COVID-19 Vaccine Timing and Co-Administration with Influenza Vaccines in Canada: A Systematic Review with Comparative Insights from G7 Countries
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
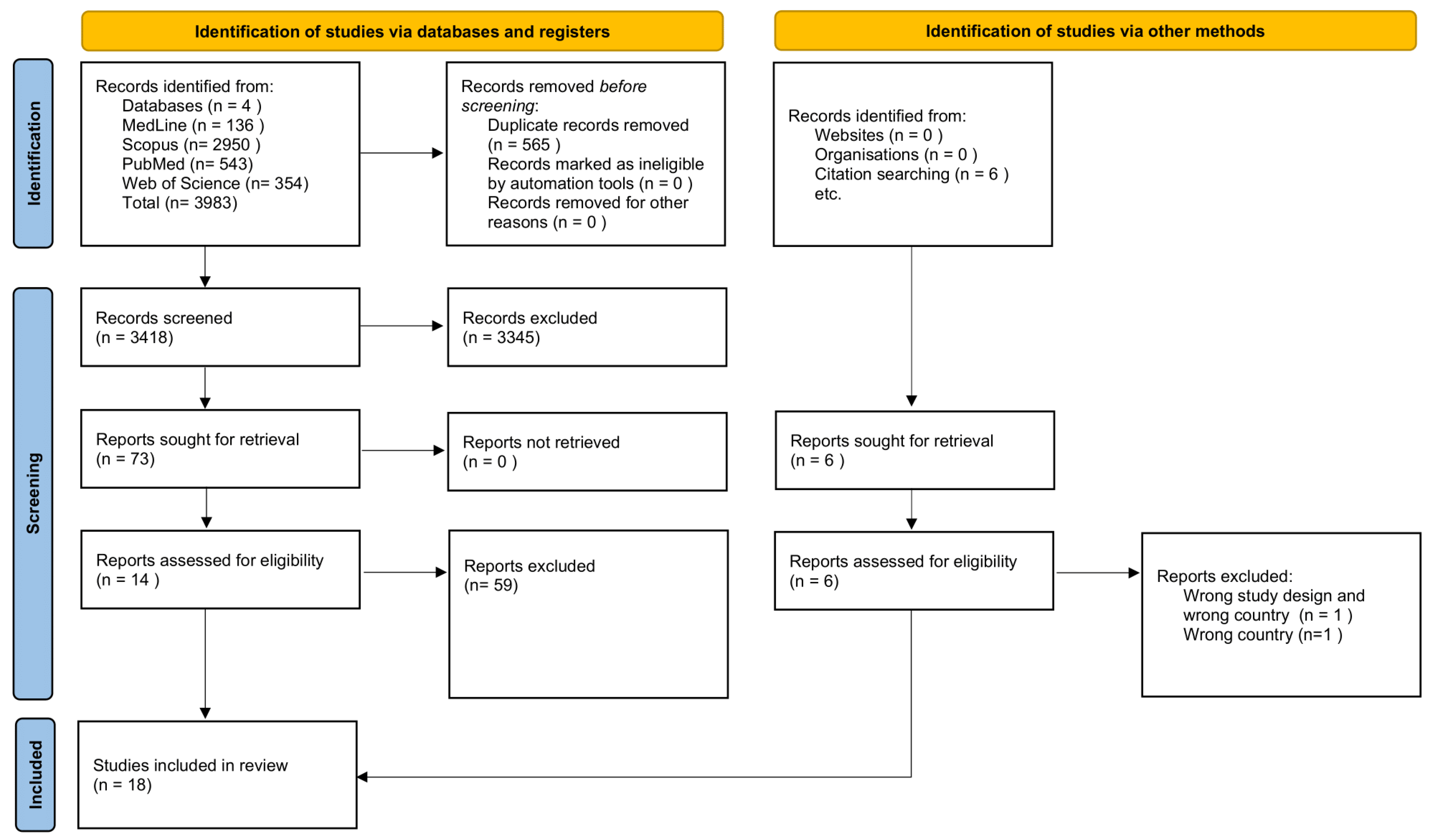
2.3. Study Selection
2.4. Data Extraction
2.5. Narrative Heterogeneity Assessment
3. Results
3.1. General Characteristics of the Included Studies
3.2. Impact of Vaccine Dosing Intervals and Administration Strategies
3.3. Integration of COVID-19 Vaccination with Other Respiratory Vaccines
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Database | Search Query |
|---|---|
| MedLine (via Web of Science) | https://www.webofscience.com/wos/medline/summary/6f68e2f4-8459-485e-8f6a-14f502875115-fa5f76ab/relevance/1 (accessed on 7 July 2024) |
| Scopus | TITLE-ABS-KEY (“2019 Novel Coronavirus*” OR 2019-ncov OR “2019 nCoV” OR “2019-nCoV Infection” OR “2019 nCoV Infection” OR “2019 Novel Coronavirus Disease” OR “2019 Novel Coronavirus Infection” OR “2019-nCoV Disease” OR “2019 nCoV Disease” OR “Coronavirus Disease 2019 Virus” OR “COVID-19 Virus*” OR “COVID 19 Virus*” OR “COVID 19” OR “COVID-19 Virus Disease” OR “COVID 19 Virus Disease” OR “COVID-19 Virus Infection” OR “COVID 19 Virus Infection” OR “Coronavirus Disease-19” OR “Coronavirus Disease 19” OR “Coronavirus Disease 2019” OR “SARS-CoV-2 Virus*” OR “SARS Coronavirus 2” OR “Severe Acute Respiratory Syndrome Coronavirus 2” OR “SARS Coronavirus 2 Infection” OR “SARS-CoV-2 Infection” OR “SARS-CoV-2 Infection” OR “Omicron variant” OR “Omicron subvariant” OR “Delta variant” OR “Delta subvariant”) AND (“vaccine booster” OR “booster shot” OR “vaccine third dose” OR ((vaccine OR vaccination OR immunization OR immunisation) AND (“fourth dose” OR “additional dose”))) AND (canad* OR alberta OR ontario OR manitoba OR “British Columbia” OR quebec OR “Nova Scotia” OR “New Brunswick” OR “Prince Edward Island” OR saskatchewan OR “Newfoundland and Labrador” OR toronto OR vancouver OR edmonton OR calgary OR winnipeg OR regina OR montreal OR moncton OR fredericton OR victoria OR charlottetown OR saskatoon OR “St. John’s” OR “Quebec City” OR halifax OR nunavut) AND (effective* OR efficac* OR immunogenic* OR “immune response” OR “humoral response” OR “antibody response” OR “antibody titer” OR neutralization OR “neutralizing antibodies” OR “duration of protection” OR “dosing timing” OR neutralisation OR “dosing interval” OR “optimal timing” OR infection* OR virulence OR severity OR hospitalization* OR hospitalized OR “transmission dynamics” OR incidence OR epidemiology OR epidemiological) AND (LIMIT-TO(AFFILCOUNTRY, “Canada”)) AND (LIMIT-TO(DOCTYPE, “ar”) OR LIMIT-TO(DOCTYPE, “cp”)) AND (LIMIT-TO(PUBYEAR, 2021) OR LIMIT-TO(PUBYEAR, 2022) OR LIMIT-TO(PUBYEAR, 2023) OR LIMIT-TO(PUBYEAR, 2024)) AND (LIMIT-TO(LANGUAGE, “English”)) AND (LIMIT-TO(EXACTKEYWORD, “Human”)) |
| PubMed | (“2019 Novel Coronavirus” OR 2019-nCoV OR “2019 nCoV” OR “2019-nCoV Infection” OR “2019 nCoV Infection” OR “2019 Novel Coronavirus Disease” OR “2019 Novel Coronavirus Infection” OR “2019-nCoV Disease” OR “2019 nCoV Disease” OR “Coronavirus Disease 2019 Virus” OR “COVID-19 Virus*” OR “COVID 19 Virus*” OR “COVID 19” OR “COVID-19 Virus Disease” OR “COVID 19 Virus Disease” OR “COVID-19 Virus Infection” OR “COVID 19 Virus Infection” OR “Coronavirus Disease-19” OR “Coronavirus Disease 19” OR “Coronavirus Disease 2019” OR “SARS-CoV-2 Virus*” OR “SARS Coronavirus 2” OR “Severe Acute Respiratory Syndrome Coronavirus 2” OR “SARS Coronavirus 2 Infection” OR “SARS-CoV-2 Infection” OR “SARS-CoV-2 Infection” OR “Omicron variant” OR “Omicron subvariant” OR “Delta variant” OR “Delta subvariant”) AND (“vaccine booster” OR “booster shot” OR “vaccine third dose” OR ((vaccine OR vaccination OR immunization OR immunisation) AND (“fourth dose” OR “additional dose”))) AND (Canad* OR Alberta OR Ontario OR Manitoba OR “British Columbia” OR Quebec OR “Nova Scotia” OR “New Brunswick” OR “Prince Edward Island” OR Saskatchewan OR “Newfoundland and Labrador” OR Toronto OR Vancouver OR Edmonton OR Calgary OR Winnipeg OR Regina OR Montreal OR Moncton OR Fredericton OR Victoria OR Charlottetown OR Saskatoon OR “St. John’s” OR “Quebec City” OR Halifax) AND (effective* OR efficac* OR immunogenic* OR “immune response” OR “humoral response” OR “antibody response” OR “antibody titer” OR neutralization OR “neutralizing antibodies” OR “duration of protection” OR “dosing timing” OR neutralisation OR “dosing interval” OR “optimal timing” OR infection* OR virulence OR severity OR hospitalization* OR hospitalized OR “transmission dynamics” OR incidence OR epidemiology OR epidemiological) AND (“17 August 2021”[PDAT]: “7 July 2024”[PDAT]) Filters: English, Humans |
| Web Of Science | https://www.webofscience.com/wos/woscc/summary/3d059cdf-83c7-48c7-b515-29a58df9b98b-fa5eba52/date-descending/1 (accessed on 7 July 2024) |
| Database | Search Query |
|---|---|
| MedLine (via Web of Science) | https://www.webofscience.com/wos/medline/summary/8ee6aea1-6199-4a03-9c29-f5874b31e393-fbd26303/relevance/1 (accessed on 7 July 2024) |
| Scopus | (“2019 Novel Coronavirus*” OR 2019-ncov OR “2019 nCoV” OR “2019-nCoV Infection” OR “2019 nCoV Infection” OR “2019 Novel Coronavirus Disease” OR “2019 Novel Coronavirus Infection” OR “2019-nCoV Disease” OR “2019 nCoV Disease” OR “Coronavirus Disease 2019 Virus” OR “COVID-19 Virus*” OR “COVID 19 Virus*” OR “COVID 19” OR “COVID-19 Virus Disease” OR “COVID 19 Virus Disease” OR “COVID-19 Virus Infection” OR “COVID 19 Virus Infection” OR “Coronavirus Disease-19” OR “Coronavirus Disease 19” OR “Coronavirus Disease 2019” OR “SARS-CoV-2 Virus*” OR “SARS Coronavirus 2” OR “Severe Acute Respiratory Syndrome Coronavirus 2” OR “SARS Coronavirus 2 Infection” OR “SARS-CoV-2 Infection” OR “SARS-CoV-2 Infection” OR “Omicron variant” OR “Omicron subvariant” OR “Delta variant” OR “Delta subvariant”) AND (“vaccine booster” OR “booster shot” OR “vaccine third dose” OR ((vaccine OR vaccination OR immunization OR immunisation) AND (“fourth dose” OR “additional dose”))) AND (france OR “French Republic” OR germany OR “Federal Republic of Germany” OR italy OR “Italian Republic” OR japan OR “Nippon” OR “Nihon” OR “United Kingdom” OR “UK” OR “Great Britain” OR england OR scotland OR wales OR “Northern Ireland” OR “United States” OR “USA” OR “United States of America” OR “US” OR “U.S.” OR “America” OR “American”) AND (effective* OR efficac* OR immunogenic* OR “immune response” OR “humoral response” OR “antibody response” OR “antibody titer” OR neutralization OR “neutralizing antibodies” OR “duration of protection” OR “dosing timing” OR neutralisation OR “dosing interval” OR “optimal timing” OR infection* OR virulence OR severity OR hospitalization* OR hospitalized OR “transmission dynamics” OR incidence OR epidemiology OR epidemiological) AND (LIMIT-TO(AFFILCOUNTRY, “United States”) OR LIMIT-TO(AFFILCOUNTRY, “United Kingdom”) OR LIMIT-TO(AFFILCOUNTRY, “Italy”) OR LIMIT-TO(AFFILCOUNTRY, “Germany”) OR LIMIT-TO(AFFILCOUNTRY, “Japan”) OR LIMIT-TO(AFFILCOUNTRY, “France”)) AND (LIMIT-TO(DOCTYPE, “ar”) OR LIMIT-TO(DOCTYPE, “cp”)) AND (LIMIT-TO(PUBYEAR, 2021) OR LIMIT-TO(PUBYEAR, 2022) OR LIMIT-TO(PUBYEAR, 2023) OR LIMIT-TO(PUBYEAR, 2024)) AND (LIMIT-TO(LANGUAGE, “English”)) |
| PubMed | (“2019 Novel Coronavirus*” OR 2019-nCoV OR “2019 nCoV” OR “2019-nCoV Infection” OR “2019 nCoV Infection” OR “2019 Novel Coronavirus Disease” OR “2019 Novel Coronavirus Infection” OR “2019-nCoV Disease” OR “2019 nCoV Disease” OR “Coronavirus Disease 2019 Virus” OR “COVID-19 Virus*” OR “COVID 19 Virus*” OR “COVID 19” OR “COVID-19 Virus Disease” OR “COVID 19 Virus Disease” OR “COVID-19 Virus Infection” OR “COVID 19 Virus Infection” OR “Coronavirus Disease-19” OR “Coronavirus Disease 19” OR “Coronavirus Disease 2019” OR “SARS-CoV-2 Virus*” OR “SARS Coronavirus 2” OR “Severe Acute Respiratory Syndrome Coronavirus 2” OR “SARS Coronavirus 2 Infection” OR “SARS-CoV-2 Infection” OR “SARS-CoV-2 Infection” OR “Omicron variant” OR “Omicron subvariant” OR “Delta variant“ OR “Delta subvariant“) AND (“vaccine booster” OR “booster shot” OR “vaccine third dose” OR ((vaccine OR vaccination OR immunization OR immunisation) AND (“fourth dose” OR “additional dose”))) AND (France OR “French Republic” OR Germany OR “Federal Republic of Germany” OR Italy OR “Italian Republic” OR Japan OR “Nippon” OR “Nihon” OR “United Kingdom” OR “UK” OR “Great Britain” OR England OR Scotland OR Wales OR “Northern Ireland” OR “United States” OR “USA” OR “United States of America” OR “US” OR “U.S.” OR “America” OR “American”) AND (effective* OR efficac* OR immunogenic* OR “immune response” OR “humoral response” OR “antibody response” OR “antibody titer” OR neutralization OR “neutralizing antibodies” OR “duration of protection” OR “dosing timing” OR neutralisation OR “dosing interval” OR “optimal timing” OR infection* OR virulence OR severity OR hospitalization* OR hospitalized OR “transmission dynamics” OR incidence OR epidemiology OR epidemiological) AND (“17 August 2021”[PDAT]: “7 July 2024”[PDAT]) Filters: Humans, English |
| Web of Science | https://www.webofscience.com/wos/woscc/summary/09409e99-aa93-4295-bb7a-e08302dfe7c6-fbd2377b/relevance/1 (accessed on 7 July 2024) |
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. COVID-19 Epidemiology Update: Current Situation. Available online: https://health-infobase.canada.ca/covid-19/current-situation.html (accessed on 30 November 2024).
- Population Reference Bureau. Countries with the Oldest Populations in the World. 2020. Available online: https://www.prb.org/resources/countries-with-the-oldest-populations-in-the-world/ (accessed on 21 March 2025).
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Gavrilov, D.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; et al. COVID-19 Pandemic. Our World in Data. 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 21 March 2025).
- Wang, Q.; Hu, S.; Du, F.; Zang, S.; Xing, Y.; Qu, Z.; Zhang, X.; Lin, L.; Hou, Z. Mapping global acceptance and uptake of COVID-19 vaccination: A systematic review and meta-analysis. Commun. Med. 2022, 2, 113. [Google Scholar] [CrossRef]
- G7 Health Ministers. G7 Health Ministers’ Communiqué, Berlin, May 2022. Available online: https://www.g7germany.de/resource/blob/974430/2042058/5651daa321517b089cdccfaffd1e37a1/2022-05-20-g7-health-ministers-communique-data.pdf (accessed on 21 March 2025).
- Wyonch, R.; Zhang, T. Damage Averted: Estimating the Effects of COVID-19 Vaccines on Hospitalizations, Mortality and Costs in Canada; Commentary-CD Howe Institute: Toronto, ON, Canada, 2022; pp. 1–27. [Google Scholar]
- Government of Canada. COVID-19 Vaccination: Vaccination Coverage—Archived Version. Available online: https://health-infobase.canada.ca/covid-19/vaccination-coverage/archive/2023-09-11/index.html (accessed on 11 September 2023).
- Pelley, L. 15% of Canadians Got Updated COVID Vaccines This Fall, New Figures Show. CBC News. 2023. Available online: https://www.cbc.ca/news/health/just-15-of-canadians-got-updated-covid-vaccines-this-fall-new-figures-show-1.7064240 (accessed on 30 November 2024).
- Public Health Agency of Canada. Guidance on the Use of COVID-19 Vaccines During the Fall of 2024. Available online: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-guidance-covid-19-vaccines-fall-2024.html (accessed on 1 December 2024).
- Centers for Disease Control and Prevention (CDC). CDC Recommends Updated COVID-19 Vaccine for Fall/Winter Virus Season. 2023. Available online: https://www.cdc.gov/media/releases/2023/p0912-COVID-19-Vaccine.html (accessed on 28 May 2025).
- Government of Canada. COVID-19 Vaccination: Vaccination Coverage. Available online: https://health-infobase.canada.ca/covid-19/vaccination-coverage/ (accessed on 31 December 2024).
- Boikos, C.; Schaible, K.; Nunez-Gonzalez, S.; Welch, V.; Hu, T.; Kyaw, M.H.; Choi, L.E.; Kamar, J.; Goebe, H.; McLaughlin, J. Co-Administration of BNT162b2 COVID-19 and Influenza Vaccines in Adults: A Global Systematic Review. Vaccines 2025, 13, 381. [Google Scholar] [CrossRef]
- World Health Organization, Regional Office for Europe. Vaccines for the Vulnerable: New WHO/Europe Study Shows the Impact of COVID-19 Vaccines in Safeguarding Health and Saving Lives. 2024. Available online: https://www.who.int/europe/news/item/23-10-2024-vaccines-for-the-vulnerable–new-who-europe-study-shows-the-impact-of-covid-19-vaccines-in-safeguarding-health-and-saving-lives (accessed on 28 May 2025).
- Meslé, M.; Brown, J.; Mook, P.; Katz, M.; Hagan, J.; Pastore, R.; Benka, B.; Redlberger-Fritz, M.; Bossuyt, N.; Stouten, V.; et al. Estimated number of lives directly saved by COVID-19 vaccination programmes in the WHO European Region from December, 2020, to March, 2023: A retrospective surveillance study. Lancet Respir. Med. 2024, 12, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, C.R.; Pandey, A.; Moghadas, S.M.; Fitzpatrick, M.C.; Singer, B.H.; Galvani, A.P. Evaluation of Strategies for Transitioning to Annual SARS-CoV-2 Vaccination Campaigns in the United States. Ann. Intern. Med. 2024, 177, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.P.; Hassler, H.B.; Dornburg, A. Optimal annual COVID-19 vaccine boosting dates following previous booster vaccination or breakthrough infection. medRxiv 2024. [Google Scholar] [CrossRef]
- Moro, P.L.; Ennulat, C.; Brown, H.; Woody, G.; Zhang, B.; Marquez, P.; Woo, E.J.; Su, J.R. Safety of Simultaneous Administration of Bivalent mRNA COVID-19 and Influenza Vaccines in the Vaccine Adverse Event Reporting System (VAERS). Drug Saf. 2024, 47, 487–493. [Google Scholar] [CrossRef]
- Jung, S.M.; Loo, S.L.; Howerton, E.; Contamin, L.; Smith, C.P.; Carcelén, E.C.; Yan, K.; Bents, S.J.; Levander, J.; Espino, J.; et al. Potential impact of annual vaccination with reformulated COVID-19 vaccines: Lessons from the US COVID-19 Scenario Modeling Hub. PLoS Med. 2024, 21, e1004387. [Google Scholar] [CrossRef]
- Lin, D.Y.; Xu, Y.; Gu, Y.; Sunny, S.K.; Moore, Z.; Zeng, D. Impact of booster vaccination interval on SARS-CoV-2 infection, hospitalization, and death. Int. J. Infect. Dis. 2024, 145, 107084. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, E.J.; Zhu, D.R.; Gunderson, A.K.; Hawke, S.; Ajeen, R.; Lodge, E.K.; Shook-Sa, B.E.; Abernathy, H.; Garrett, H.E.; King, E.; et al. Magnitude and Durability of the Antibody Response to mRNA-Based Vaccination Among SARS-CoV-2 Seronegative and Seropositive Health Care Personnel. Open Forum Infect. Dis. 2024, 11, ofae009. [Google Scholar] [CrossRef] [PubMed]
- Grewal, R.; Nguyen, L.; Buchan, S.A.; Wilson, S.E.; Costa, A.P.; Kwong, J.C. Effectiveness and duration of protection of a fourth dose of coronavirus disease 2019 messenger RNA vaccine among long-term care residents in Ontario, Canada. J. Infect. Dis. 2023, 227, 977–980. [Google Scholar] [CrossRef]
- Grewal, R.; Kitchen, S.A.; Nguyen, L.; Buchan, S.A.; Wilson, S.E.; Costa, A.P.; Kwong, J.C. Effectiveness of a fourth dose of COVID-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: Test negative design study. BMJ 2022, 378, e071502. [Google Scholar] [CrossRef] [PubMed]
- Wiemken, T.L.; Khan, F.; Nguyen, J.L.; Jodar, L.; McLaughlin, J.M. Is COVID-19 seasonal? A time series modeling approach. medRxiv 2022. [Google Scholar] [CrossRef]
- Wiemken, T.L.; Khan, F.; Puzniak, L.; Yang, W.; Simmering, J.; Polgreen, P.; Nguyen, J.L.; Jodar, L.; McLaughlin, J.M. Seasonal trends in COVID-19 cases, hospitalizations, and mortality in the United States and Europe. Sci. Rep. 2023, 13, 3886. [Google Scholar] [CrossRef]
- Mehta, D.; Sun, T.; Wang, J.; Situ, A.; Park, Y. Comparison of healthcare resource use and cost between influenza and COVID-19 vaccine coadministration and influenza vaccination only. J. Med. Econ. 2024, 27, 1190–1196. [Google Scholar] [CrossRef]
- Bartsch, S.M.; Weatherwax, C.; Wasserman, M.R.; Chin, K.L.; Martinez, M.F.; Velmurugan, K.; Singh, R.D.; John, D.C.; Heneghan, J.L.; Gussin, G.M.; et al. How the Timing of Annual COVID-19 Vaccination of Nursing Home Residents and Staff Affects Its Value. J. Am. Med Dir. Assoc. 2024, 25, 639–646. [Google Scholar] [CrossRef]
- Stoddard, M.; Yuan, L.; Sarkar, S.; van Egeren, D.; Mangalaganesh, S.; Nolan, R.P.; Rogers, M.S.; Hather, G.; White, L.F.; Chakravarty, A. The impact of vaccination frequency on COVID-19 public health outcomes: A model-based analysis. medRxiv 2023. [Google Scholar] [CrossRef]
- Reifferscheid, L.; Lee, J.S.W.; MacDonald, N.E.; Sadarangani, M.; Assi, A.; Lemaire-Paquette, S.; MacDonald, S.E. Transition to endemic: Acceptance of additional COVID-19 vaccine doses among Canadian adults in a national cross-sectional survey. BMC Public Health 2022, 22, 1745. [Google Scholar] [CrossRef]
- Park, H.J.; Gonsalves, G.S.; Tan, S.T.; Kelly, J.D.; Rutherford, G.W.; Wachter, R.M.; Schechter, R.; Paltiel, A.D.; Lo, N.C. Comparing frequency of booster vaccination to prevent severe COVID-19 by risk group in the United States. Nat. Commun. 2024, 15, 1883. [Google Scholar] [CrossRef] [PubMed]
- Fisman, D.N.; Amoako, A.; Simmons, A.; Tuite, A.R. Impact of immune evasion, waning and boosting on dynamics of population mixing between a vaccinated majority and unvaccinated minority. PLoS ONE 2024, 19, e0297093. [Google Scholar] [CrossRef]
- Aydillo, T.; Balsera-Manzanero, M.; Rojo-Fernandez, A.; Escalera, A.; Salamanca-Rivera, C.; Pachón, J.; Del Mar Muñoz-García, M.; Sánchez-Cordero, M.J.; Sánchez-Céspedes, J.; García-Sastre, A.; et al. Concomitant administration of seasonal influenza and COVID-19 mRNA vaccines. Emerg. Microbes Infect. 2024, 13, 2292068. [Google Scholar] [CrossRef] [PubMed]
- Barouch, S.E.; Chicz, T.M.; Blanc, R.; Barbati, D.R.; Parker, L.J.; Tong, X.; Li, W.; McNamara, R.P. Concurrent administration of COVID-19 and influenza vaccines enhances Spike-specific antibody responses. Open Forum Infect. Dis. 2024, 11, ofae144. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Kamei, K.; Matsuda, H.; Takahashi, C.; Yang, J.; Wada, K.; Yonemoto, N. Cost-effectiveness analysis of COVID-19 booster vaccination with BNT162b2 in Japan. Expert Rev. Vaccines 2024, 23, 349–361. [Google Scholar] [CrossRef]
| Databases | Number of Retrieved Records | Search Terms |
|---|---|---|
| Medline | 20 | See Table A1 |
| Scopus | 221 | See Table A1 |
| PubMed | 54 | See Table A1 |
| Web of Science | 33 | See Table A1 |
| Total | 328 |
| Databases | Number of Retrieved Records | Search Terms |
|---|---|---|
| Medline | 116 | See Table A2 |
| Scopus | 2729 | See Table A2 |
| PubMed | 489 | See Table A2 |
| Web of Science | 321 | See Table A2 |
| Total | 3655 |
| Criteria | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Humans | Non-humans, in vitro studies, animal models |
| Exposure | COVID-19 booster vaccination strategy (e.g., timing, co-administration with influenza) | Studies not focused on booster administration |
| Comparator | Age group, population type, comorbidities, vaccine type, dosing interval | Irrelevant comparators or no clear comparison |
| Outcomes | Efficacy, effectiveness, immunogenicity, epidemiologic impact (hospitalizations, infections, disease severity) | Non-epidemiological outcomes or irrelevant endpoints |
| Setting | Study conducted in Canada or G7 countries (France, Germany, Italy, Japan, United Kingdom, United States) | Study conducted outside of Canada or G7 countries |
| Study Design | Observational studies (cohort, case-control, cross-sectional) and simulation modeling studies relevant to policy evaluation | RCTs, qualitative, ecological studies, case reports, experimental studies, reviews, congress abstracts, and posters |
| Authors | Characteristics | ||||
|---|---|---|---|---|---|
| Institution | Participants | Country | Method | Result | |
| Susanna E. Barouch et al. [35] | Ragon Institute of MGH, MIT, and Harvard; Univ. of Massachusetts at Lowell | Concurrent and separate administration groups | USA | Analyzed IgG1 and neutralization responses | Higher durable SARS-CoV-2 response, no interference with influenza |
| Emily J. Ciccone et al. [23] | Univ. of North Carolina, Columbia Univ., Duke Univ., and RTI International | Healthcare personnel grouped by serostatus | USA | Blood sampling and antibody testing | Higher initial response in seropositive individuals, persistence up to 9 months |
| Dan-Yu Lin et al. [22] | University of North Carolina, CDC Foundation | 5,769,205 adults, age-based grouping | USA | Linked health data, Cox regression models | Shorter booster intervals reduced hospitalizations and death |
| Pedro L. Moro et al. [20] | CDC, FDA | 3689 participants, diverse age range | USA | Analyzed Vaccine Adverse Event Reporting System | No unexpected adverse events; common side effects included fatigue |
| Chad R. Wells et al. [18] | Yale School of Public Health | Synthetic population model of U.S. demographics | USA | Age-structured dynamic transmission model | A second dose reduced hospitalizations by 123,869 cases, saving billion |
| Laura Reifferscheid et al. [31] | Univ. of Alberta, BMC Public Health | 6010 Canadian adults, national sample | Canada | Online cross-sectional survey with logistic regression | 70% acceptance for third dose, co-administration with flu vaccine favored |
| David N. Fisman et al. [33] | Dalla Lana School of Public Health, Public Health Agency of Canada | Simulated vaccinated and unvaccinated sub-populations | Canada | Compartmental model (SIR) with waning immunity and booster effects | Vaccinated had lower infection risk; contact with unvaccinated increased risk |
| Timothy L. Wiemken et al. [27] | Pfizer Inc., Columbia Univ., Univ. of Iowa | COVID-19 cases, hospitalizations, and mortality data from U.S. and Europe | USA, UK, France, Germany, Italy | Time series decomposition using Prophet model | Seasonal COVID-19 peaks observed; supports aligning annual boosters with flu season |
| Timothy L Wiemken et al. [26] | Pfizer Inc. | COVID-19 cases in US and Europe | USA, Uk, France, Germany, Italy | Time series modeling for COVID-19 seasonality analysis | COVID-19 rates peaked during winter respiratory season, supporting annual boosters |
| Sarah M. Bartsch et al. [29] | CUNY Graduate School of Public Health, California Association of Long Term Care Medicine, Univ. of California Irvine, Johns Hopkins Univ. | Nursing home residents and staff | USA | Agent-based model simulating nursing home COVID-19 spread, vaccination timing, and economic outcomes | Late summer/early fall vaccination was cost-effective, averting 102–105 cases when initiated between July and October |
| Teresa Aydillo et al. [34] | Icahn School of Medicine at Mount Sinai, University of Seville | 128 volunteers receiving influenza and/or COVID-19 vaccines | USA | Antibody and T-cell response quantification in three vaccination groups: flu only, flu and COVID-19 in the same arm, and flu and COVID-19 in different arms | Concomitant vaccination was safe, with higher antibody response when administered in different arms, particularly for H3N2 influenza |
| Ramandip Grewal et al. [24] | Public Health Ontario, ICES, Univ. of Toronto | Adults aged 50+ in Ontario, Canada | Canada | Test-negative design estimating vaccine effectiveness by time since booster dose | Boosters restored protection against severe outcomes to 92–97% shortly after the dose but waned over time, especially during BA.4/BA.5 Omicron sublineages |
| Ramandip Grewal et al. [25] | ICES, Univ. of Toronto, McMaster Univ. | Long-term care residents aged 60+ in Ontario, Canada | Canada | Test-negative design comparing fourth vs. third dose effectiveness in long-term care residents | Fourth dose provided additional protection against infection, symptomatic infection, and severe outcomes compared to third dose |
| Jeffrey P. Townsend et al. [19] | Yale School of Public Health, Georgia Institute of Technology, Univ. of North Carolina at Charlotte | Simulated individuals with prior booster or breakthrough infections | United States | Longitudinal antibody data and projections for optimal booster timing | Suggested delayed boosting after breakthrough infections based on regional infection rates |
| Sung-mok Jung et al. [21] | Univ. of North Carolina at Chapel Hill, Johns Hopkins Bloomberg School of Public Health, Univ. of Pittsburgh, Pennsylvania State Univ., Northeastern Univ., NIH | Model-based projections with age-based vaccination coverage | USA | Scenario modeling to predict hospitalizations and deaths under different immune escape and vaccination scenarios | Reformulated vaccines significantly reduce morbidity and mortality, especially in high-risk groups during winter |
| Hailey J. Park et al. [32] | Stanford Univ., Yale School of Public Health, Univ. of California, San Francisco, California Dept. of Public Health | Simulation of age-based and immunocompromised populations | USA | Microsimulation model analyzing effects of booster frequency on severe COVID-19 across age and risk groups | Semiannual boosters benefit older and immunocompromised groups most, with limited impact for younger populations |
| Darshan Mehta et al. [28] | Moderna Inc. | Adults aged 50+ receiving influenza and/or COVID-19 vaccines | USA | Retrospective cohort study using insurance claims data to evaluate healthcare utilization and costs | Same-day co-administration reduced COVID-19-related hospitalizations and overall medical costs |
| Madison Stoddard et al. [30] | Fractal Therapeutics, Harvard Medical School, Boston Children’s Hospital | Simulated population with varying vaccination frequencies | USA | Agent-based model for varying COVID-19 booster frequencies and effectiveness | Higher booster frequency showed benefits for population-level COVID-19 control |
| Study | Population (Canada vs. Specific G7 Countries) | Dosing Intervals | Key Findings on Effectiveness (e.g., Infection Rates, Hospitalizations) | Safety Outcomes |
|---|---|---|---|---|
| Wiemken et al. [27] | USA, UK, France, Germany, Italy | Annual boosters timed with flu season | COVID-19 rates peaked in winter; supporting synchronized COVID-19 and flu vaccination | Safety not assessed |
| Mehta et al. [28] | USA | Single-dose and combined influenza-COVID-19 vaccination for those 50+ | Reduced hospitalizations and overall healthcare costs with combined vaccination | Low risk of adverse events |
| Stoddard et al. [30] | USA | Frequent boosters modeled (every 3–6 months) for populations with faster antibody waning | Projected to maintain vaccine efficacy and reduce infection and death risk under waning immunity scenarios | Yearly boosters with improved durability projected to reduce variation and enhance protection |
| Fisman et al. [33] | Canada | Booster vaccination modeled with intervals ranging from 2 to 24 months, including annual schedules | Lower infection rates among vaccinated vs. unvaccinated populations across all scenarios | Not reported |
| Barouch et al. [35] | USA | Same-day (concurrent) vs different-day COVID-19 and influenza vaccination | Enhanced and sustained spike-specific antibody responses for COVID-19 with concurrent administration; no reduction in influenza response | Safety not assessed in this study |
| Aydillo et al. [34] | USA | Same-day co-administration of COVID-19 and influenza vaccines | Maintained antibody responses for both vaccines; enhanced response to influenza H3N2 when administered in separate arms | Mild reactogenicity profile; no safety concerns observed |
| Moro et al. [20] | USA | Same-day co-administration of COVID-19 and influenza vaccines | Effectiveness not assessed | No unexpected safety signals; most reported events were mild (e.g., fatigue, headache) |
| Park et al. [32] | USA | Semiannual vs. annual vs. one-time boosters in older and immunocompromised populations | Semiannual boosters substantially reduced severe COVID-19 outcomes in high-risk groups; annual boosters showed limited impact for younger adults | Safety outcomes not explicitly modeled; focus was on projected effectiveness |
| Lin et al. [22] | USA | Booster intervals of 6, 9, and 12 months | Shorter intervals associated with significantly lower risks of infection, hospitalization, and death; strongest effects observed in older adults | Not reported |
| Country | Type of Vaccines | Co-Administration Strategy | Key Findings | Authors |
|---|---|---|---|---|
| USA | COVID-19, Influenza | Same-day vs. different-day administration of COVID-19 and influenza vaccines | Enhanced antibody response; no interference with influenza response | Susanna E. Barouch et al. [35] |
| USA | COVID-19, Influenza | Same-day administration | No unexpected adverse effects; mild side effects like fatigue | Pedro L. Moro et al. [20] |
| Canada | COVID-19, Influenza | Public acceptance of co-administration with influenza vaccines | Older adults and individuals with chronic illness showed higher acceptance of both additional COVID-19 doses and co-administration with influenza vaccines | Laura Reifferscheid et al. [31] |
| USA | COVID-19, Influenza | Same-day co-administration in fall campaigns | Positive public response; antibody response maintained | Teresa Aydillo et al. [34] |
| USA | COVID-19, Influenza | Same-day co-administration vs. influenza vaccine only (adults 50+) | Lower all-cause and COVID-19–related hospitalizations; reduced medical costs with co-administration | Mehta et al. [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al hashimi, F.; Shuaib, S.E.; Bragazzi, N.L.; Chen, S.; Wu, J. COVID-19 Vaccine Timing and Co-Administration with Influenza Vaccines in Canada: A Systematic Review with Comparative Insights from G7 Countries. Vaccines 2025, 13, 670. https://doi.org/10.3390/vaccines13070670
Al hashimi F, Shuaib SE, Bragazzi NL, Chen S, Wu J. COVID-19 Vaccine Timing and Co-Administration with Influenza Vaccines in Canada: A Systematic Review with Comparative Insights from G7 Countries. Vaccines. 2025; 13(7):670. https://doi.org/10.3390/vaccines13070670
Chicago/Turabian StyleAl hashimi, Farah, Sherif Eneye Shuaib, Nicola Luigi Bragazzi, Shengyuan Chen, and Jianhong Wu. 2025. "COVID-19 Vaccine Timing and Co-Administration with Influenza Vaccines in Canada: A Systematic Review with Comparative Insights from G7 Countries" Vaccines 13, no. 7: 670. https://doi.org/10.3390/vaccines13070670
APA StyleAl hashimi, F., Shuaib, S. E., Bragazzi, N. L., Chen, S., & Wu, J. (2025). COVID-19 Vaccine Timing and Co-Administration with Influenza Vaccines in Canada: A Systematic Review with Comparative Insights from G7 Countries. Vaccines, 13(7), 670. https://doi.org/10.3390/vaccines13070670







