Production and Immune Response Against Pandemic Influenza Candidate Vaccines as Preparedness Against the Circulating H5N1 Influenza Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequence Analysis
2.2. Strains
2.3. Vaccine Preparation
2.4. Determination of HA Content
2.5. Vaccine Immunization
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Hemagglutination Inhibition (HI) Assay
2.8. Microneutralization (MN) Assay
2.9. Statistical Analysis
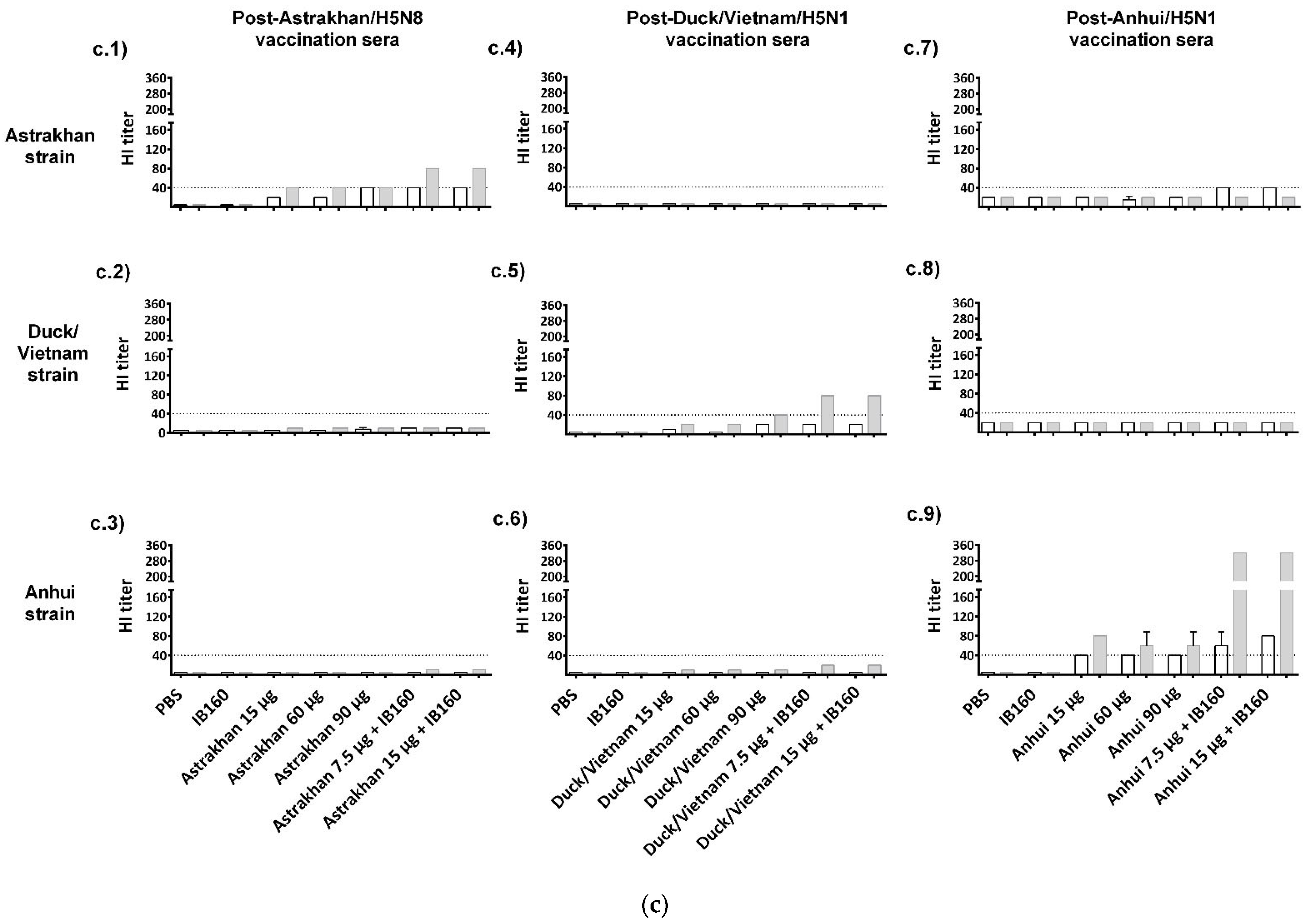
3. Results
3.1. Selection of the Best CVVs Matching the Circulating H5N1 Viruses
3.2. Production of CVV Monovalent Bulks
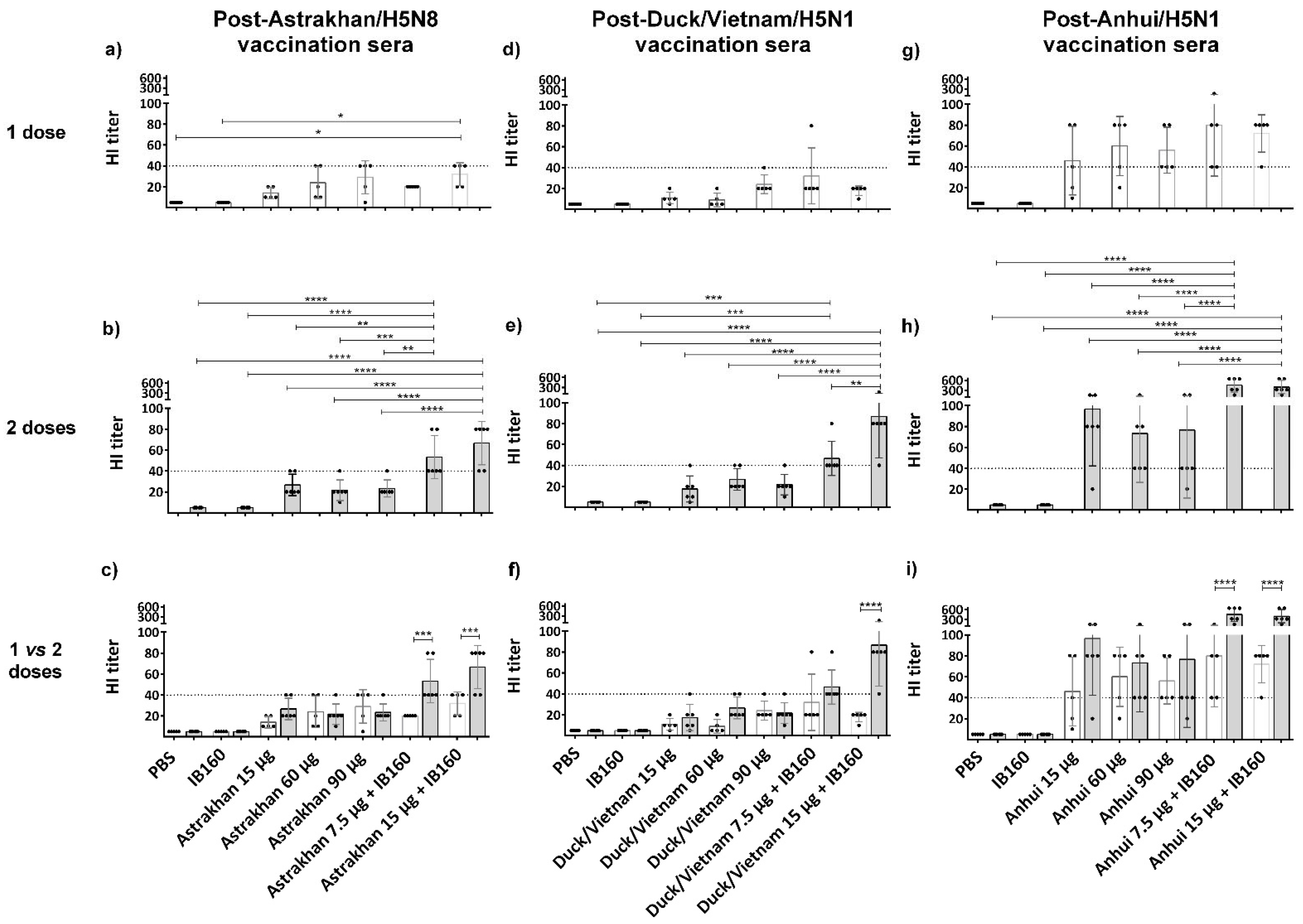
3.3. Immunogenicity of Influenza CVV Formulations
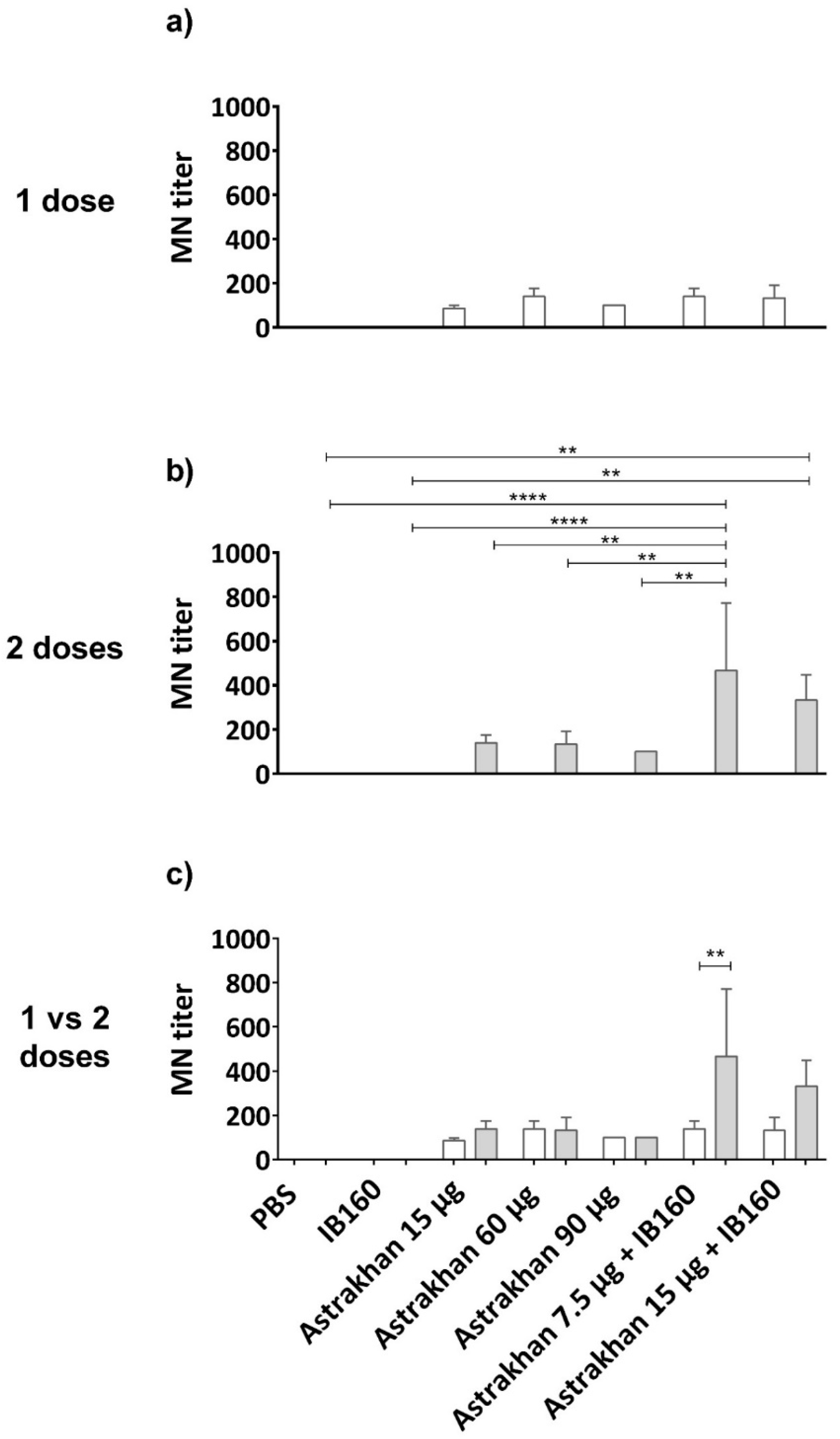
3.4. Seroneutralization of Brazilian Wild-Type H5N1 Virus Clade 2.3.4.4b
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control); EURL (European Union Reference Laboratory for Avian Influenza). Scientific report: Avian influenza overview December 2023–March 2024. EFSA J. 2024, 22, 8754. [Google Scholar] [CrossRef]
- Peacock, T.P.; Moncla, L.; Dudas, G.; VanInsberghe, D.; Sukhova, K.; Lloyd-Smith, J.O.; Worobey, M.; Lowen, A.C.; Nelson, M.I. The global H5N1 influenza panzootic in mammals. Nature 2024, 637, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kupferschmidt, K. Bird flu spread between mink is a ‘warning bell’. Science 2023, 379, 316–317. [Google Scholar] [CrossRef]
- Puryear, W.; Sawatzki, K.; Hill, N.; Foss, A.; Stone, J.J.; Doughty, L.; Walk, D.; Gilbert, K.; Murray, M.; Cox, E.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Outbreak in New England Seals, United States. Emerg. Infect. Dis. 2023, 29, 786–791. [Google Scholar] [CrossRef]
- Leguia, M.; Garcia-Glaessner, A.; Muñoz-Saavedra, B.; Juarez, D.; Barrera, P.; Calvo-Mac, C.; Jara, J.; Silva, W.; Ploog, K.; Amaro, L.; et al. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat. Commun. 2023, 14, 5489. [Google Scholar] [CrossRef] [PubMed]
- WHO. Influenza A(H5N1) in Cats—Poland Influenza A(H5N1) in Cats—Poland. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON476 (accessed on 8 November 2024).
- Kim, I.-H.; Nam, J.-H.; Kim, C.-K.; Choi, Y.J.; Lee, H.; An, B.M.; Lee, N.-J.; Jeong, H.; Lee, S.-Y.; Yeo, S.-G.; et al. Pathogenicity of Highly Pathogenic Avian Influenza A(H5N1) Viruses Isolated from Cats in Mice and Ferrets, South Korea, 2023. Emerg. Infect. Dis. 2024, 30, 2033–2041. [Google Scholar] [CrossRef]
- Burrough, E.R.; Magstadt, D.R.; Petersen, B.; Timmermans, S.J.; Gauger, P.C.; Zhang, J.; Siepker, C.; Mainenti, M.; Li, G.; Thompson, A.C.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg. Infect. Dis. 2024, 30, 1335–1343. [Google Scholar] [CrossRef]
- Epidemiological Update Avian Influenza A(H5N1) in the Americas Region, 4 March 2025. Available online: https://www.paho.org/sites/default/files/2025-03/2025-mar-4-phe-epidupdate-avianinfluenza-eng-final.pdf (accessed on 23 April 2025).
- Webby, R.J.; Uyeki, T.M. An Update on Highly Pathogenic Avian Influenza A(H5N1) Virus, Clade 2.3.4.4b. J. Infect. Dis. 2024, 230, 533–542. [Google Scholar] [CrossRef]
- Technical Report: June 2024 Highly Pathogenic Avian Influenza A(H5N1) Viruses. Available online: https://www.cdc.gov/bird-flu/php/technical-report/h5n1-06052024.html?CDC_AAref_Val=https://www.cdc.gov/flu/avianflu/spotlights/2023-2024/h5n1-technical-report-06052024.htm (accessed on 26 June 2024).
- Wille, M.; Barr, I.G. Resurgence of avian influenza virus. Science 2022, 376, 459–460. [Google Scholar] [CrossRef]
- WHO/OIE/FAO H5N1 Evolution Working Group. Continued evolution of highly pathogenic avian influenza A (H5N1): Updated nomenclature. Influenza Other Respir. Viruses 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26 (Suppl. S4), D49–D53. [Google Scholar] [CrossRef] [PubMed]
- USA FDA, “Vaccine Licensed for Use in the United States”, 7 May 2022. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states (accessed on 30 May 2023).
- Vaccines for Pandemic Influenza. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/pandemic-influenza/vaccines-pandemic-influenza (accessed on 30 May 2023).
- Tosh, C.; Nagarajan, S.; Murugkar, H.V.; Bhatia, S.; Kulkarni, D.D. Evolution and spread of avian influenza H5N1 viruses. Adv. Anim. Vet. Sci. 2 2014, 4S, 33–41. [Google Scholar] [CrossRef]
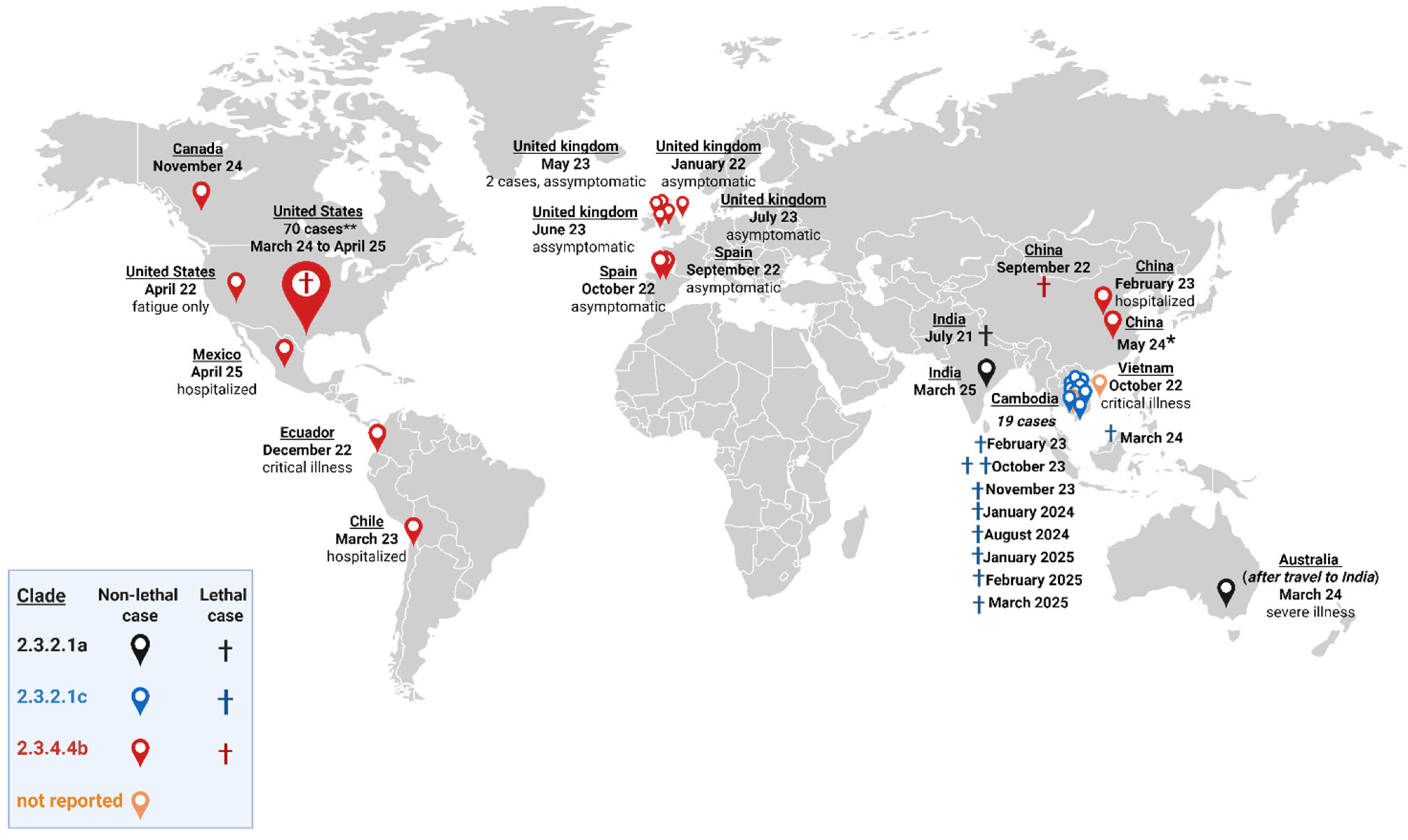
- Past Reported Global Human Cases with Highly Pathogenic Avian Influenza A(H5N1) (HPAI H5N1) by Country, 1997–2025. Available online: https://www.cdc.gov/bird-flu/php/avian-flu-summary/chart-epi-curve-ah5n1.html (accessed on 23 April 2025).
- Garg, S.; Reinhart, K.; Couture, A.; Kniss, K.; Davis, C.T.; Kirby, M.K.; Murray, E.L.; Zhu, S.; Kraushaar, V.; Wadford, D.A.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infections in Humans. N. Engl. J. Med. 2025, 392, 843–854. [Google Scholar] [CrossRef]
- Elbe, S.; Buckland-Merrett, G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob. Chall. 2017, 1, 33–46. Available online: https://gisaid.org/ (accessed on 24 April 2025). [CrossRef] [PubMed]
- The Influenza Virus Resource at the National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/genomes/FLU/Database/nph-select.cgi?go=database (accessed on 7 February 2025).
- Cumulative Number of Confirmed Human Cases for Avian Influenza A(H5N1) Reported to WHO, 2003–2025, 20 January 2025. Available online: https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who--2003-2025--20-january-2025 (accessed on 3 February 2025).
- WHO Global Influenza Program, Zoonotic Influenza: Candidate Vaccine Viruses and Potency Testing Reagents. Available online: https://www.who.int/teams/global-influenza-programme/vaccines/who-recommendations/zoonotic-influenza-viruses-and-candidate-vaccine-viruses (accessed on 5 April 2023).
- Medina-Armenteros, Y.; Cajado-Carvalho, D.; das Neves Oliveira, R.; Apetito Akamatsu, M.; Lee Ho, P. Selecting the Best Candidate Vaccine Virus for Potential Pandemic H5N1 Currently in Circulation. Figshare. Dataset 2023. [CrossRef]
- Medina-Armenteros, Y.; Cajado-Carvalho, D.; das Neves Oliveira, R.; Apetito Akamatsu, M.; Lee Ho, P. Recent Occurrence, Diversity, and Candidate Vaccine Virus Selection for Pandemic H5N1: Alert Is in the Air. Vaccines 2024, 12, 1044. [Google Scholar] [CrossRef]
- Akamatsu, M.A.; Sakihara, V.A.; Carvalho, B.P.; Abrantes, A.d.P.; Takano, M.A.S.; Adami, E.A.; Yonehara, F.S.; Carneiro, P.d.S.; Rico, S.; Schanoski, A.; et al. Preparedness against pandemic influenza: Production of an oil-in-water emulsion adjuvant in Brazil. PLoS ONE 2020, 15, e0233632. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher sequence analysis tools framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. Available online: https://www.ebi.ac.uk/jdispatcher (accessed on 9 May 2024). [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinformatics 2009, 10, 421. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 January 2025). [CrossRef]
- Summary of Status of Development and Availability of A(H5N1) Candidate Vaccine Viruses and Potency Testing Reagents. Available online: https://cdn.who.int/media/docs/default-source/cvv-southern-hemisphere-2025/h5n1_summary_a_h5n1_cvv_20240927.pdf?sfvrsn=f7f9ff20_1 (accessed on 15 November 2024).
- Summary of Status of Development and Availability of A(H5) Non–A(H5N1) Candidate Vaccine Viruses and Potency Testing Reagents. Available online: https://cdn.who.int/media/docs/default-source/influenza/cvvs/cvv-zoonotic-northern-hemisphere-2025-2026/h5-non-h5n1_cvv_-20250228.pdf?sfvrsn=273530c8_3 (accessed on 27 May 2025).
- WHO. Recommendations for the Production and Control of Influenza Vaccine (Inactivated), World Health Organization, WHO Technical Report Series. 2005. Available online: https://cdn.who.int/media/docs/default-source/biologicals/vaccine-quality/recommendations-for-the-production-and-control-of-influenza-vaccine-(inactivated)b0ed4c58-8154-496d-bf91-624734826500.pdf?sfvrsn=cfcd1432_1&download=true (accessed on 30 May 2023).
- Miyaki, C.; Meros, M.; Precioso, A.R.; Raw, I. Influenza vaccine production for Brazil: A classic example of successful North-South bilateral technology transfer. Vaccine 2011, 29 (Suppl. S1), A12–A15. [Google Scholar] [CrossRef]
- Schild, G.C.; Wood, J.M.; Newman, R.W. A single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen. Proposals for an assay method for the haemagglutinin content of influenza vaccines. Bull. World Health Organ. 1975, 52, 223–231. [Google Scholar]
- Hornbeck, P. Enzyme-Linked Immunoabsorbent Assays. In Current Protocols in Immunology; Section I Assay for antibody production; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1991; Volume 1, 2.1.1–2.1.22. [Google Scholar]
- WHO Global Influenza Surveillance Network: Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. Part 2, Appendix 2F (Serological Diagnosis of Influenza by Haemagglutination Inhibition Testing). Available online: https://iris.who.int/bitstream/handle/10665/44518/9789241548090_eng.pdf (accessed on 30 May 2023).
- de Araújo, A.C.; Silva, L.M.N.; Cho, A.Y.; Repenning, M.; Amgarten, D.; de Moraes, A.P.; Malta, F.; Miller, M.; Dorlass, E.G.; Palameta, S.; et al. Incursion of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus, Brazil, 2023. Emerg. Infect. Dis. 2024, 30, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Tignor, G.H.; Mifune, K.; Motohashi, T. Isolation and assay of rabies serogroup viruses in CER cells. Intervirology 1977, 8, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Coswig, L.T.; dos Santos, M.B.; Hafez, H.M.; Ferreira, H.L.; Arns, C.W. Propagation of avian metapneumovirus subtypes A and B using chicken embryo related and other cell systems. J. Virol. Methods 2010, 167, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Spilki, F.R.; de Almeida, R.S.; Campalans, J.; Arns, C.W. Susceptibility of different cell lines to infection with bovine respiratory syncytial virus. J. Virol. Methods 2006, 131, 130–133. [Google Scholar] [CrossRef]
- Baz, M.; Luke, C.J.; Cheng, X.; Jin, H.; Subbarao, K. H5N1 vaccines in humans. Virus Res. 2013, 178, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Hobson, D.; Curry, R.L.; Beare, A.S.; Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. 1972, 70, 767–777. [Google Scholar] [CrossRef]
- Hammershaimb, E.A.D.; Campbell, J.D. Vaccine Development. Pediatr. Clin. North Am. 2024, 71, 529–549. [Google Scholar] [CrossRef]
- Ly, H. Recent global outbreaks of highly pathogenic and low-pathogenicity avian influenza A virus infections. Virulence 2024, 15, 2383478. [Google Scholar] [CrossRef]
- Federal and State Veterinary Agencies Share Update on HPAI Detections in Oregon Backyard Farm, Including First H5N1 Detections in Swine. Available online: https://www.aphis.usda.gov/news/agency-announcements/federal-state-veterinary-agencies-share-update-hpai-detections-oregon (accessed on 31 October 2024).
- Plaza, P.I.; Gamarra-Toledo, V.; Euguí, J.; Lambertucci, S.A. Recent Changes in Patterns of Mammal Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus Worldwide. Emerg. Infect. Dis. 2024, 30, 444–452. [Google Scholar] [CrossRef]
- Imai, M.; Watanabe, T.; Hatta, M.; Das, S.C.; Ozawa, M.; Shinya, K.; Zhong, G.; Hanson, A.; Katsura, H.; Watanabe, S.; et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 2012, 486, 420–428. [Google Scholar] [CrossRef]
- Herfst, S.; Schrauwen, E.J.; Linster, M.; Chutinimitkul, S.; De Wit, E.; Munster, V.J.; Sorrell, E.M.; Bestebroer, T.M.; Burke, D.F.; Smith, D.J.; et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012, 336, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Restori, K.H.; Septer, K.M.; Field, C.J.; Patel, D.R.; VanInsberghe, D.; Raghunathan, V.; Lowen, A.C.; Sutton, T.C. Risk assessment of a highly pathogenic H5N1 influenza virus from mink. Nat. Commun. 2024, 15, 4112. [Google Scholar] [CrossRef]
- Belser, J.A.; Sun, X.; Pulit-Penaloza, J.A.; Maines, T.R. Fatal Infection in Ferrets after Ocular Inoculation with Highly Pathogenic Avian Influenza A(H5N1) Virus. Emerg. Infect. Dis. 2024, 30, 1484–1487. [Google Scholar] [CrossRef]
- Gu, C.; Maemura, T.; Guan, L.; Eisfeld, A.J.; Biswas, A.; Kiso, M.; Uraki, R.; Ito, M.; Trifkovic, S.; Wang, T.; et al. A human isolate of bovine H5N1 is transmissible and lethal in animal models. Nature 2024, 636, 711–718. [Google Scholar] [CrossRef]
- Ison, M.G.; Marrazzo, J. The Emerging Threat of H5N1 to Human Health. N. Engl. J. Med. 2024, 92, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Jassem, A.N.; Roberts, A.; Tyson, J.; Zlosnik, J.E.; Russell, S.L.; Caleta, J.M.; Eckbo, E.J.; Gao, R.; Chestley, T.; Grant, J.; et al. Critical Illness in an Adolescent with Influenza A(H5N1) Virus Infection. N. Engl. J. Med. 2024, 392, 927–929. [Google Scholar] [CrossRef]
- Mahase, E. Bird flu: US reports first human death in person infected with H5N1. BMJ 2025, 388, r28. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Penaloza, J.A.; Brock, N.; Belser, J.A.; Sun, X.; Pappas, C.; Kieran, T.J.; Thakur, P.B.; Zeng, H.; Cui, D.; Frederick, J.; et al. Highly pathogenic avian influenza A(H5N1) virus of clade 2.3.4.4b isolated from a human case in Chile causes fatal disease and transmits between co-housed ferrets. Emerg. Microbes Infect. 2024, 13, 2332667. [Google Scholar] [CrossRef]
- Winokur, P.L.; Hegmann, T.E.; Keitel, W.A.; Bernstein, D.I.; Frey, S.E.; Bryant, C. DMID 15-0064 Study Group. Safety and Immunogenicity of a monovalent inactivated influenza A/H5N8 virus vaccine given with and without AS03 or MF59 adjuvants in healthy adults. Clin. Infect. Dis 2023, ciac983. [Google Scholar] [CrossRef]
- Khurana, S.; King, L.R.; Manischewitz, J.; Posadas, O.; Mishra, A.K.; Liu, D.; Beigel, J.H.; Rappuoli, R.; Tsang, J.S.; Golding, H. Licensed H5N1 vaccines generate cross-neutralizing antibodies against highly pathogenic H5N1 clade 2.3.4.4b influenza virus. Nat. Med. 2024, 30, 2771–2776. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Zhang, Y.; Zhang, G. Current Progress in the Science of Novel Adjuvant Nano-Vaccine-Induced Protective Immune Responses. Pathogens 2024, 13, 441. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gong, H.; Sun, Q.; Yang, J.; Yan, X.; Xu, F. Research progress on emulsion vaccine adjuvants. Heliyon 2024, 10, e24662. [Google Scholar] [CrossRef] [PubMed]
- Furey, C.; Scher, G.; Ye, N.; Kercher, L.; DeBeauchamp, J.; Crumpton, J.C.; Jeevan, T.; Patton, C.; Franks, J.; Rubrum, A.; et al. Development of a nucleoside-modified mRNA vaccine against clade 2.3.4.4b H5 highly pathogenic avian influenza virus. Nat. Commun. 2024, 15, 4350. [Google Scholar] [CrossRef] [PubMed]

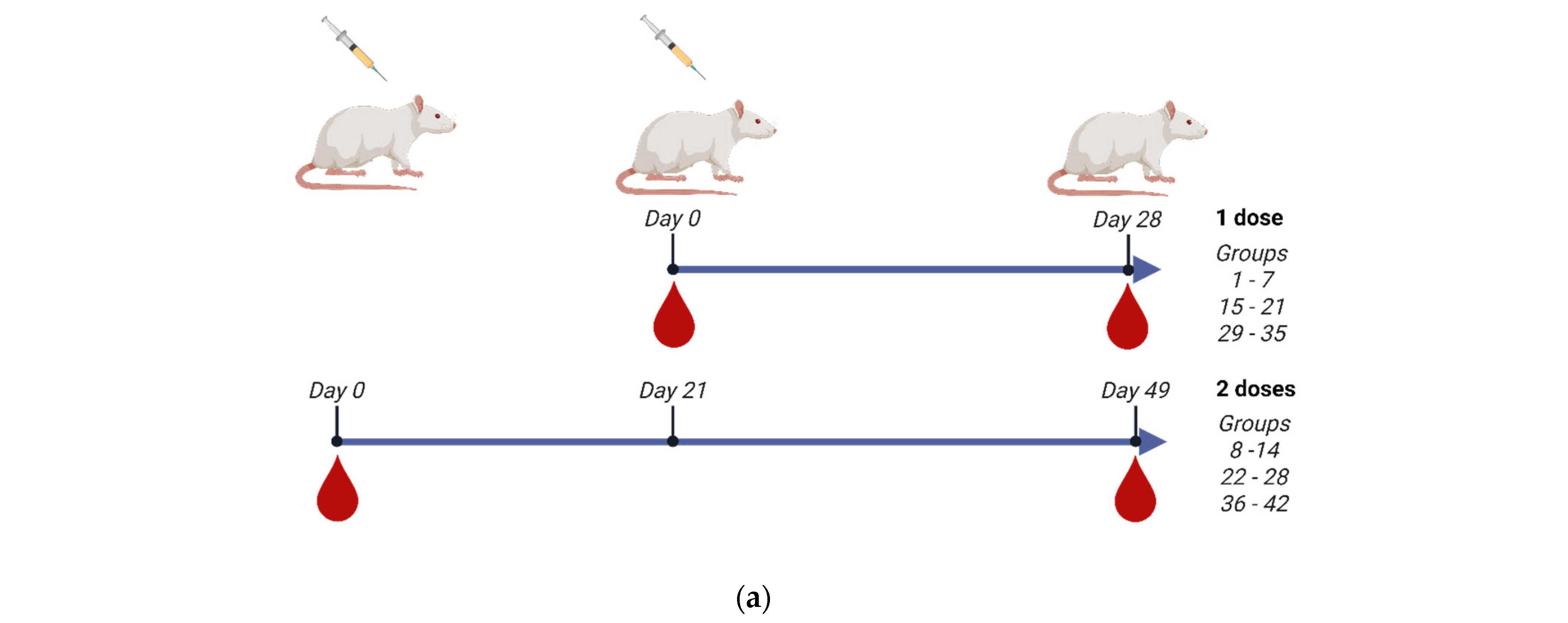
| Formulations’ Abbreviated Codes | |||||||
|---|---|---|---|---|---|---|---|
| A/Astrakhan/3212/2020 (H5N8) [IDCDC-RG71A] | A/duck/Vietnam/NCVD-1584/2012 (H5N1) [NIBRG-301] | A/Anhui/1/2005 (H5N1) [IBCDC-RG6] | Number of Doses | Number of Animals/ Group | |||
| Groups | Astrakhan | Groups | Duck/Vietnam | Groups | Anhui | ||
| 1 | PBS | 15 | PBS | 29 | PBS | 1 | 5 |
| 2 | IB160 | 16 | IB160 | 30 | IB160 | 1 | 5 |
| 3 | Astrakhan 15 µg | 17 | Duck/Vietnam 15 µg | 31 | Anhui 15 µg | 1 | 5 |
| 4 | Astrakhan 60 µg | 18 | Duck/Vietnam 60 µg | 32 | Anhui 60 µg | 1 | 5 |
| 5 | Astrakhan 90 µg | 19 | Duck/Vietnam 90 µg | 33 | Anhui 90 µg | 1 | 5 |
| 6 | Astrakhan 7.5 µg + IB160 | 20 | Duck/Vietnam 7.5 µg + IB160 | 34 | Anhui 7.5 µg + IB160 | 1 | 5 |
| 7 | Astrakhan 15 µg + IB160 | 21 | Duck/Vietnam 15 µg + IB160 | 35 | Anhui 15 µg + IB160 | 1 | 5 |
| 8 | PBS | 22 | PBS | 36 | PBS | 2 | 6 |
| 9 | IB160 | 23 | IB160 | 37 | IB160 | 2 | 6 |
| 10 | Astrakhan 15 µg | 24 | Duck/Vietnam 15 µg | 38 | Anhui 15 µg | 2 | 6 |
| 11 | Astrakhan 60 µg | 25 | Duck/Vietnam 60 µg | 39 | Anhui 60 µg | 2 | 6 |
| 12 | Astrakhan 90 µg | 26 | Duck/Vietnam 90 µg | 40 | Anhui 90 µg | 2 | 6 |
| 13 | Astrakhan 7.5 µg + IB160 | 27 | Duck/Vietnam 7.5 µg + IB160 | 41 | Anhui 7.5 µg + IB160 | 2 | 6 |
| 14 | Astrakhan 15 µg + IB160 | 28 | Duck/Vietnam 15 µg + IB160 | 42 | Anhui 15 µg + IB160 | 2 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee Ho, P.; Medina-Armenteros, Y.; Mendonça Munhoz Dati, L.; Cajado-Carvalho, D.; Savio Silva, C.; Fernandes Campos, P.; Abreu, P.A.E.; Tavares de Castro, J.; Tonolli, P.N.; Fujimori, M.; et al. Production and Immune Response Against Pandemic Influenza Candidate Vaccines as Preparedness Against the Circulating H5N1 Influenza Viruses. Vaccines 2025, 13, 620. https://doi.org/10.3390/vaccines13060620
Lee Ho P, Medina-Armenteros Y, Mendonça Munhoz Dati L, Cajado-Carvalho D, Savio Silva C, Fernandes Campos P, Abreu PAE, Tavares de Castro J, Tonolli PN, Fujimori M, et al. Production and Immune Response Against Pandemic Influenza Candidate Vaccines as Preparedness Against the Circulating H5N1 Influenza Viruses. Vaccines. 2025; 13(6):620. https://doi.org/10.3390/vaccines13060620
Chicago/Turabian StyleLee Ho, Paulo, Yordanka Medina-Armenteros, Lívia Mendonça Munhoz Dati, Daniela Cajado-Carvalho, Christian Savio Silva, Pollyanna Fernandes Campos, Patrícia Antonia Estima Abreu, Júlia Tavares de Castro, Paulo Newton Tonolli, Mahyumi Fujimori, and et al. 2025. "Production and Immune Response Against Pandemic Influenza Candidate Vaccines as Preparedness Against the Circulating H5N1 Influenza Viruses" Vaccines 13, no. 6: 620. https://doi.org/10.3390/vaccines13060620
APA StyleLee Ho, P., Medina-Armenteros, Y., Mendonça Munhoz Dati, L., Cajado-Carvalho, D., Savio Silva, C., Fernandes Campos, P., Abreu, P. A. E., Tavares de Castro, J., Tonolli, P. N., Fujimori, M., Silveira Martins Rosa, R., Palameta, S., Edward Miller, M., Sakihara, V. A., de Lima Valadares, F., Lauretti Ferreira, F., Pereira Carvalho Holanda, B., Gonçalves de Macedo, D., Comone, P., ... das Neves Oliveira, R. (2025). Production and Immune Response Against Pandemic Influenza Candidate Vaccines as Preparedness Against the Circulating H5N1 Influenza Viruses. Vaccines, 13(6), 620. https://doi.org/10.3390/vaccines13060620







