Troubled Times, Changing Tides: A Seroprevalence Study on Meningococcal Immunity in France Between 2016 and 2024
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Purification of Meningococcal Capsular Polysaccharides
2.3. Quantitation of N. meningitidis Polysaccharide IgG Antibodies (Anti-Nm IgG) Titers Assay
2.4. Statistical Analysis
2.5. Ethics Approval
2.6. Role of Funding Source
3. Results
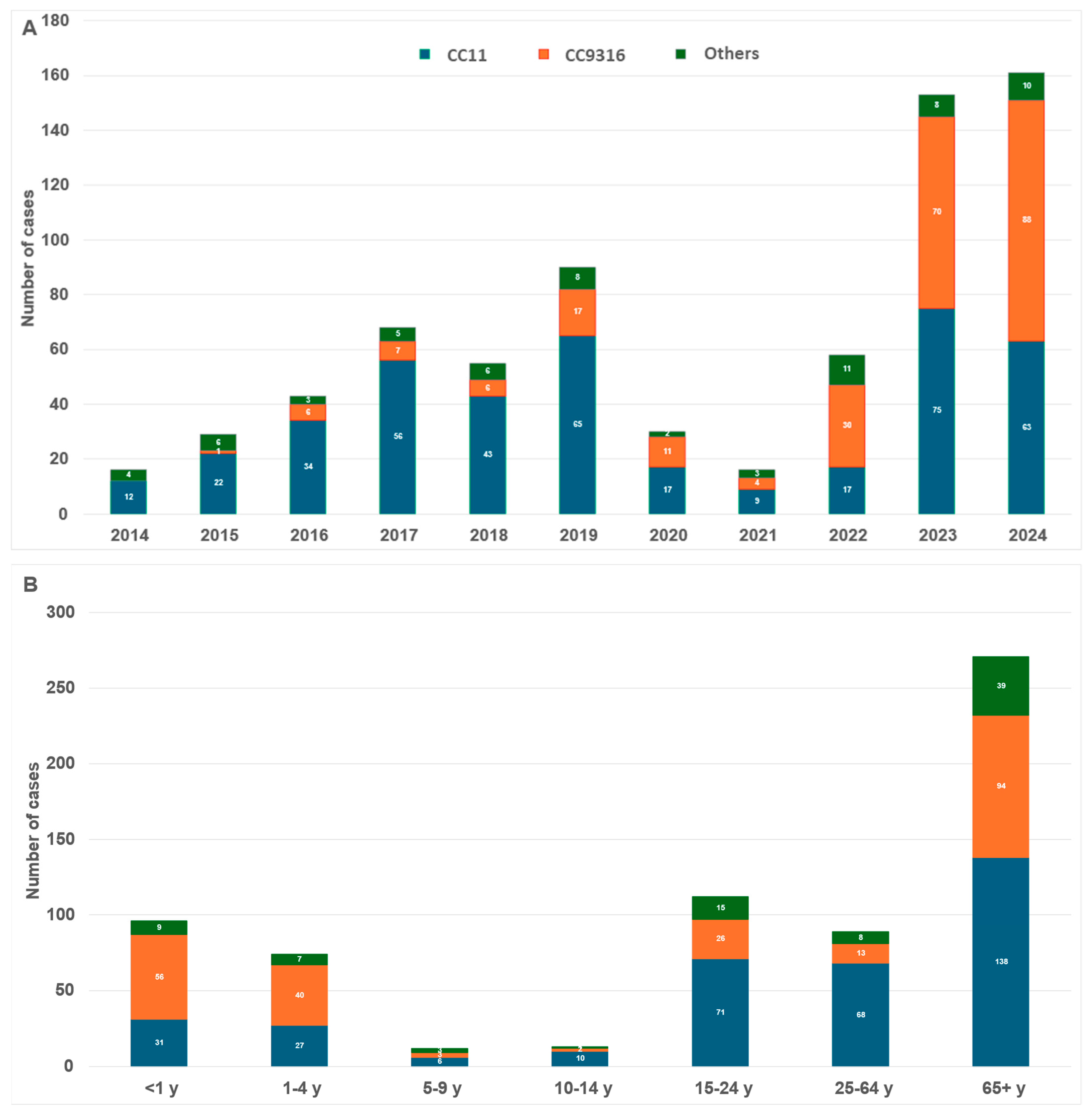
3.1. Serogroup X
3.2. Serogroup C
3.3. Serogroup B
3.4. Serogroup W
3.5. Serogroup Y
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christensen, H.; May, M.; Bowen, L.; Hickman, M.; Trotter, C.L. Meningococcal carriage by age: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Cruz, E.; Lopatynsky-Reyes, E.Z. Association between Meningococcal Meningitis and Santa Ana Winds in Children and Adolescents from Tijuana, Mexico: A Need for Vaccination. Trop. Med. Infect. Dis. 2023, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.; Hong, E.; Denizon, M.; Falguières, M.; Terrade, A.; Deghmane, A.-D.; Taha, M.-K. The Rapid Rebound of Invasive Meningococcal Disease in France at the end of 2022. J. Infect. Public Health 2023, 16, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Rameix-Welti, M.-A.; Zarantonelli, M.L.; Giorgini, D.; Ruckly, C.; Marasescu, M.; van der Werf, S.; Alonso, J.-M.; Naffakh, N.; Taha, M.-K. Influenza A virus neuraminidase enhances meningococcal adhesion to epithelial cells through interaction with sialic acid-containing meningococcal capsules. Infect. Immun. 2009, 77, 3588–3595. [Google Scholar] [CrossRef]
- Michel, J.; Stoica, M.A.; Aouiti-Trabelsi, M.; De Oliveira, F.; Hong, E.; Joly, L.M.; Deghmane, A.E.; Plantier, J.C.; Taha, M.K. Prevalence of Respiratory Pathogens in COVID Patients. J. Biotechnol. Biomed. 2023, 6, 450–459. [Google Scholar]
- Taine, M.; Offredo, L.; Drouin, J.; Toubiana, J.; Weill, A.; Zureik, M.; Dray-Spira, R. Mandatory infant vaccinations in France during the COVID-19 pandemic in 2020. Front. Pediatr. 2021, 9, 666848. [Google Scholar] [CrossRef]
- Clark, S.A.; Campbell, H.; Ribeiro, S.; Bertran, M.; Walsh, L.; Walker, A.; Willerton, L.; Lekshmi, A.; Bai, X.; Lucidarme, J.; et al. Epidemiological and strain characteristics of invasive meningococcal disease prior to, during and after COVID-19 pandemic restrictions in England. J. Infect. 2023, 87, 385–391. [Google Scholar] [CrossRef]
- Kuitunen, I.; Artama, M.; Haapanen, M.; Renko, M. Respiratory virus circulation in children after relaxation of COVID-19 restrictions in fall 2021-A nationwide register study in Finland. J. Med. Virol. 2022, 94, 4528–4532. [Google Scholar] [CrossRef]
- Meyer Sauteur, P.M.; Beeton, M.L.; on behalf of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Mycoplasma and Chlamydia Infections (ESGMAC); The ESGMAC Mycoplasma Pneumoniae Surveillance (MAPS) Study Group. Mycoplasma pneumoniae: Delayed re-emergence after COVID-19 pandemic restrictions. Lancet Microbe 2024, 5, e100–e101. [Google Scholar] [CrossRef]
- Cohen, R.; Ashman, M.; Taha, M.K.; Varon, E.; Angoulvant, F.; Levy, C.; Rybak, A.; Ouldali, N.; Guiso, N.; Grimprel, E. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect. Dis. Now. 2021, 51, 418–423. [Google Scholar] [CrossRef]
- Cohen, R.; Levy, C.; Rybak, A.; Angoulvant, F.; Ouldali, N.; Grimprel, E. Immune debt: Recrudescence of disease and confirmation of a contested concept. Infect. Dis. Now. 2023, 53, 104638. [Google Scholar] [CrossRef] [PubMed]
- Ang, H.J.; Menegale, F.; Preziosi, G.; Pariani, E.; Migliari, M.; Pellegrinelli, L.; Sechi, G.M.; Buoro, S.; Merler, S.; Cereda, D.; et al. Reconstructing the impact of COVID-19 on the immunity gap and transmission of respiratory syncytial virus in Lombardy, Italy. EBioMedicine 2023, 95, 104745. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, L.; Ma, Y.; Wang, H.; Xu, X.; Wang, Y.; Hao, C.; Jiang, W. Evidence of immunity gap: Decline in antibodies against M. pneumoniae during the COVID-19 pandemic. J. Infect. 2024, 89, 106209. [Google Scholar] [CrossRef] [PubMed]
- République Française. Décret n° 2024-694 du 5 Juillet 2024 Relatif à L’obligation Vaccinale Contre les Méningocoques de Type B et ACWY. JORF n° 0159 du 6 Juillet 2024 [Internet]. Paris: Journal Officiel de la République Française; 5 July 2024. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000049888947 (accessed on 13 June 2025).
- Parent du Chatelet, I.; Deghmane, A.E.; Antona, D.; Hong, E.; Fonteneau, L.; Taha, M.K.; Lévy-Bruhl, D. Characteristics and changes in invasive meningococcal disease epidemiology in France, 2006–2015. J. Infect. 2017, 74, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Findlow, J.; Htar, M.T.T.; Villena, R.; Balmer, P. Invasive Meningococcal Disease in the Post-COVID World: Patterns of Disease Rebound. Vaccines 2025, 13, 165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clark, S.; Campbell, H.; Mensah, A.A.; Lekshmi, A.; Walker, A.; Ribeiro, S.; Walsh, L.; Willerton, L.; Bai, X.; Lucidarme, J.; et al. An Increase in Group B Invasive Meningococcal Disease Among Adolescents and Young Adults in England Following Easing of COVID-19 Containment Measures. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3998164 (accessed on 16 December 2021). [CrossRef]
- Nato, F.; Mazie, J.C.; Fournier, J.M.; Slizewicz, B.; Sagot, N.; Guibourdenche, M.; Postic, D.; Riou, J.Y. Production of polyclonal and monoclonal antibodies against group A, B, and C capsular polysaccharides of Neisseria meningitidis and preparation of latex reagents. J. Clin. Microbiol. 1991, 29, 1447–1452. [Google Scholar] [CrossRef]
- Santé Publique France. Vaccination Coverage Data for Meningococcal C by Age Group; Santé Publique France: Saint-Maurice, France, 2025. Available online: https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/articles/donnees-de-couverture-vaccinale-meningocoque-c-par-groupe-d-age (accessed on 22 April 2025).
- Pollard, A.J.; Frasch, C. Development of natural immunity to Neisseria meningitidis. Vaccine 2001, 19, 1327–1346. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.G.; Snape, M.D. A multicomponent serogroup B meningococcal vaccine is licensed for use in Europe: What do we know, and what are we yet to learn? Expert Rev. Vaccines 2013, 12, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Galli, E.; Mantegazza, C.; Fabiano, V.; Zuccotti, G.V. The multicomponent meningococcal serogroup B vaccine (4CMenB): Origin, composition, health impact and unknown aspects. Future Microbiol. 2015, 10, 1579–1598. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, L.; Vesikari, T.; Absalon, J.; Beeslaar, J.; Ward, B.J.; Senders, S.; Eiden, J.J.; Jansen, K.U.; Anderson, A.S.; York, L.J.; et al. B1971009 and B1971016 Trial Investigators. A Bivalent Meningococcal B Vaccine in Adolescents and Young Adults. N. Engl. J. Med. 2017, 377, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.E.; Bonanni, P.; Martinón-Torres, F.; Richmond, P.C.; Safadi, M.A.P.; Salisbury, D.M.; Charos, A.; Schley, K.; Findlow, J.; Balmer, P. Meningococcal Disease in the Post-COVID-19 Era: A Time to Prepare. Infect. Dis. Ther. 2023, 12, 2649–2663. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.I.; Tessier, E.; White, J.M.; Woodruff, M.; Knowles, C.; Bates, C.; Parry, J.; Walker, J.L.; Scott, J.A.; Smeeth, L.; et al. Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020. Eurosurveillance 2020, 25, 2000848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cunniff, L.; Alyanak, E.; Fix, A.; Novak, M.; Peterson, M.; Mevis, K.; Eiden, A.L.; Bhatti, A. The impact of the COVID-19 pandemic on vaccination uptake in the United States and strategies to recover and improve vaccination rates: A review. Hum. Vaccin. Immunother. 2023, 19, 2246502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamson, E.; Forbes, C.; Wittkopf, P.; Pandey, A.; Mendes, D.; Kowalik, J.; Czudek, C.; Mugwagwa, T. Impact of pandemics and disruptions to vaccination on infectious diseases epidemiology past and present. Hum. Vaccin. Immunother. 2023, 19, 2219577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadley, L.; Karachaliou Prasinou, A.; Christensen, H.; Ramsay, M.; Trotter, C. Modelling the impact of COVID-19 and routine MenACWY vaccination on meningococcal carriage and disease in the UK. Epidemiol. Infect. 2023, 151, e98. [Google Scholar] [CrossRef]
- Santé Publique France. Données de Couverture Vaccinale Méningocoque C par Groupe D’âge [Internet]. Available online: https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/articles/donnees-de-couverture-vaccinale-meningocoque-c-par-groupe-d-age#:~:text=A%20partir%20des%20donn%C3%A9es%20du,7%2D31%2C0%5D%20ont (accessed on 22 April 2025).
- Taha, M.K.; Deghmane, A.E. Impact of COVID-19 pandemic and the lockdown on invasive meningococcal disease. BMC Res. Notes 2020, 13, 399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feldman, C.; Anderson, R. Meningococcal pneumonia: A review. Pneumonia 2019, 11, 3. [Google Scholar] [CrossRef]
- Santé Publique France. Infections Invasives à Méningocoque en France en 2023 [Internet]. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-a-prevention-vaccinale/infections-invasives-a-meningocoque/documents/bulletin-national2/infections-invasives-a-meningocoque-en-france-en-2023 (accessed on 2 May 2024).
- Taha, S.; Fantoni, G.; Hong, E.; Terrade, A.; Doucoure, O.; Deghmane, A.E.; Taha, M.K. Characterization of Unusual Serogroups of Neisseria meningitidis. Microorganisms 2024, 12, 2528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ministère du Travail, de la Santé, des Solidarités et des Familles; Ministère de l’Économie, des Finances et de la Souveraineté Industrielle et Numérique. Arrêté du 2 Avril 2025 Modifiant la Liste des Spécialités Pharmaceutiques Remboursables aux Assurés Sociaux. JORF n°0081 du 4 Avril 2025, Texte n° 8 [Internet]. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000051427381 (accessed on 4 April 2025).

| Age Group | n (%) | Titer for NmB GM (CI95%) | Titer for NmC GM (CI95%) | Titer for NmW GM (CI95%) | Titer for NmY GM (CI95%) | Titer for NmX GM (CI95%) |
|---|---|---|---|---|---|---|
| <1 year | 27 (16.3) | 0.2043 | 0.2067 | 0.6394 | 0.1681 | 0.2363 |
| [0.1952; 0.2137] | [0.1964; 0.2174] | [0.6165; 0.6632] | [0.1606; 0.176] | [0.2208; 0.2529] | ||
| 1–4 years | 40 (24.1) | 0.2149 | 0.2087 | 0.6425 | 0.1625 | 0.2386 |
| [0.2086; 0.2214] | [0.2011; 0.2166] | [0.6126; 0.6739] | [0.1523; 0.1733] | [0.23; 0.2476] | ||
| 5–9 years | 10 (6.0) | 0.2134 | 0.1948 | 0.6575 | 0.17 | 0.2226 |
| [0.193; 0.2359] | [0.1787; 0.2124] | [0.6223; 0.6946] | [0.1599; 0.1808] | [0.1988; 0.2494] | ||
| 10–14 years | 13 (7.8) | 0.2197 | 0.1993 | 0.6861 | 0.1685 | 0.2351 |
| [0.2049; 0.2356] | [0.185; 0.2148] | [0.6358; 0.7405] | [0.149; 0.1904] | [0.2156; 0.2563] | ||
| 15–24 years | 11 (6.6) | 0.2042 | 0.1899 | 0.6294 | 0.1638 | 0.2213 |
| [0.1873; 0.2226] | [0.1752; 0.2059] | [0.5912; 0.67] | [0.1516; 0.1769] | [0.2003; 0.2445] | ||
| 25–44 years | 18 (10.8) | 0.1937 | 0.179 | 0.616 | 0.1267 | 0.195 |
| [0.1606; 0.2337] | [0.1466; 0.2187] | [0.5712; 0.6643] | [0.08167; 0.1967] | [0.16; 0.2376] | ||
| 45–65 years | 27 (16.3) | 0.2183 | 0.1948 | 0.6514 | 0.1606 | 0.2227 |
| [0.2075; 0.2296] | [0.1856; 0.2044] | [0.6278; 0.6759] | [0.1497; 0.1723] | [0.2084; 0.238] | ||
| ≥65 years | 20 (12.0) | 0.2085 | 0.1827 | 0.6141 | 0.1615 | 0.217 |
| [0.1961; 0.2216] | [0.1698; 0.1966] | [0.5642; 0.6685] | [0.1502; 0.1736] | [0.204; 0.2308] |
| Vaccination | Pre-NPIs | NPIs | Post-NPIs | Since January 2025 |
|---|---|---|---|---|
| NmC | Mandatory since 2018 | Mandatory | Mandatory | No |
| NmACWY | No | No | No | Mandatory * |
| NmB | No | No | Recommended since 2022 | Mandatory * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, S.; Terrade, A.; Doucoure, O.; Deghmane, A.-E.; Taha, M.-K. Troubled Times, Changing Tides: A Seroprevalence Study on Meningococcal Immunity in France Between 2016 and 2024. Vaccines 2025, 13, 647. https://doi.org/10.3390/vaccines13060647
Taha S, Terrade A, Doucoure O, Deghmane A-E, Taha M-K. Troubled Times, Changing Tides: A Seroprevalence Study on Meningococcal Immunity in France Between 2016 and 2024. Vaccines. 2025; 13(6):647. https://doi.org/10.3390/vaccines13060647
Chicago/Turabian StyleTaha, Samy, Aude Terrade, Oumar Doucoure, Ala-Eddine Deghmane, and Muhamed-Kheir Taha. 2025. "Troubled Times, Changing Tides: A Seroprevalence Study on Meningococcal Immunity in France Between 2016 and 2024" Vaccines 13, no. 6: 647. https://doi.org/10.3390/vaccines13060647
APA StyleTaha, S., Terrade, A., Doucoure, O., Deghmane, A.-E., & Taha, M.-K. (2025). Troubled Times, Changing Tides: A Seroprevalence Study on Meningococcal Immunity in France Between 2016 and 2024. Vaccines, 13(6), 647. https://doi.org/10.3390/vaccines13060647





