Public Health Impact of Potential Infant MenACWY Vaccination Strategies in Spain
Abstract
1. Introduction
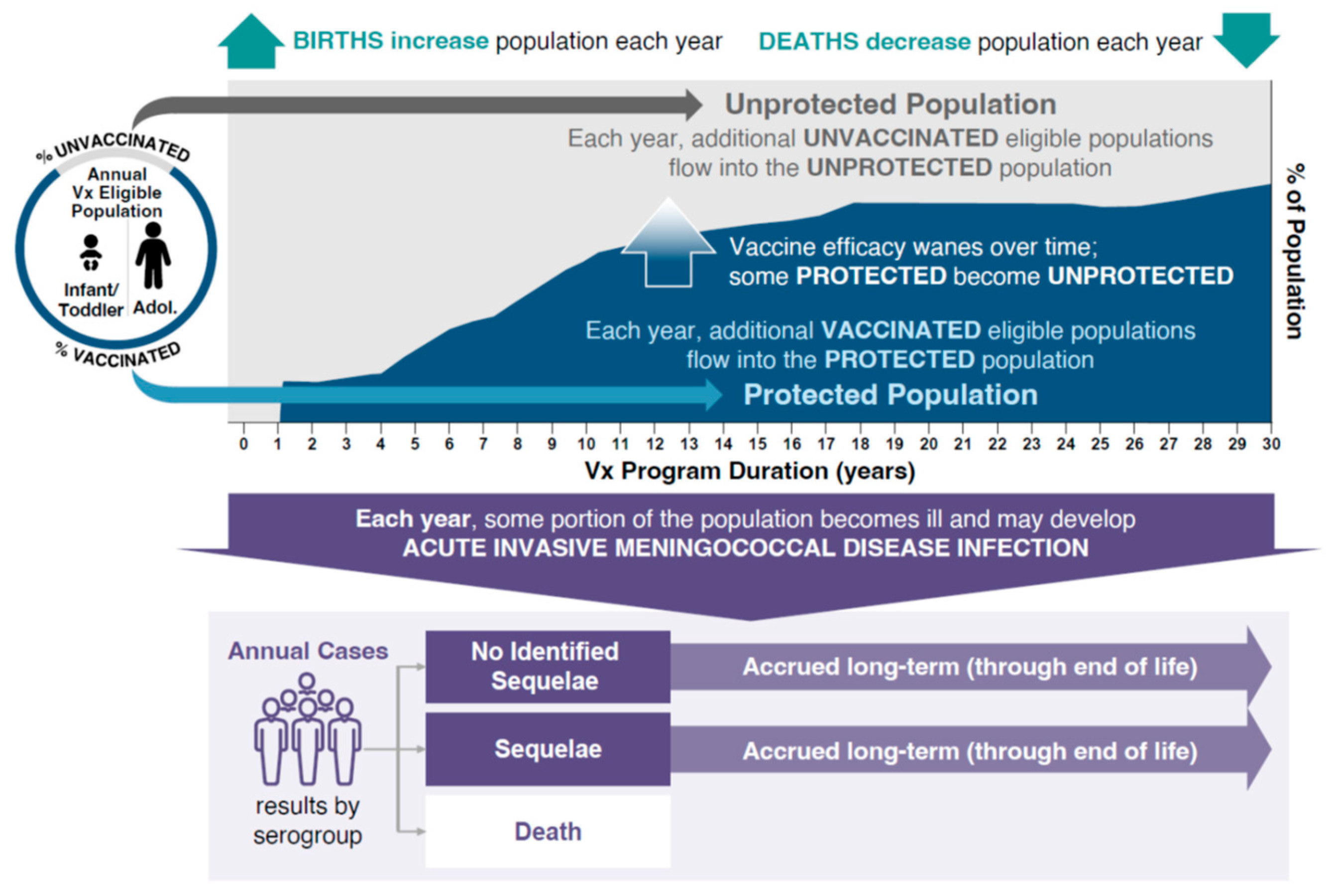
2. Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parikh, S.R.; Campbell, H.; Bettinger, J.A.; Harrison, L.H.; Marshall, H.S.; Martinon-Torres, F.; Safadi, M.A.; Shao, Z.; Zhu, B.; von Gottberg, A.; et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J. Infect. 2020, 81, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Santoreneos, R.; Giles, L.; Haji Ali Afzali, H.; Marshall, H. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine 2019, 37, 2768–2782. [Google Scholar] [CrossRef] [PubMed]
- Pardo de Santayana, C.; Tin Tin Htar, M.; Findlow, J.; Balmer, P. Epidemiology of invasive meningococcal disease worldwide from 2010–2019: A literature review. Epidemiol. Infect. 2023, 151, e57. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, K.J.; Muller, D.; Schumacher, S.; Beck, E.; Meszaros, K.; Koerber, F. Systematic review of invasive meningococcal disease: Sequelae and quality of life impact on patients and their caregivers. Infect. Dis. Ther. 2018, 7, 421–438. [Google Scholar] [CrossRef]
- Acevedo, R.; Bai, X.; Borrow, R.; Caugant, D.A.; Carlos, J.; Ceyhan, M.; Christensen, H.; Climent, Y.; De Wals, P.; Dinleyici, E.C.; et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: Epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev. Vaccines 2019, 18, 15–30. [Google Scholar] [CrossRef]
- Nuttens, C.; Findlow, J.; Balmer, P.; Swerdlow, D.L.; Tin Tin Htar, M. Evolution of invasive meningococcal disease epidemiology in Europe, 2008 to 2017. Eurosurveillance 2022, 27, 2002075. [Google Scholar] [CrossRef]
- Stinson, C.; Burman, C.; Presa, J.; Abalos, M. Atypical presentation of invasive meningococcal disease caused by serogroup W meningococci. Epidemiol. Infect. 2020, 148, e12. [Google Scholar] [CrossRef]
- Campbell, H.; Andrews, N.; Parikh, S.; Ribeiro, S.; Gray, S.; Lucidarme, J.; Ramsay, M.E.; Borrow, R.; Ladhani, S.N. Variable clinical presentation by the main capsular groups causing invasive meningococcal disease in England. J. Infect. 2020, 80, 182–189. [Google Scholar] [CrossRef]
- Soneira, M.S.; Alférez, M.D.R.C.; Portero, R.C. Meningococcal Disease. Seasons 2018–2019, 2019–2020; Vol. 29|n.° 4 35-51; Carlos III Health Institute: Madrid, Spain, 2021. [Google Scholar]
- Soneira, M.S.; Alférez, M.D.R.C.; Portero, R.C. Meningococcal Disease. 2020–2021 Season; Vol. 30|n.° 4 37-49; Carlos III Health Institute: Madrid, Spain, 2022. [Google Scholar]
- Brueggemann, A.B.; Jansen van Rensburg, M.J.; Shaw, D.; McCarthy, N.D.; Jolley, K.A.; Maiden, M.C.J.; van der Linden, M.P.G.; Amin-Chowdhury, Z.; Bennett, D.E.; Borrow, R.; et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet Digit. Health 2021, 3, e360–e370. [Google Scholar] [CrossRef]
- Shen, S.; Findlow, J.; Peyrani, P. Global epidemiology of meningococcal disease-causing serogroups before and after the COVID-19 pandemic: A narrative review. Infect. Dis. Ther. 2024, 13, 2489–2507. [Google Scholar] [CrossRef]
- Moraga-Llop, F.; Andradas, E.; Blesa-Baviera, L.C.; Canton, R.; Gonzalez Del Castillo, J.; Martinon-Torres, F.; Moya, E.; Trilla, A.; Vazquez, J.; Villena, R.J.; et al. Meningococcal meningitis in Spain in the Horizon 2030: A position paper. Rev. Esp. Quimioter. 2024, 37, 285–298. [Google Scholar] [CrossRef] [PubMed]
- National Center for Epidemiology. Instituto de Salud Carlos III (ISCIII). [Weekly Online Newsletter. Number 52. Year 2024]. Available online: https://cne.isciii.es/documents/d/cne/is_n-52-20241226_web (accessed on 27 January 2025).
- Tzanakaki, G.; Cabrnochova, H.; Delic, S.; Draganescu, A.; Hilfanova, A.; Onozo, B.; Pokorn, M.; Skoczynska, A.; Tesovic, G. Invasive meningococcal disease in South-Eastern European countries: Do we need to revise vaccination strategies? Hum. Vaccines Immunother. 2024, 20, 2301186. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.A.; Borrow, R. Herd protection against meningococcal disease through vaccination. Microorganisms 2020, 8, 1675. [Google Scholar] [CrossRef]
- Ministry of Health Spain. Vaccines and Vaccination Program. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/calendario/home.htm (accessed on 8 August 2024).
- Alvarez Garcia, F.J.; Iofrio de Arce, A.; Alvarez Aldean, J.; Garces-Sanchez, M.; Garrote Llanos, E.; Montesdeoca Melian, A.; Navarro Gomez, M.; Pineda Solas, V.; Rivero Calle, I.; Ruiz-Contreras, J.; et al. Immunisation schedule of the Spanish Association of Pediatrics: 2024 recommendations. An. Pediatr. (Engl. Ed.) 2024, 100, 34–45. [Google Scholar] [CrossRef]
- Xunta de Galicia Dirección Xeral de Saúde Pública. Calendario de Inmunización ao Longo de Toda a Vida. Available online: https://www.sergas.es/Saude-publica/Calendario-comun-vacinacion (accessed on 3 June 2025).
- Junta de Andalucía Consejería de Salud y Consumo. Vaccination Schedule Andalusia. Available online: https://www.andavac.es/calendario-vacunaciones/ (accessed on 25 March 2024).
- Junta de Castilla y León Consejería de Sanidad. Nuevo Calendario de Vacunaciones de Inmunizaciones Para Toda la Vida- Castilla y León 2024. Available online: https://www.saludcastillayleon.es/profesionales/es/vacunaciones/nuevo-calendario-vacunaciones-inmunizaciones-toda-vida-cast (accessed on 25 March 2024).
- Villena, R.; Kriz, P.; Tin Tin Htar, M.; Burman, C.; Findlow, J.; Balmer, P.; Jodar, L. Real-world impact and effectiveness of MenACWY-TT. Hum. Vaccines Immunother. 2023, 19, 2251825. [Google Scholar] [CrossRef]
- Ohm, M.; Hahné, S.J.M.; van der Ende, A.; Sanders, E.A.M.; Berbers, G.A.M.; Ruijs, W.L.M.; van Sorge, N.M.; de Melker, H.E.; Knol, M.J. Vaccine impact and effectiveness of meningococcal serogroup ACWY conjugate vaccine implementation in the Netherlands: A nationwide surveillance study. Clin. Infect. Dis. 2022, 74, 2173–2180. [Google Scholar] [CrossRef]
- European Medicines Agency. Nimenrix Meningococcal Groups A, C, W-135 and Y Conjugate Vaccine. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/nimenrix (accessed on 8 August 2024).
- NeisVac-C Vaccine (Meningococcal Group C-TT Conjugate Vaccine, Adsorbed); Full Prescribing Information, Pfizer Canada Ltd.: Kirkland, QC, Canada, 2015.
- Schley, K.; Kowalik, J.C.; Sullivan, S.M.; Vyse, A.; Czudek, C.; Tichy, E.; Findlow, J. Assessing the role of infant and toddler MenACWY immunisation in the UK: Does the adolescent MenACWY programme provide sufficient protection? Vaccines 2023, 11, 940. [Google Scholar] [CrossRef]
- Borja-Tabora, C.F.C.; Peyrani, P.; Webber, C.; Van der Wielen, M.; Cheuvart, B.; De Schrevel, N.; Bianco, V.; Aris, E.; Cutler, M.; Li, P.; et al. A phase 2b/3b MenACWY-TT study of long-term antibody persistence after primary vaccination and immunogenicity and safety of a booster dose in individuals aged 11 through 55 years. BMC Infect. Dis. 2020, 20, 426. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Peyrani, P.; Webber, C.; Van Der Wielen, M.; Cheuvart, B.; De Schrevel, N.; Aris, E.; Cutler, M.; Li, P.; Perez, J.L. Ten-year antibody persistence and booster response to MenACWY-TT vaccine after primary vaccination at 1–10 years of age. Hum. Vaccines Immunother. 2020, 16, 1280–1291. [Google Scholar] [CrossRef]
- European Medicine Agency. Nimenrix. Annex I: Summary of Product Characteristics. Last updated 16 December 2024. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/nimenrix#product-info (accessed on 4 February 2025).
- European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 28 June 2024).
- Spanish Ministry of Health. Vaccines and Vaccination Program. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/coberturas/docs/Todas_las_tablas2020.pdf (accessed on 8 October 2024).
- Advisory Committee on Vaccines and Immunisations of the Spanish Association of Paediatrics. Coberturas de Vacunación Frente a Meningitis C y Meninigitis ACWY en Adolescentes. Comunidades Autónomas. Año 2020. Available online: https://vacunasaep.org/sites/vacunasaep.org/files/minsanidad_coberturas-vacunales-2020-tabla07.pdf (accessed on 12 September 2024).
- Burman, C.; Knuf, M.; Safadi, M.A.P.; Findlow, J. Antibody persistence and revaccination recommendations of MenACWY-TT: A review of clinical studies assessing antibody persistence up to 10 years after vaccination. Expert Rev. Vaccines 2024, 23, 614–635. [Google Scholar] [CrossRef]
- Campbell, H.; Edelstein, M.; Andrews, N.; Borrow, R.; Ramsay, M.; Ladhani, S. Emergency meningococcal ACWY vaccination program for teenagers to control group W meningococcal disease, England, 2015–2016. Emerg. Infect. Dis. 2017, 23, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Campbell, H.; Andrews, N.; Parikh, S.R.; White, J.; Edelstein, M.; Bai, X.; Lucidarme, J.; Borrow, R.; Ramsay, M.E.; Ladhani, S.N. Impact of an adolescent meningococcal ACWY immunisation programme to control a national outbreak of group W meningococcal disease in England: A national surveillance and modelling study. Lancet Child Adolesc. Health 2022, 6, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; May, M.; Bowen, L.; Hickman, M.; Trotter, C.L. Meningococcal carriage by age: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 853–861. [Google Scholar] [CrossRef]
- Carr, J.P.; MacLennan, J.M.; Plested, E.; Bratcher, H.B.; Harrison, O.B.; Aley, P.K.; Bray, J.E.; Camara, S.; Rodrigues, C.M.C.; Davis, K.; et al. Impact of meningococcal ACWY conjugate vaccines on pharyngeal carriage in adolescents: Evidence for herd protection from the UK MenACWY programme. Clin. Microbiol. Infect. 2022, 28, 1649.e1–1649.e8. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Forsten, A.; Boutriau, D.; Bianco, V.; Van der Wielen, M.; Miller, J.M. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum. Vaccines Immunother. 2012, 8, 1892–1903. [Google Scholar] [CrossRef]
- Knuf, M.; Baine, Y.; Bianco, V.; Boutriau, D.; Miller, J.M. Antibody persistence and immune memory 15 months after priming with an investigational tetravalent meningococcal tetanus toxoid conjugate vaccine (MenACWY-TT) in toddlers and young children. Hum. Vaccines Immunother. 2012, 8, 866–872. [Google Scholar] [CrossRef]
- Vesikari, T.; Forsten, A.; Bianco, V.; Van der Wielen, M.; Miller, J.M. Immunogenicity, safety and antibody persistence of a booster dose of quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine compared with monovalent meningococcal serogroup C vaccine administered four years after primary vaccination using the same vaccines. Pediatr. Infect. Dis. J. 2015, 34, e298–e307. [Google Scholar] [CrossRef]
- Knuf, M.; Helm, K.; Kolhe, D.; Van Der Wielen, M.; Baine, Y. Antibody persistence and booster response 68 months after vaccination at 2–10 years of age with one dose of MenACWY-TT conjugate vaccine. Vaccine 2018, 36, 3286–3295. [Google Scholar] [CrossRef]
- French Ministry of Labor, Health and Solidarity. [The Vaccination Schedule]. Available online: https://sante.gouv.fr/prevention-en-sante/preserver-sa-sante/vaccination/calendrier-vaccinal?lang=en (accessed on 9 August 2024).
- Koski, S.; Martinon-Torres, F.; Rämet, M.; Zolotas, L.; Newton, R.; Maansson, R.; Cuter, M.P.P.; Findlow, J.; Balmer, P.; Jodar, L.; et al. Meningitis Research Foundation Conference 2023 Poster Abstract Book. #P21 A Phase 3B, Open-Label Study to Evaluate the Safety and Immunogenicity of MenACWY-TT Vaccine in Healthy Infants Given at 3 and 12 Months of Age. Available online: https://www.meningitis.org/getmedia/41dcca45-856d-45cc-97f1-00465ea928c6/MRF-Conference-2023-Poster-Abstract-Book_v2 (accessed on 29 January 2025).
- European Centre for Disease Prevention and Control. Vaccine Scheduler. Vaccine Schedules in All Countries of the European Union. Available online: https://vaccine-schedule.ecdc.europa.eu/ (accessed on 29 January 2025).
- Government of Malta. Primary HealthCare. Available online: https://primaryhealthcare.gov.mt/en/ (accessed on 7 May 2025).
- National Center for Epidemiology. Instituto de Salud Carlos III (ISCIII). [Weekly Online Newsletter. Number 42. Year 2023]. Available online: https://cne.isciii.es/documents/d/cne/is_n-42-20231017_web-pdf (accessed on 12 May 2025).
- Ladhani, S.N.; Campbell, H.; Andrews, N.; Parikh, S.R.; White, J.; Edelstein, M.; Clark, S.A.; Lucidarme, J.; Borrow, R.; Ramsay, M.E. First real-world evidence of meningococcal group B vaccine, 4CMenB, protection against meningococcal group W disease: Prospective Enhanced National Surveillance, England. Clin. Infect. Dis. 2021, 73, e1661–e1668. [Google Scholar] [CrossRef]
- Harris, S.L.; Tan, C.; Andrew, L.; Hao, L.; Liberator, P.A.; Absalon, J.; Anderson, A.S.; Jones, T.R. The bivalent factor H binding protein meningococcal serogroup B vaccine elicits bactericidal antibodies against representative non-serogroup B meningococci. Vaccine 2018, 36, 6867–6874. [Google Scholar] [CrossRef]
- Abitbol, V.; Martinon-Torres, F.; Taha, M.K.; Nolan, T.; Muzzi, A.; Bambini, S.; Borrow, R.; Toneatto, D.; Serino, L.; Rappuoli, R.; et al. 4CMenB journey to the 10-year anniversary and beyond. Hum. Vaccines Immunother. 2024, 20, 2357924. [Google Scholar] [CrossRef] [PubMed]
- Castilla, J.; Garcia Cenoz, M.; Abad, R.; Sanchez-Cambronero, L.; Lorusso, N.; Izquierdo, C.; Canellas Llabres, S.; Roig, J.; Malvar, A.; Gonzalez Carril, F.; et al. Effectiveness of a meningococcal group B vaccine (4CMenB) in children. N. Engl. J. Med. 2023, 388, 427–438. [Google Scholar] [CrossRef] [PubMed]
| Base-Case Incidence Rate | High Serogroup Incidence Scenario | |||||
|---|---|---|---|---|---|---|
| Age Range | MenC 1999–2006 | MenW 2016–2019 | MenY 2016–2019 | High MenC 1999–2000 | High MenW 2018–2019 | High MenY 2018–2019 |
| 0–<3 mo | 2.57 | 0.40 | 0.11 | 5.14 | 0.60 | 0.23 |
| 3–<12 mo | 5.14 | 0.80 | 0.23 | 10.29 | 1.19 | 0.45 |
| 1–<2 y | 6.04 | 0.23 | 0.08 | 12.03 | 0.36 | 0.08 |
| 2–<3 y | 4.95 | 0.17 | 0.06 | 9.76 | 0.28 | 0.07 |
| 3–<4 y | 3.85 | 0.12 | 0.05 | 7.49 | 0.20 | 0.05 |
| 4–<5 y | 2.76 | 0.07 | 0.04 | 5.21 | 0.11 | 0.04 |
| 5–<15 y | 1.06 | 0.04 | 0.07 | 1.86 | 0.07 | 0.08 |
| 15–<25 y | 0.67 | 0.14 | 0.10 | 1.10 | 0.20 | 0.09 |
| 25–<50 y | 0.10 | 0.03 | 0.02 | 0.19 | 0.05 | 0.03 |
| 50–<65 y | 0.10 | 0.08 | 0.04 | 0.19 | 0.14 | 0.06 |
| 65–<100 y | 0.14 | 0.23 | 0.17 | 0.26 | 0.34 | 0.27 |
| Age at Vaccination | ||||||
|---|---|---|---|---|---|---|
| Vaccination Strategy | 2 mo | 3 mo | 4 mo | 6 mo | 12 mo | 12 y |
| Reference strategy | ||||||
| MenC vaccine at 4 and 12 mo + MenACWY-TT at 12 y | C 90% | C 92% | ACWY 94% | |||
| MenACWY-TT strategies | ||||||
| MenACWY-TT at 2, 4, 12 mo + 12 y | ACWY 85% | ACWY 92% | ACWY 92% | ACWY 94% | ||
| MenACWY-TT at 4 and 12 mo + 12 y | ACWY 85% | ACWY 92% | ACWY 94% | |||
| MenACWY-TT at 3 and 12 mo + 12 y | ACWY 85% | ACWY 92% | ACWY 94% | |||
| MenACWY-TT at 6 and 12 mo + 12 y | ACWY 89% | ACWY 92% | ACWY 94% | |||
| Vaccination Dosing Strategies | Cases | Deaths | Cases with LTS |
|---|---|---|---|
| Reference strategy | |||
| MenC vaccine at 4 and 12 mo + MenACWY-TT at 12 y | 5273 | 833 | 2124 |
| MenACWY-TT strategies | Add’l cases prevented * | Add’l deaths prevented * | Add’l cases with LTS prevented * |
| MenACWY-TT at 2, 4, and 12 mo + 12 y | 103 | 17 | 41 |
| MenACWY-TT at 4 and 12 mo + 12 y | 60 | 11 | 24 |
| MenACWY-TT at 3 and 12 mo + 12 y | 80 | 14 | 32 |
| MenACWY-TT at 6 and 12 mo + 12 y | 20 | 4 | 7 |
| High-Incidence Scenario * | Reference Strategy | MenACWY-TT Strategies | |||
|---|---|---|---|---|---|
| MenC Vaccine at 4 and 12 mo + MenACWY-TT at 12 y | At 2, 4, and 12 mo + 12 y | At 4 and 12 mo + 12 y | At 3 and 12 mo + 12 y | At 6 and 12 mo + 12 y | |
| High MenC | |||||
| Cases | 7465 | 7337 | 7415 | 7379 | 7489 |
| Deaths | 1164 | 1143 | 1155 | 1149 | 1167 |
| Cases with LTS | 3014 | 2963 | 2995 | 2980 | 3025 |
| Additional cases prevented | — | 128 | 50 | 87 | −24 |
| Additional deaths prevented | — | 21 | 9 | 15 | −2 |
| Additional cases with LTS prevented | — | 51 | 20 | 34 | −11 |
| High MenW | |||||
| Cases | 6156 | 6023 | 6068 | 6047 | 6112 |
| Deaths | 1008 | 985 | 992 | 988 | 999 |
| Cases with LTS | 2463 | 2410 | 2429 | 2420 | 2446 |
| Additional cases prevented | — | 133 | 88 | 109 | 45 |
| Additional deaths prevented | — | 23 | 16 | 20 | 9 |
| Additional cases with LTS prevented | — | 53 | 34 | 43 | 17 |
| High MenY | |||||
| Cases | 5854 | 5742 | 5786 | 5766 | 5828 |
| Deaths | 900 | 882 | 889 | 886 | 895 |
| Cases with LTS | 2370 | 2325 | 2343 | 2335 | 2360 |
| Additional cases prevented | — | 112 | 68 | 89 | 26 |
| Additional deaths prevented | — | 18 | 12 | 15 | 5 |
| Additional cases with LTS prevented | — | 45 | 27 | 35 | 10 |
| High MenC/W/Y | |||||
| Cases | 8930 | 8762 | 8844 | 8806 | 8923 |
| Deaths | 1407 | 1379 | 1391 | 1385 | 1403 |
| Cases with LTS | 3599 | 3532 | 3566 | 3550 | 3597 |
| Additional cases prevented | — | 168 | 86 | 124 | 7 |
| Additional deaths prevented | — | 28 | 16 | 22 | 3 |
| Additional cases with LTS prevented | — | 67 | 34 | 49 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schley, K.; Findlow, J.; Molina, C.; Sullivan, S.M.; Tichy, E. Public Health Impact of Potential Infant MenACWY Vaccination Strategies in Spain. Vaccines 2025, 13, 642. https://doi.org/10.3390/vaccines13060642
Schley K, Findlow J, Molina C, Sullivan SM, Tichy E. Public Health Impact of Potential Infant MenACWY Vaccination Strategies in Spain. Vaccines. 2025; 13(6):642. https://doi.org/10.3390/vaccines13060642
Chicago/Turabian StyleSchley, Katharina, Jamie Findlow, Carlos Molina, Shannon M. Sullivan, and Eszter Tichy. 2025. "Public Health Impact of Potential Infant MenACWY Vaccination Strategies in Spain" Vaccines 13, no. 6: 642. https://doi.org/10.3390/vaccines13060642
APA StyleSchley, K., Findlow, J., Molina, C., Sullivan, S. M., & Tichy, E. (2025). Public Health Impact of Potential Infant MenACWY Vaccination Strategies in Spain. Vaccines, 13(6), 642. https://doi.org/10.3390/vaccines13060642






