar, S.G.; Nascimento, J.P.; Iguchi, T.; Lozana Jde, A.; Farias, R.H. Collaborative Group for the Study of Yellow Fever Vaccines. Immunogenicity of WHO-17D and Brazilian 17DD yellow fever vaccines: A randomized trial. Rev. Saude Publica 2004, 38, 671–678. [Google Scholar] [CrossRef]
- Monath, T.P.; Soike, K.; Levenbook, I.; Zhang, Z.X.; Arroyo, J.; Delagrave, S.; Myers, G.; Barrett, A.D.; Shope, R.E.; Ratterree, M.; et al. Recombinant, chimaeric live, attenuated vaccine (ChimeriVax) incorporating the envelope genes of Japanese encephalitis (SA14-14-2) virus and the capsid and nonstructural genes of yellow fever (17D) virus is safe, immunogenic and protective in non-human primates. Vaccine 1999, 17, 1869–1882. [Google Scholar]
- Julander, J.G.; Trent, D.W.; Monath, T.P. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine 2011, 29, 6008–6016. [Google Scholar] [CrossRef]
- Mantel, N.; Piras-Douce, F.; Chautard, E.; Marcos-Lopez, E.; Bodinham, C.L.; Cosma, A.; Courtois, V.; Dhooge, N.; Gautheron, S.; Kaufmann, S.H.E.; et al. Cynomolgus macaques as a translational model of human immune responses to yellow fever 17D vaccination. J. Virol. 2024, 98, e0151623. [Google Scholar] [CrossRef]
- Maeki, T.; Tajima, S.; Ando, N.; Wakimoto, Y.; Hayakawa, K.; Kutsuna, S.; Kato, F.; Taniguchi, S.; Nakayama, E.; Lim, C.K.; et al. Analysis of cross-reactivity among flaviviruses using sera of patients with dengue showed the importance of neutralization tests with paired serum samples for the correct interpretations of serological test results for dengue. J. Infect. Chemother. 2023, 29, 469–474. [Google Scholar] [CrossRef]
- Moi, M.L.; Lim, C.K.; Kotaki, A.; Takasaki, T.; Kurane, I. Discrepancy in dengue virus neutralizing antibody titers between plaque reduction neutralizing tests with Fcgamma receptor (FcgammaR)-negative and FcgammaR-expressing BHK-21 cells. Clin. Vaccine Immunol. 2010, 17, 402–407. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1106. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Um, J.; Nix, C.D.; Messer, W.B.; Zhu, Y.; Park, J.S.; Collins, M.H.; Chin, B. Long-term immunity after vaccination against yellow fever in Korean travelers. Jpn. J. Infect. Dis. 2025, 78, 79–84. [Google Scholar] [CrossRef]
- Akondy, R.S.; Akondy, R.S.; Monson, N.D.; Miller, J.D.; Edupuganti, S.; Teuwen, D.; Wu, H.; Quyyumi, F.; Garg, S.; Altman, J.D.; et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J. Immunol. 2009, 183, 7919–7930. [Google Scholar] [CrossRef]
- Fuertes Marraco, S.A.; Fuertes Marraco, S.A.; Soneson, C.; Cagnon, L.; Gannon, P.O.; Allard, M.; Abed Maillard, S.; Montandon, N.; Rufer, N.; Waldvogel, S.; et al. Long-lasting stem cell–like memory CD8+ T cells with a naïve-like profile upon yellow fever vaccination. Sci. Transl. Med. 2015, 7, 282ra48. [Google Scholar] [CrossRef]
- Schnyder, J.L.; Bache, B.E.; Welkers, M.R.A.; Spijker, R.; Schaumburg, F.; Goorhuis, A.; Grobusch, M.P.; de Jong, H.K. Yellow fever breakthrough infections after yellow fever vaccination: A systematic review and meta-analysis. Lancet Microbe 2024, 5, 100937. [Google Scholar] [CrossRef]
- Monath, T.P.; Cetron, M.S.; McCarthy, K.; Nichols, R.; Archambault, W.T.; Weld, L.; Bedford, P. Yellow fever 17D vaccine safety and immunogenicity in the elderly. Hum. Vaccin. 2005, 1, 207–214. [Google Scholar] [CrossRef]
- Rosenstein, M.D.; de Visser, A.W.; Visser, L.G.; Roukens, A.H.E. Long-term immunity after a single yellow fever vaccination in travelers vaccinated at 60 years or older: A 10-year follow-up study. J. Travel. Med. 2021, 28, taab126. [Google Scholar] [CrossRef]
- Burkhard, J.; Ciurea, A.; Gabay, C.; Hasler, P.; Müller, R.; Niedrig, M.; Fehr, J.; Villiger, P.; Visser, L.G.; de Visser, A.W.; et al. Long-term immunogenicity after yellow fever vaccination in immunosuppressed and healthy individuals. Vaccine 2020, 38, 3610–3617. [Google Scholar] [CrossRef]
- Michel, R.; Berger, F.; Ravelonarivo, J.; Dussart, P.; Dia, M.; Nacher, M.; Rogier, S.; Moua, D.; Sarr, F.D.; Diop, O.M.; et al. Observational study on immune response to yellow fever and measles vaccines in 9 to 15-month old children. Is it necessary to wait 4 weeks between two live attenuated vaccines? Vaccine 2015, 33, 2301–2306. [Google Scholar] [CrossRef]
- Grobusch, M.P.; van Aalst, M.; Goorhuis, A. Yellow fever vaccination -Once in a lifetime? Travel. Med. Infect. Dis. 2017, 15, 1–2. [Google Scholar] [CrossRef]
- Visser, L.G.; Veit, O.; Chen, L.H. Waning immunity after single-dose yellow fever vaccination: Who needs a second shot? J. Travel. Med. 2019, 26, tay134. [Google Scholar] [CrossRef]
- Kareko, B.W.; Booty, B.L.; Nix, C.D.; Lyski, Z.L.; Slifka, M.K.; Amanna, I.J.; Messer, W.B. Persistence of neutralizing antibody responses among yellow fever virus 17D vaccinees living in a nonendemic setting. J. Infect. Dis. 2020, 221, 2018–2025. [Google Scholar] [CrossRef]
- Staples, J.E.; Barrett, A.D.T.; Wilder-Smith, A.; Hombach, J. Review of data and knowledge gaps regarding yellow fever vaccine-induced immunity and duration of protection. NPJ Vaccines 2020, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Campi-Azevedo, A.C.; Peruhype-Magalhaes, V.; Coelho-Dos-Reis, J.G.; Antonelli, L.R.; Costa-Pereira, C.; Speziali, E.; Reis, L.R.; Lemos, J.A.; Ribeiro, J.G.L.; Bastos Camacho, L.A.; et al. 17DD Yellow fever revaccination and heightened long-term immunity in populations of disease-endemic areas, Brazil. Emerg. Infect. Dis. 2019, 25, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, J.L.; de Jong, H.K.; Bache, B.E.; Schaumburg, F.; Grobusch, M.P. Long-term immunity following yellow fever vaccination: A systematic review and meta-analysis. Lancet Glob. Health 2024, 12, e445–e456. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for Yellow Fever Vaccine Booster Doses. Available online: https://www.cdc.gov/acip/grade/yf-vac-boost.html (accessed on 17 May 2025).
- WHO. For Further Information on the EYE Initiative. Available online: https://www.who.int/initiatives/eye-strategy#:~:text=The%20EYE%20strategy%20is%20a,prevent%20international%20spread%3B%20and (accessed on 17 May 2025).
| Characteristics | N (%) | Seroprevalence % (95%CI) | GMT (95%CI) |
|---|---|---|---|
| Total | 65 | 100 (94.5–100) | 55.7 (44.1–70.3) |
| Sex | |||
| Male | 35 (53.85) | 100 (90.0–100) | 60.6 (44.2–83.4) |
| Female | 30 (46.15) | 100 (88.4–100) | 50.4 (35.1–72.4) |
| p = 1 | p = 0.435 | ||
| Age at YF vaccination (years) | |||
| <40 | 40 (61.5) | 100 (91.2–100) | 51.9 (39.1–68.9) |
| ≥40 | 25 (38.5) | 100 (86.3–100) | 62.3 (40.6–95.7) |
| p = 1 | p = 0.449 | ||
| Age at registration (years) | |||
| <40 | 35 (53.85) | 100 (81.5–100) | 58.8 (38.9–88.9) |
| ≥40 | 30 (46.15) | 100 (92.5–100) | 54.5 (40.7–72.9) |
| p = 1 | p = 0.775 | ||
| Time elapsed since YF vaccination (years) | |||
| 5–9 | 35 (53.85) | 100 (90.0–100) | 68.3 (49.8–93.8) |
| 10–26 | 30 (46.15) | 100 (88.4–100) | 43.9 (31.0–61.9) |
| p = 1 | p = 0.059 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukushima, S.; Lim, C.K.; Hamada, A. A Single Dose of Yellow Fever Vaccine Provides Long-Term Immunity in Japanese Travelers. Vaccines 2025, 13, 675. https://doi.org/10.3390/vaccines13070675
Fukushima S, Lim CK, Hamada A. A Single Dose of Yellow Fever Vaccine Provides Long-Term Immunity in Japanese Travelers. Vaccines. 2025; 13(7):675. https://doi.org/10.3390/vaccines13070675
Chicago/Turabian StyleFukushima, Shinji, Chang Kweng Lim, and Atsuo Hamada. 2025. "A Single Dose of Yellow Fever Vaccine Provides Long-Term Immunity in Japanese Travelers" Vaccines 13, no. 7: 675. https://doi.org/10.3390/vaccines13070675
APA StyleFukushima, S., Lim, C. K., & Hamada, A. (2025). A Single Dose of Yellow Fever Vaccine Provides Long-Term Immunity in Japanese Travelers. Vaccines, 13(7), 675. https://doi.org/10.3390/vaccines13070675






