CEA-Functionalized Gold Nanoparticles as a Nanovaccine Platform: In Vitro Evaluation of Cytocompatibility, Cellular Uptake, and Antigen Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Gold Nanoparticles
2.2. Functionalization of Gold Nanoparticles with Colon Cancer-Targeting Molecule
2.3. In Vitro Studies—Cell Suspension Preparation and Maintenance
2.4. In Vitro Exposure of the Cell Suspension to the Vaccine Nanoconstruct
2.5. Evaluation of Antigen Trafficking in the Exposed Cell Suspension
3. Results
3.1. Synthesis and Characterization of Gold Nanoparticles
3.2. Functionalization: Physical and Chemical Characterization of the CEA-AuNP
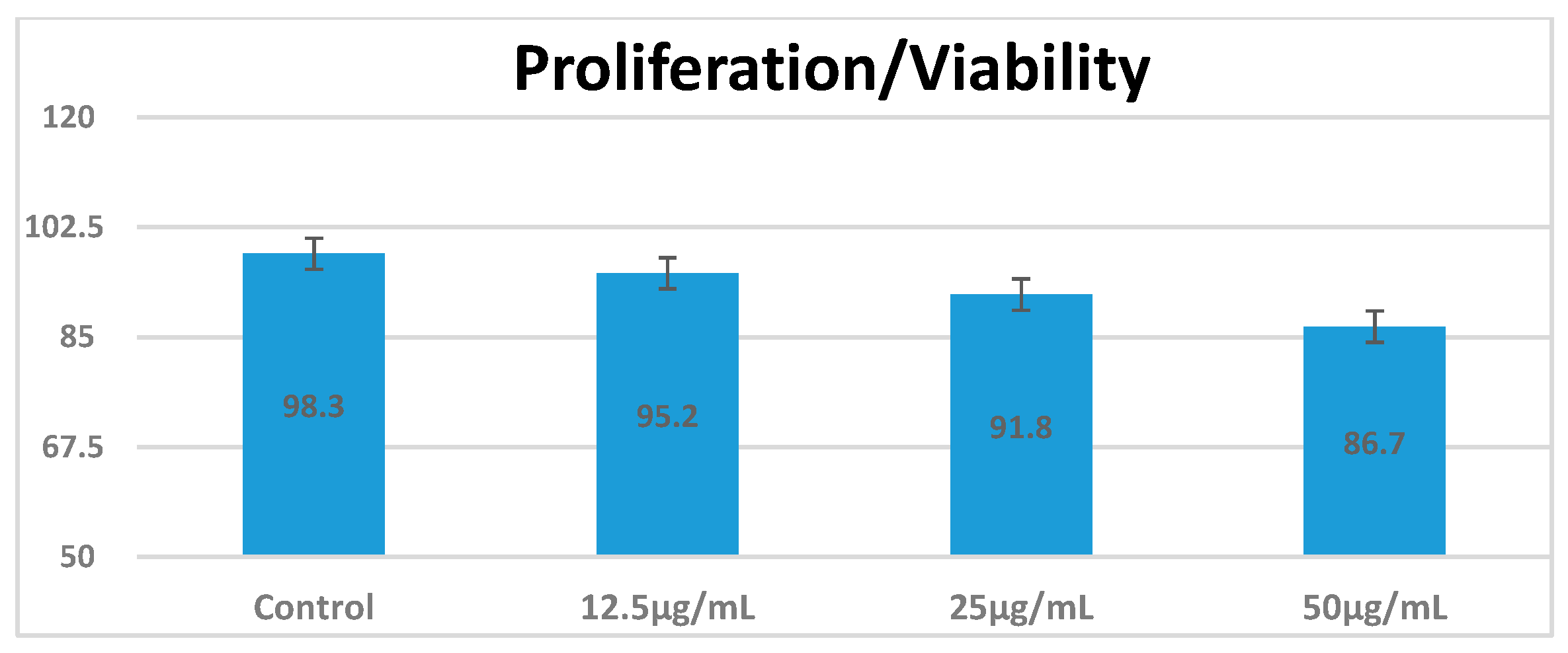
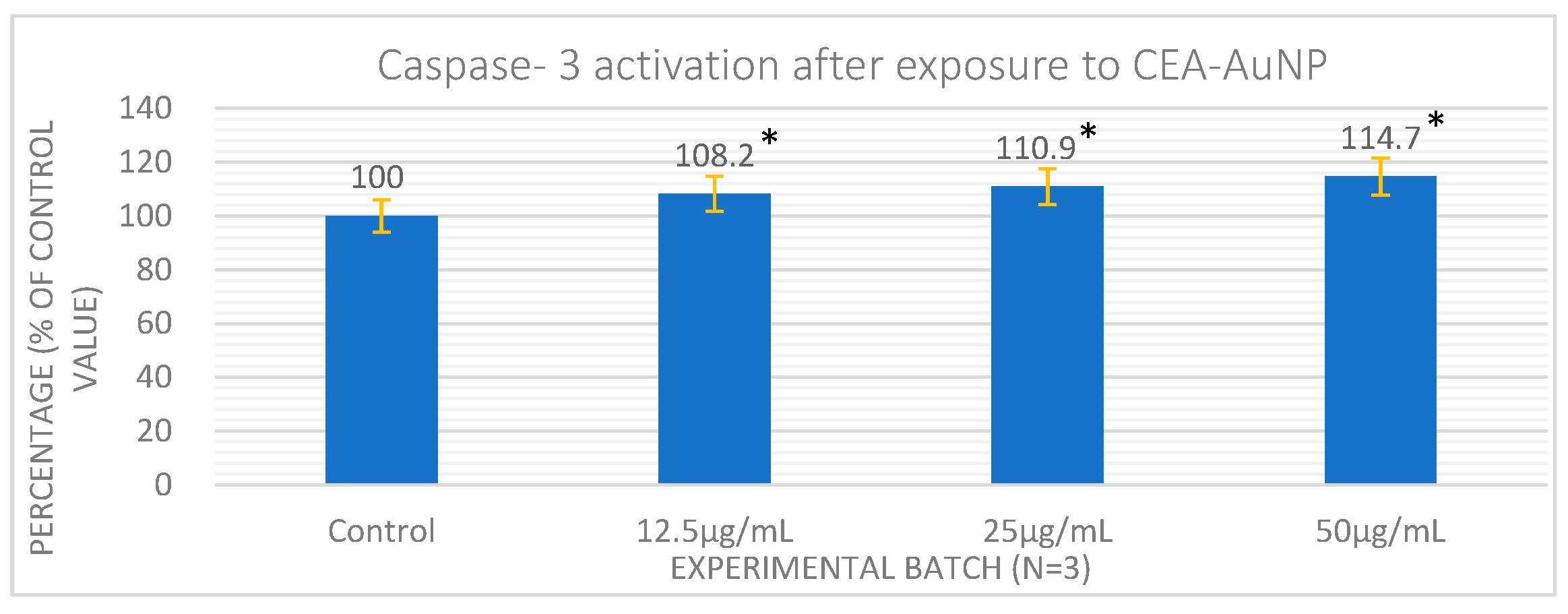
3.3. Cell Viability an Apoptosis Following Exposure to CEA-AuNPs
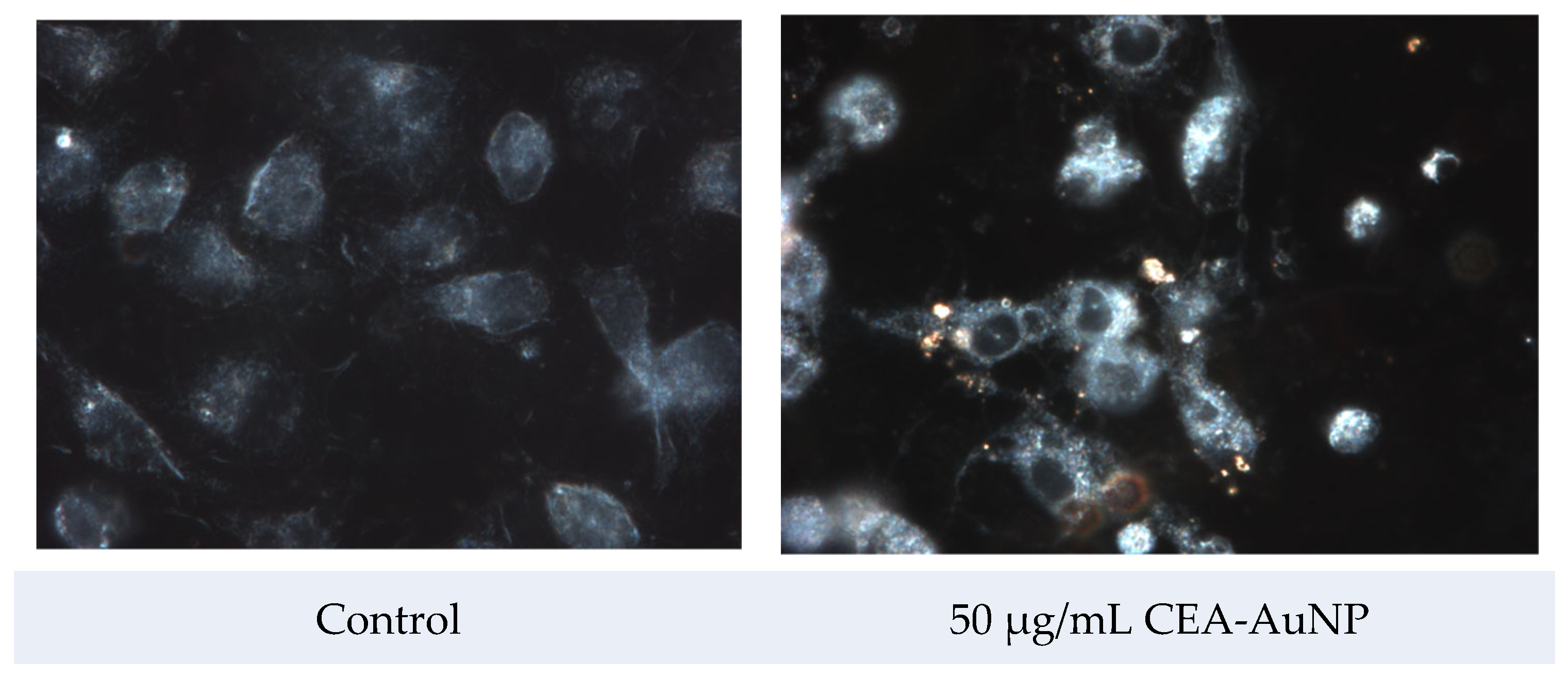
3.4. Assessment of Antigen Processing by Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNP | Gold nanoparticle |
| CEA | Carcinoembrionic antigen |
| CEA-AuNPs | CEA-functionalized gold nanoparticles |
| MHC | Major histocompatibility complex |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness-A Literature Review. Cells 2023, 12, 2159. [Google Scholar] [CrossRef]
- Sobhani, N.; Scaggiante, B.; Morris, R.; Chai, D.; Catalano, M.; Tardiel-Cyril, D.R.; Neeli, P.; Roviello, G.; Mondani, G.; Li, Y. Therapeutic cancer vaccines: From biological mechanisms and engineering to ongoing clinical trials. Cancer Treat. Rev. 2022, 109, 102429. [Google Scholar] [CrossRef] [PubMed]
- Kankanala, V.L.Z.M.; Mukkamalla, S.K.R. Carcinoembryonic Antigen. [Updated 2024 Dec 11]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK578172/ (accessed on 28 April 2025).
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann. Coloproctology 2019, 35, 294–305. [Google Scholar] [CrossRef]
- Choi, S.H.; Yang, S.Y.; Han, Y.D.; Cho, M.S.; Hur, H.; Lee, K.Y.; Kim, N.K.; Min, B.S. Carcinoembryonic antigen levels of tumor-draining venous blood as a prognostic marker in colon cancer. Korean J. Clin. Oncol. 2017, 13, 68–74. [Google Scholar] [CrossRef]
- Bhagat, A.; Lyerly, H.K.; Morse, M.A.; Hartman, Z.C. CEA vaccines. Hum. Vaccines Immunother. 2023, 19, 2291857. [Google Scholar] [CrossRef]
- Wen, R.; Umeano, A.C.; Kou, Y.; Xu, J.; Farooqi, A.A. Nanoparticle systems for cancer vaccine. Nanomedicine 2019, 14, 627–648. [Google Scholar] [CrossRef]
- Almeida, J.P.; Figueroa, E.R.; Drezek, R.A. Gold nanoparticle mediated cancer immunotherapy. Nanomedicine 2014, 10, 503–514. [Google Scholar] [CrossRef]
- Almeida, J.P.M.; Lin, A.Y.; Figueroa, E.R.; Foster, A.E.; Drezek, R.A. In vivo gold nanoparticle delivery of peptide vaccine induces anti-tumor immune response in prophylactic and therapeutic tumor models. Small 2015, 11, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Cao-Milan, R.; Liz-Marzan, L.M. Gold nanoparticle conjugates: Recent advances toward clinical applications. Expert Opin. Drug Deliv. 2014, 11, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Q.; Liang, L.; Li, J.; Wang, K.; Li, J.; Lv, M.; Chen, N.; Song, H.; Lee, J.; et al. Real-time visualization of clustering and intracellular transport of gold nanoparticles by correlative imaging. Nat. Commun. 2017, 8, 15646. [Google Scholar] [CrossRef]
- Mocan, T.; Matea, C.; Tabaran, F.; Iancu, C.; Orasan, R.; Mocan, L. In Vitro Administration of Gold Nanoparticles Functionalized with MUC-1 Protein Fragment Generates Anticancer Vaccine Response via Macrophage Activation and Polarization Mechanism. J. Cancer 2015, 6, 583–592. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Zdrehus, R.; Delcea, C.; Mocan, L. Role of Biofunctionalized Nanoparticles in Digestive Cancer Vaccine Development. Pharmaceutics 2024, 16, 410. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Humbert, C.; Pluchery, O.; Lacaze, E.; Tadjeddine, A.; Busson, B. Optical spectroscopy of functionalized gold nanoparticles assemblies as a function of the surface coverage. Gold Bull. 2013, 46, 299–309. [Google Scholar] [CrossRef]
- Thambiraj, S.; Hema, S.; Shankaran, D.R. Functionalized gold nanoparticles for drug delivery applications. Mater. Today Proc. 2018, 5, 16763–16773. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Saenmuangchin, R.; Siripinyanond, A. Flow field-flow fractionation for hydrodynamic diameter estimation of gold nanoparticles with various types of surface coatings. Anal. Bioanal. Chem. 2018, 410, 6845–6859. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Ahn, S.; Lee, J.; Kim, J.Y.; Choi, M.; Gujrati, V.; Kim, H.; Kim, J.; Shin, E.-C.; Jon, S. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Control. Release 2017, 256, 56–67. [Google Scholar] [CrossRef]
- Nguyen, B.; Tolia, N.H. Protein-based antigen presentation platforms for nanoparticle vaccines. npj Vaccines 2021, 6, 70. [Google Scholar] [CrossRef]
- Wei, Y.; Quan, L.; Zhou, C.; Zhan, Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomedicine 2018, 13, 1495–1512. [Google Scholar]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle characterization: What to measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Gadag, S.; Garg, S.; Nayak, U.Y. Understanding the effect of functionalization on loading capacity and release of drug from mesoporous silica nanoparticles: A computationally driven study. ACS Omega 2022, 7, 8229–8245. [Google Scholar] [CrossRef]
- Baharara, J.; Ramezani, T.; Divsalar, A.; Mousavi, M.; Seyedarabi, A. Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna J. Med. Biotechnol. 2016, 8, 75. [Google Scholar]
- McNamara, K.; Tofail, S.A. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Bharathala, S.; Sharma, P. Biomedical applications of nanoparticles. In Nanotechnology in Modern Animal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–132. [Google Scholar]
- Johnson, S.; Nguyen, V.; Coder, D. Assessment of cell viability. Curr. Protoc. Cytom. 2013, 64, 9.2.1–9.2.26. [Google Scholar] [CrossRef]
- Conners, C.M.; Bhethanabotla, V.R.; Gupta, V.K. Concentration-dependent effects of alendronate and pamidronate functionalized gold nanoparticles on osteoclast and osteoblast viability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 21–29. [Google Scholar] [CrossRef]
- Grippin, A.J.; Sayour, E.J.; Mitchell, D.A. Translational nanoparticle engineering for cancer vaccines. Oncoimmunology 2017, 6, e1290036. [Google Scholar] [CrossRef]
- Ma, W.; Jing, L.; Valladares, A.; Mehta, S.L.; Wang, Z.; Li, P.A.; Bang, J.J. Silver nanoparticle exposure induced mitochondrial stress, caspase-3 activation and cell death: Amelioration by sodium selenite. Int. J. Biol. Sci. 2015, 11, 860. [Google Scholar] [CrossRef]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Gaume, B.; Bergmann-Leitner, E.S.; Leitner, W.W.; Robert, E.G.; Catez, F.; Smith, C.L.; Youle, R.J. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 2001, 1, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Creagh, E.M.; Conroy, H.; Martin, S.J. Caspase-activation pathways in apoptosis and immunity. Immunol. Rev. 2003, 193, 10–21. [Google Scholar] [CrossRef]
- Gogolák, P.; Réthi, B.; Hajas, G.; Rajnavölgyi, É. Targeting dendritic cells for priming cellular immune responses. J. Mol. Recognit. 2003, 16, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gregg, J.L.; Wang, N.; Zhou, D.; O’Donnell, P.; Blum, J.S.; Crotzer, V.L. Compartmentalization of class II antigen presentation: Contribution of cytoplasmic and endosomal processing. Immunol. Rev. 2005, 207, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Tonigold, M.; Mailänder, V. Endocytosis and intracellular processing of nanoparticles in dendritic cells: Routes to effective immunonanomedicines. Nanomedicine 2016, 11, 2625–2630. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Costantino, L.; Italiani, P. Interaction of nanoparticles with immunocompetent cells: Nanosafety considerations. Nanomedicine 2012, 7, 121–131. [Google Scholar] [CrossRef]
- Li, L.; Yan, X.; Xia, M.; Shen, B.; Cao, Y.; Wu, X.; Sun, J.; Zhang, Y.; Zhang, M. Nanoparticle/nanocarrier formulation as an antigen: The immunogenicity and antigenicity of itself. Mol. Pharm. 2021, 19, 148–159. [Google Scholar] [CrossRef]
- Baljon, J.J.; Wilson, J.T. Bioinspired vaccines to enhance MHC class-I antigen cross-presentation. Curr. Opin. Immunol. 2022, 77, 102215. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Liu, Y.; Chen, X.; Liu, Q.; Jia, J.; Yang, T.; Qiu, S.; Ma, G. Immune responses to vaccines involving a combined antigen–nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials 2014, 35, 6086–6097. [Google Scholar] [CrossRef]
- Liang, J.; Yao, L.; Liu, Z.; Chen, Y.; Lin, Y.; Tian, T. Nanoparticles in Subunit Vaccines: Immunological Foundations, Categories, and Applications. Small 2025, 21, 2407649. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Lee, I.H.; Kwon, H.K.; An, S.; Kim, D.; Kim, S.; Yu, M.K.; Lee, J.; Lee, T.; Im, S.; Jon, S. Imageable antigen-presenting gold nanoparticle vaccines for effective cancer immunotherapy in vivo. Angew. Chem. Int. Ed. 2012, 51, 8800. [Google Scholar] [CrossRef]
- Silva, J.M.; Vandermeulen, G.; Oliveira, V.G.; Pinto, S.N.; Rodrigues, C.; Salgado, A.; Afonso, C.A.; Viana, A.S.; Jérôme, C.; Silva, L.C.; et al. Development of Functionalized Nanoparticles for Vaccine Delivery to Dendritic Cells: A Mechanistic Approach. Nanomedicine 2014, 9, 2639–2656. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdrehus, R.-S.; Mocan, T.; Sabau, L.I.; Matea, C.T.; Tăbăran, F.; Pop, T.; Delcea, C.; Mosteanu, O.; Mocan, L. CEA-Functionalized Gold Nanoparticles as a Nanovaccine Platform: In Vitro Evaluation of Cytocompatibility, Cellular Uptake, and Antigen Processing. Vaccines 2025, 13, 668. https://doi.org/10.3390/vaccines13070668
Zdrehus R-S, Mocan T, Sabau LI, Matea CT, Tăbăran F, Pop T, Delcea C, Mosteanu O, Mocan L. CEA-Functionalized Gold Nanoparticles as a Nanovaccine Platform: In Vitro Evaluation of Cytocompatibility, Cellular Uptake, and Antigen Processing. Vaccines. 2025; 13(7):668. https://doi.org/10.3390/vaccines13070668
Chicago/Turabian StyleZdrehus, Razvan-Septimiu, Teodora Mocan, Lavinia Ioana Sabau, Cristian Tudor Matea, Flaviu Tăbăran, Teodora Pop, Cristian Delcea, Ofelia Mosteanu, and Lucian Mocan. 2025. "CEA-Functionalized Gold Nanoparticles as a Nanovaccine Platform: In Vitro Evaluation of Cytocompatibility, Cellular Uptake, and Antigen Processing" Vaccines 13, no. 7: 668. https://doi.org/10.3390/vaccines13070668
APA StyleZdrehus, R.-S., Mocan, T., Sabau, L. I., Matea, C. T., Tăbăran, F., Pop, T., Delcea, C., Mosteanu, O., & Mocan, L. (2025). CEA-Functionalized Gold Nanoparticles as a Nanovaccine Platform: In Vitro Evaluation of Cytocompatibility, Cellular Uptake, and Antigen Processing. Vaccines, 13(7), 668. https://doi.org/10.3390/vaccines13070668





