Safety and Immunogenicity of a Canine Distemper DNA Vaccine Formulated with Lipid Nanoparticles in Dogs, Foxes, and Raccoon Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Construction of Recombinant Plasmids
2.3. Plasmid DNA Identification
2.4. DNA-LNP Vaccine Preparation
2.5. DNA-LNP Vaccine Determination
2.6. Ethics Statement
2.7. Mouse Studies
2.8. Dog Studies
2.9. Fox and Raccoon Dog Studies
2.10. Serum CDV NAb Detection
2.11. Mouse Serum Cytokines Detection
2.12. Statistical Analysis
3. Results
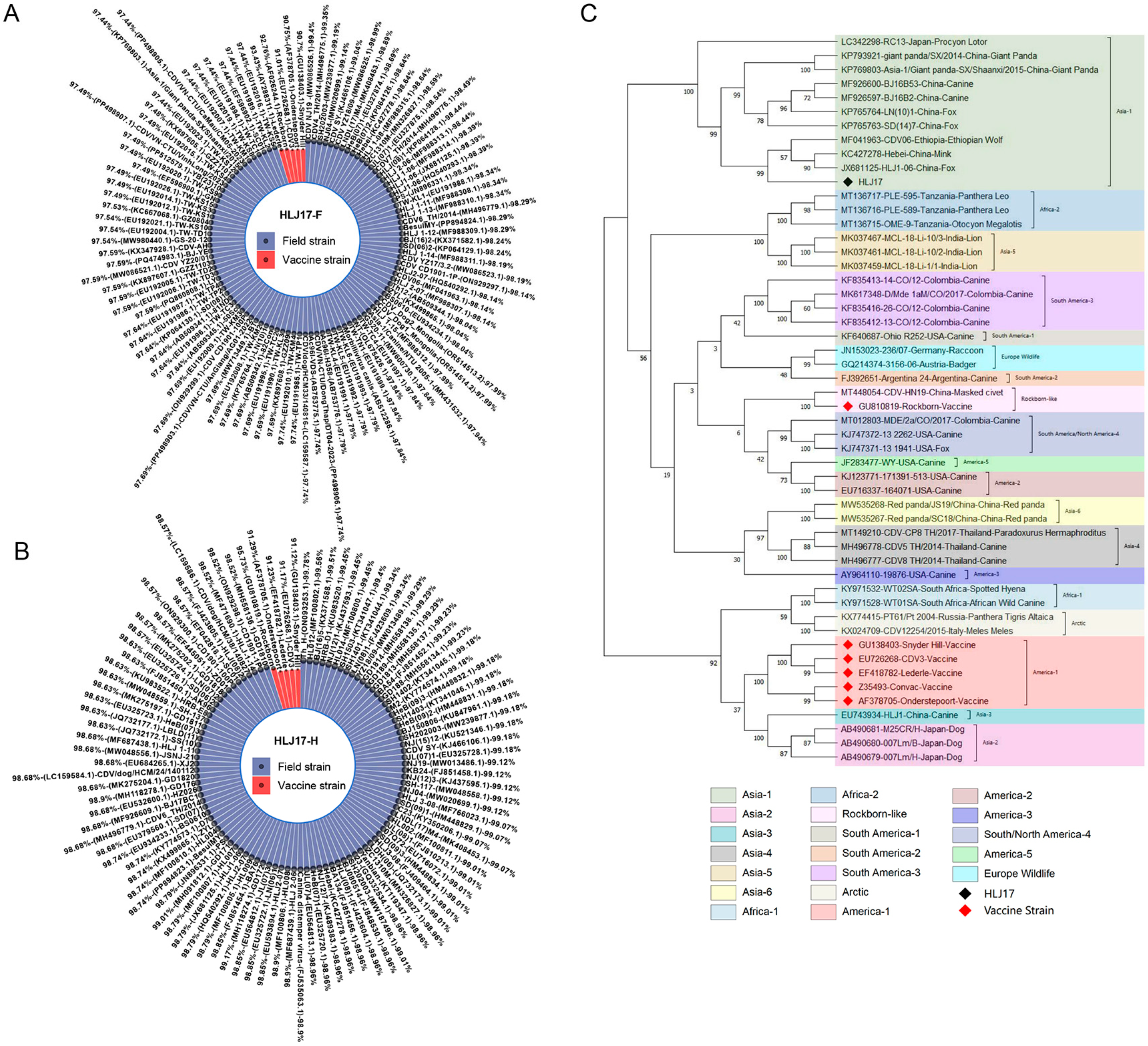
3.1. The Sequence Source and Analysis of CDV F and H Genes
3.2. Generation of the Recombinant Plasmids Encoding HLJ17H or HLJ17F Gene
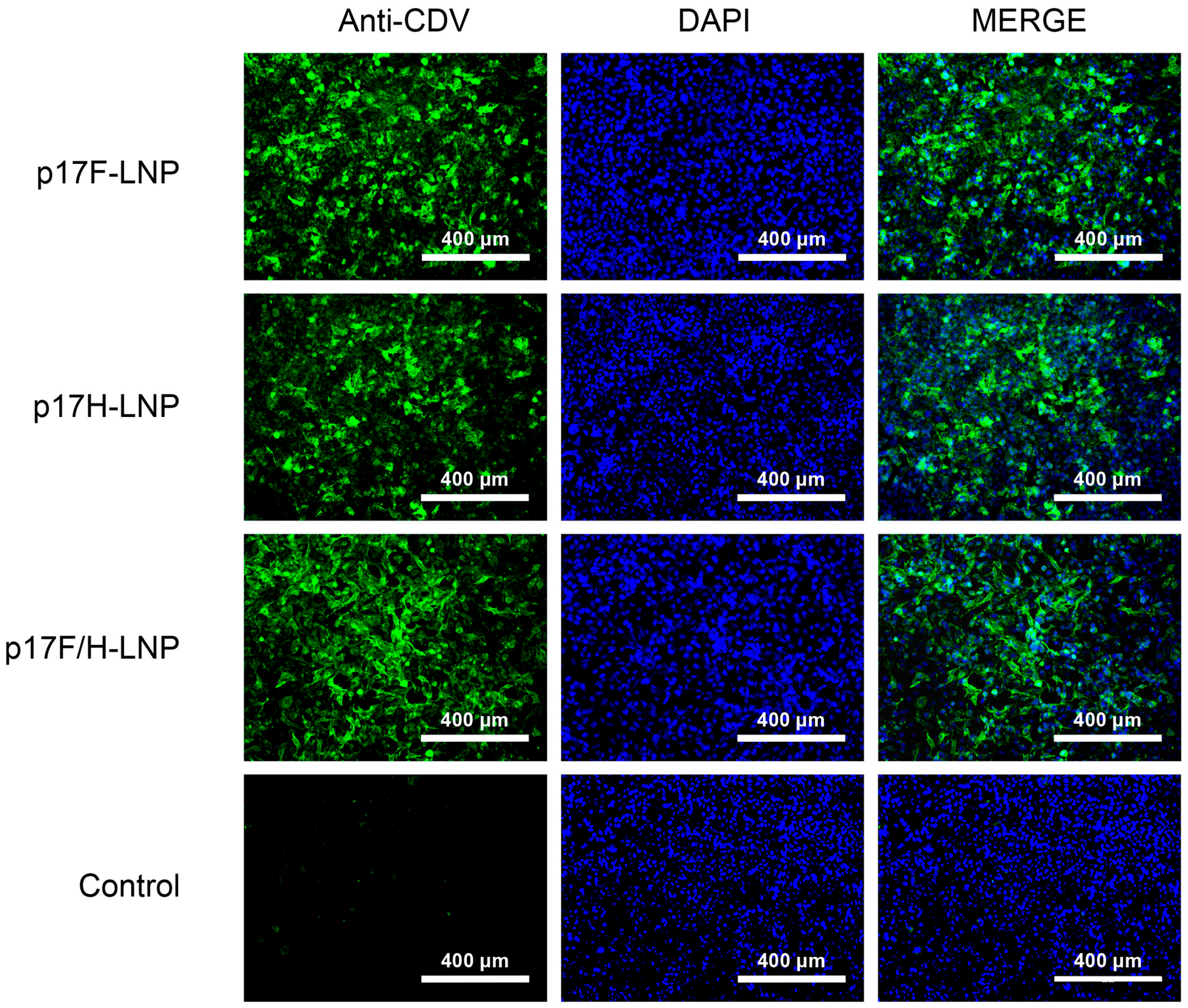
3.3. Formulation of DNA-LNP Vaccine
3.4. The DNA-LNP Formulations Are Safe and Induce Strong Humoral Immune Responses in Mice
3.5. The DNA-LNP Candidate Vaccines Induce Specific Cytokines Responses and Humoral Immune Responses in Mice
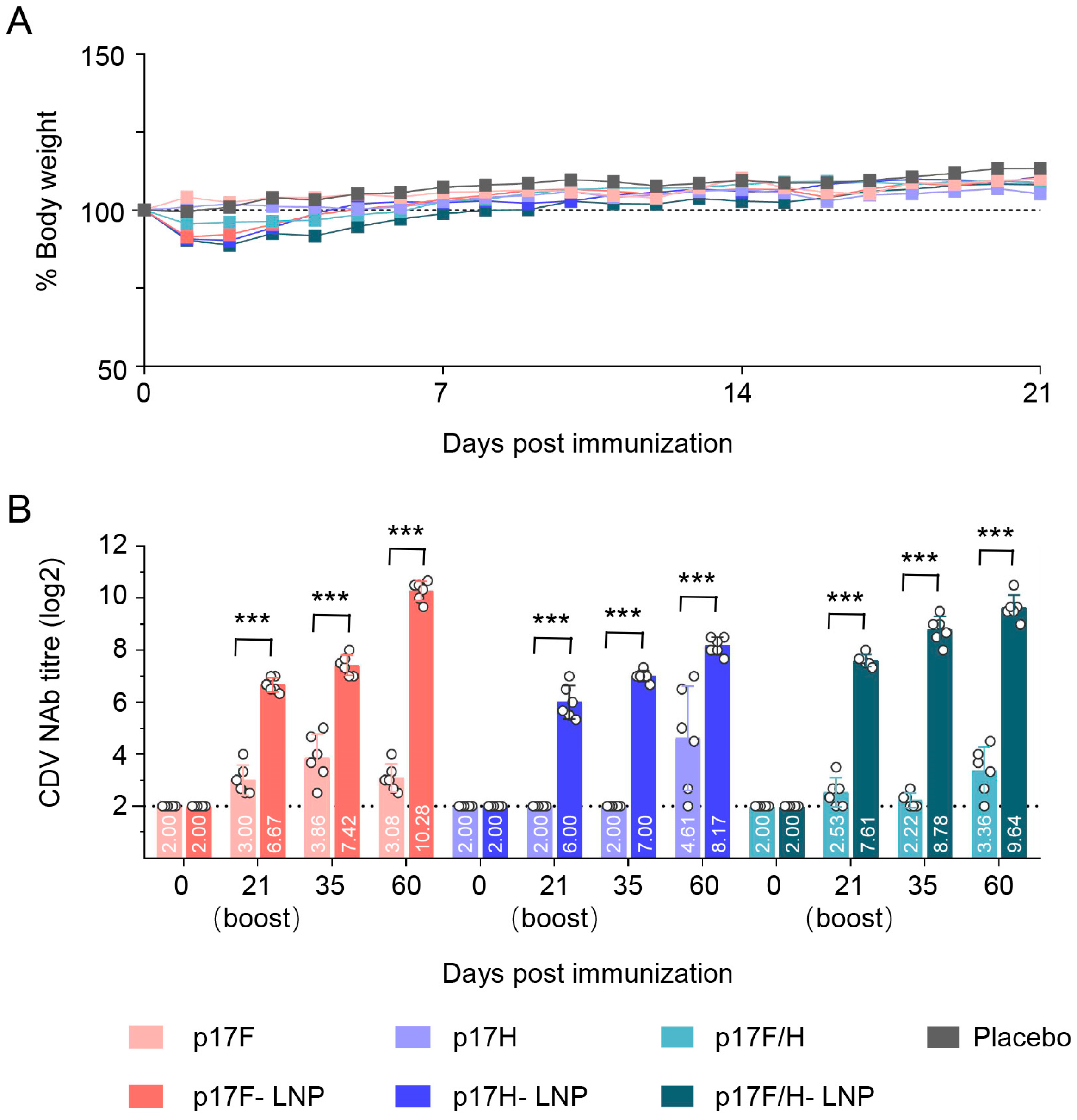
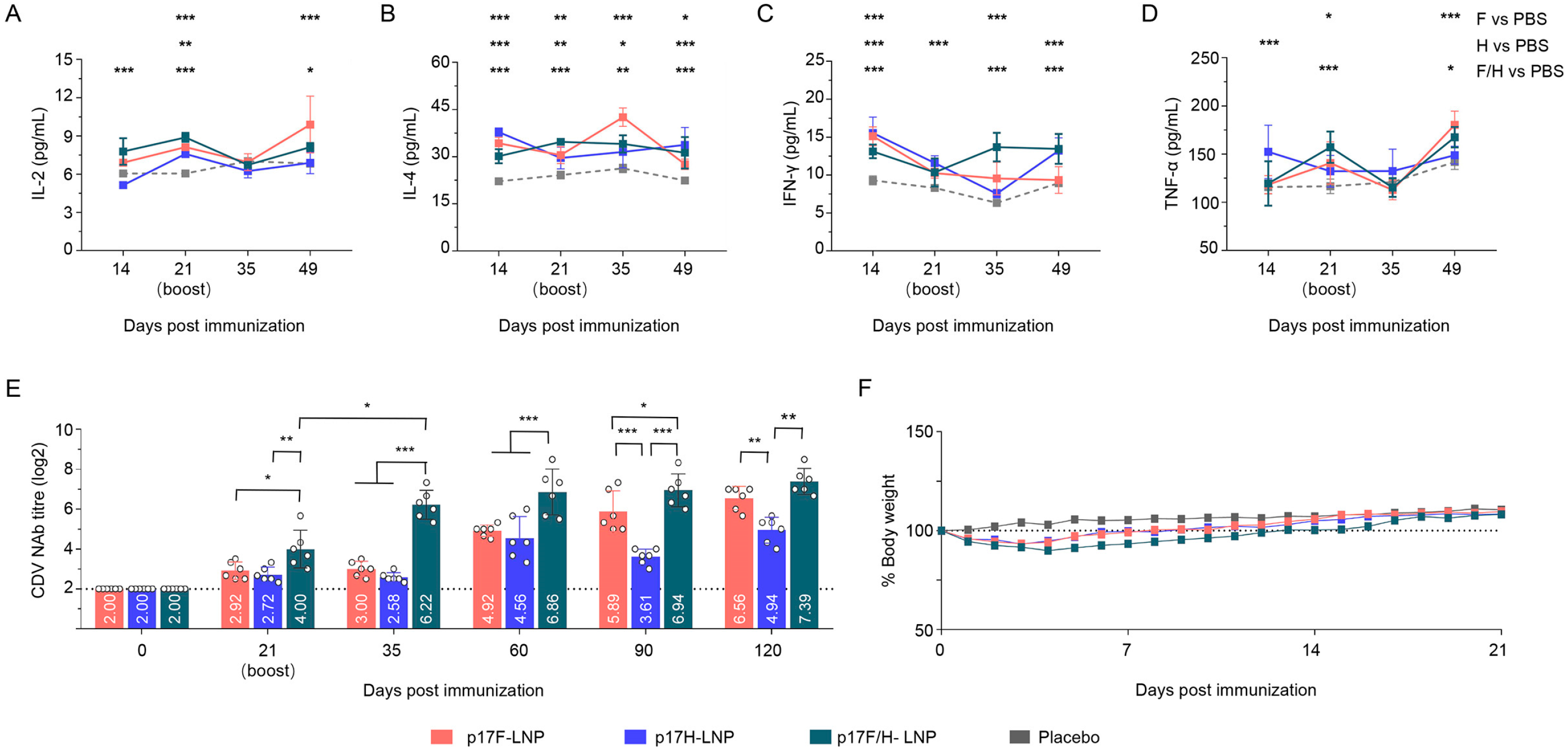
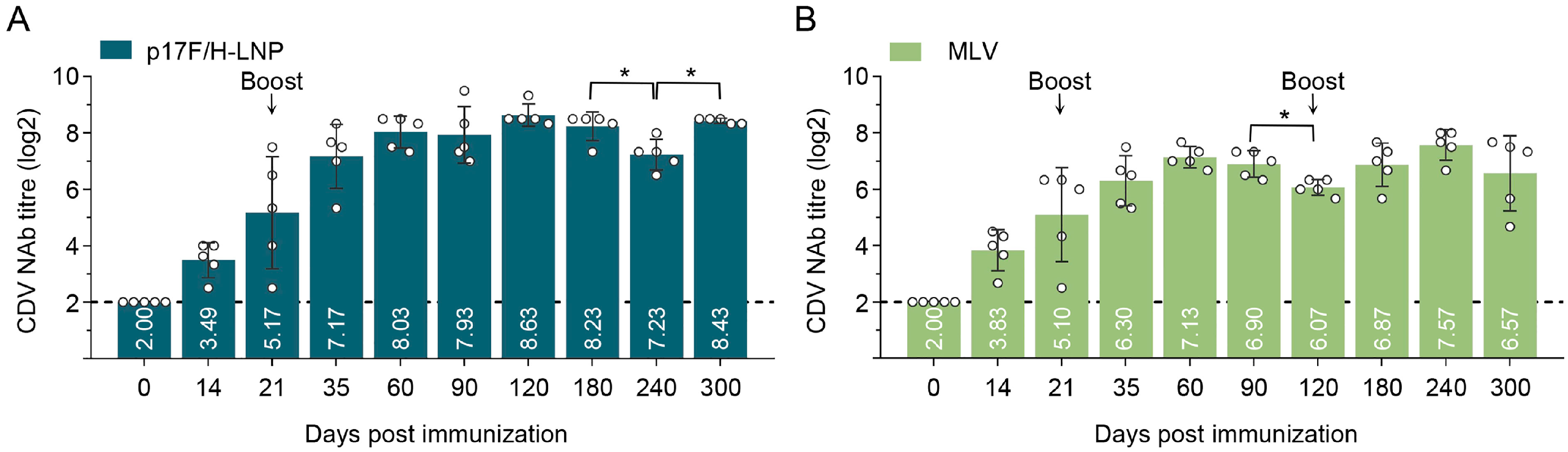
3.6. Immunogenicity of p17F/H-LNP Vaccine in Dogs
3.7. Immunogenicity of p17F/H-LNP Vaccine in Foxes and Raccoon Dogs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliver, S.; Bevan, S.; Christian, K.P. Canine Distemper Virus Pathogenesis in the Ferret Model. In Measles and Related Morbilliviruses: Methods and Protocols; Springer: New York, NY, USA, 2024; pp. 197–208. [Google Scholar]
- Charlotte, L.; Ingo, S.; Christina, P.; Armend, C.; Kristel, K.; Somporn, T.; Wolfgang, B.; Frauke, S. New aspects of the pathogenesis of canine distemper leukoencephalitis. Viruses 2014, 6, 2571. [Google Scholar] [CrossRef] [PubMed]
- Michele, L.; Gabriela Molinari, D.; Alexandre Mendes, A.; Selwyn Arlington, H.; Luciana, S.; Kelly Cristiane Ito, Y.; Fabiana Marques, B.; Alice Fernandes, A.; Amauri Alcindo, A. Canine distemper virus active infection in order Pilosa, family Myrmecophagidae, species Tamandua tetradactyla. Vet. Microbiol. 2018, 220, 7–11. [Google Scholar] [CrossRef]
- Na, F.; Yicong, Y.; Tiecheng, W.; Peter, W.; Jianzhong, W.; Yuanguo, L.; Zhe, S.; Yuwei, G.; Xianzhu, X. Fatal canine distemper virus infection of giant pandas in China. Sci. Rep. 2016, 6, 27518. [Google Scholar] [CrossRef]
- Sarin, S.; Anuwat, W.; Nattarun, C.; Luxsana, P.; Anurux, S.; Patarapol, M.; Benjaporn, B.; Chalisa, M.; Ruangrat, B.; Jarupa, T.; et al. Canine distemper outbreak and laryngeal paralysis in captive tigers (Panthera tigris). BMC Vet. Res. 2025, 21, 33. [Google Scholar] [CrossRef]
- Andreas, B.; Wolfgang, B.; Peter, W. Cross-species transmission of canine distemper virus-an update. One Health 2015, 1, 49–59. [Google Scholar] [CrossRef]
- Mara, H. Endangered species. Captive pandas succumb to killer virus. Science 2015, 347, 700–701. [Google Scholar] [CrossRef]
- Eman, A.; Amy, L.H.; Gina, D.G.; Rebecca, P.W. Antigenic analysis of genetic variants of Canine distemper virus. Vet. Microbiol. 2018, 219, 154–160. [Google Scholar] [CrossRef]
- Rebecca, P.W. Canine Distemper Virus in Endangered Species: Species Jump, Clinical Variations, and Vaccination. Pathogens 2023, 12, 57. [Google Scholar] [CrossRef]
- Zheng, F.; Hao, S.; Qianyu, W.; Zichen, L.; Shaotong, T.; Yingyun, W.; Yuanheng, W.; Gang, L.; Yipeng, J. Risk of canine distemper virus vaccination of domestic dogs in giant panda habitat to giant pandas. Sci. Rep. 2024, 14, 29761. [Google Scholar] [CrossRef]
- Pegah, Y.; Abbas, K.; Narges, M.; Armin, B.; Mitra, A.; Pooya, F.; Fereshteh, R.; Hossein, K. Nanoparticle-mediated enhancement of DNA Vaccines: Revolutionizing immunization strategies. Int. J. Biol. Macromol. 2025, 302, 140558. [Google Scholar] [CrossRef]
- Guimaraes, L.C.; Costa, P.A.C.; Júnior, S.R.A.S.; Ferreira, H.A.S.; Braga, A.C.S.; de Oliveira, L.C.; Figueiredo, M.M.; Shepherd, S.; Hamilton, A.; Queiroz-Junior, C.M.; et al. Nanoparticle-based DNA vaccine protects against SARS-CoV-2 variants in female preclinical models. Nat. Commun. 2024, 15, 590. [Google Scholar] [CrossRef] [PubMed]
- Wenjie, W.; Zhenwei, B.; Yakun, L.; Xingxia, X.; Jing, Q.; Yeping, T.; Yumei, Z.; Suquan, S.; Liping, Y. Development of a monoclonal antibody recognizing novel linear neutralizing epitope on H protein of canine distemper virus vaccine strains (America-1 genotype). Int. J. Biol. Macromol. 2023, 246, 125584. [Google Scholar] [CrossRef]
- Vito, M.; Gabrielle, E.; Canio, B. Canine distemper virus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 787–797. [Google Scholar] [CrossRef]
- Jianzhong, W.; Lina, L.; Xianchun, Z.; Chunliu, W.; Guangmei, Z.; Guilian, Y.; Yanlong, J.; Wentao, Y.; Haibin, H.; Chunwei, S.; et al. Immunogenicity and protective efficacy of a novel bacterium-like particle-based vaccine displaying canine distemper virus antigens in mice and dogs. Microbiol. Spectr. 2024, 12, e03477-23. [Google Scholar] [CrossRef]
- Zhenwei, B.; Wenjie, W.; Xingxia, X. Structure and function of a novel lineage-specific neutralizing epitope on H protein of canine distemper virus. Front. Microbiol. 2023, 13, 1088243. [Google Scholar] [CrossRef]
- Mislay, A.; Lisa, A.; Mojtaba, K.; Nadine, A.-E.; Francesco, O.; Jürgen, S.-S.; Andreas, Z.; Richard, K.P.; Philippe, P. Molecular determinants defining the triggering range of prefusion F complexes of canine distemper virus. J. Virol. 2013, 88, 2951–2966. [Google Scholar] [CrossRef]
- Chenchen, G.; Jun, S.; Jigui, W.; Qianqian, X.; Jing, W.; Jun, X.; Weiquan, L. Fusion protein and hemagglutinin of canine distemper virus co-induce apoptosis in canine mammary tumor cells. J. Cancer Res. Clin. Oncol. 2023, 149, 9903–9918. [Google Scholar] [CrossRef]
- da Fontoura Budaszewski, R.; Hudacek, A.; Sawatsky, B.; Krämer, B.; Yin, X.; Schnell, M.J.; von Messling, V. Inactivated Recombinant Rabies Viruses Displaying Canine Distemper Virus Glycoproteins Induce Protective Immunity against Both Pathogens. J. Virol. 2017, 91, e02077-16. [Google Scholar] [CrossRef]
- Martella, V.; Cirone, F.; Elia, G.; Lorusso, E.; Decaro, N.; Campolo, M.; Desario, C.; Lucente, M.S.; Bellacicco, A.L.; Blixenkrone-Møller, M.; et al. Heterogeneity within the hemagglutinin genes of canine distemper virus (CDV) strains detected in Italy. Vet. Microbiol. 2006, 116, 301–309. [Google Scholar] [CrossRef]
- Imhoff, H.; von Messling, V.; Herrler, G.; Haas, L. Canine distemper virus infection requires cholesterol in the viral envelope. J. Virol. 2007, 81, 4158–4165. [Google Scholar] [CrossRef]
- Liang, J.; Wang, T.; Wang, Q.; Wang, X.; Fan, X.; Hu, T.; Leng, X.; Shi, K.; Li, J.; Gong, Q.; et al. Prevalence of canine distemper in minks, foxes and raccoon dogs from 1983 to 2023 in Asia, North America, South America and Europe. Front. Vet. Sci. 2024, 11, 1394631. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Zhou, K.; Yan, Y.; Li, C.; Ni, B.; Liu, J.; Wang, Y.; Zhang, X.; Wang, D.; Lv, L.; et al. A preparation method for mRNA-LNPs with improved properties. J. Control. Release 2023, 364, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Carey, S.; Clercx, C.; Kohn, B.; MarsilIo, F.; Thiry, E.; Freyburger, L.; Schulz, B.; Walker, D.J. Aetiology of Canine Infectious Respiratory Disease Complex and Prevalence of its Pathogens in Europe. J. Comp. Pathol. 2020, 176, 86–108. [Google Scholar] [CrossRef] [PubMed]
- Marlen, M.-G.; Julian, R.-S. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar] [CrossRef]
- Jolene, A.G.; David, L.P.; Davor, O.; Douglas, C.G.; Claire, M.J. Genetic characterization of canine distemper virus from wild and domestic animal submissions to diagnostic facilities in Canada. Prev. Vet. Med. 2021, 198, 105535. [Google Scholar] [CrossRef]
- Jolene, A.G.; David, L.P.; Davor, O.; Claire, M.J. Comparison of two surveillance components for investigating the epidemiology of canine distemper virus in raccoons (Procyon lotor). J. Wildl. Dis. 2021, 57, 104–115. [Google Scholar] [CrossRef]
- July, D.-V.; Nicolás, S.; Ximena, A.O.-C.; Julián, R.-S. Evolution and Interspecies Transmission of Canine Distemper Virus—An Outlook of the Diverse Evolutionary Landscapes of a Multi-Host Virus. Viruses 2019, 11, 582. [Google Scholar] [CrossRef]
- Sakai, K.; Nagata, N.; Ami, Y.; Seki, F.; Suzaki, Y.; Iwata-Yoshikawa, N.; Suzuki, T.; Fukushi, S.; Mizutani, T.; Yoshikawa, T.; et al. Lethal canine distemper virus outbreak in cynomolgus monkeys in Japan in 2008. J. Virol. 2013, 87, 1105–1114. [Google Scholar] [CrossRef]
- Rendon-Marin, S.; Higuita-Gutiérrez, L.; Ruiz-Saenz, J. Morbillivirus canisSafety and Immunogenicity of Vaccines for Domestic and Wild Animals: A Scoping Review. Viruses 2024, 16, 1078. [Google Scholar] [CrossRef]
- Vega-Mariño, P.; Olson, J.; Howitt, B.; Criollo, R.; Figueroa, L.; Orlando, S.; Cruz, M.; Garcia-Bereguiain, M. A recent distemper virus outbreak in the growing canine populations of Galapagos Islands: A persistent threat for the endangered Galapagos Sea Lion. Front. Vet. Sci. 2023, 10, 1154625. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, Y.; Chen, J.; Zheng, J.; Sun, D. Viral Pathogenesis, Recombinant Vaccines, and Oncolytic Virotherapy: Applications of the Canine Distemper Virus Reverse Genetics System. Viruses 2020, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, Y.; Sui, P.; Pan, H.; Shi, Y.; Chen, J.; Zhang, H.; Wang, X.; Tao, R.; Liu, M.; et al. DNA Vaccine Co-Expressing Hemagglutinin and IFN-γ Provides Partial Protection to Ferrets against Lethal Challenge with Canine Distemper Virus. Viruses 2023, 15, 1873. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Nielsen, L.; Aasted, B.; Pertoldi, C.; Blixenkrone-Møller, M. Canine distemper virus DNA vaccination of mink can overcome interference by maternal antibodies. Vaccine 2015, 33, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, S.; Dema, B.; Sanchez-Martinez, A.; Montalvo Zurbia-Flores, G.; Rollier, C. DNA Vaccines: History, Molecular Mechanisms and Future Perspectives. J. Mol. Biol. 2023, 435, 168297. [Google Scholar] [CrossRef]
- Poria, R.; Kala, D.; Nagraik, R.; Dhir, Y.; Dhir, S.; Singh, B.; Kaushik, N.; Noorani, M.; Kaushal, A.; Gupta, S. Vaccine development: Current trends and technologies. Life Sci. 2024, 336, 122331. [Google Scholar] [CrossRef]
- Liao, H.; Shen, K.; Yang, C.; Chiu, F.; Chiang, C.; Chai, K.; Huang, W.; Ho, H.; Chen, Y.; Huang, M.; et al. Lipid nanoparticle-encapsulated DNA vaccine robustly induce superior immune responses to the mRNA vaccine in Syrian hamsters. Mol. Ther. Methods Clin. Dev. 2024, 32, 101169. [Google Scholar] [CrossRef]
- Nguyen, T.; Lai, D.; Sillman, S.; Petro-Turnquist, E.; Weaver, E.; Vu, H. Lipid nanoparticle-encapsulated DNA vaccine confers protection against swine and human-origin H1N1 influenza viruses. mSphere 2024, 9, e0028324. [Google Scholar] [CrossRef]
- Lai, D.; Nguyen, T.; Poonsuk, K.; McVey, D.; Vu, H. Lipid nanoparticle-encapsulated DNA vaccine encoding African swine fever virus p54 antigen elicits robust immune responses in pigs. Vet. Microbiol. 2025, 305, 110508. [Google Scholar] [CrossRef]
- Xu, K.; Lei, W.; Kang, B.; Yang, H.; Wang, Y.; Lu, Y.; Lv, L.; Sun, Y.; Zhang, J.; Wang, X.; et al. A novel mRNA vaccine, SYS6006, against SARS-CoV-2. Front. Immunol. 2022, 13, 1051576. [Google Scholar] [CrossRef]
- Rosa, S.; Prazeres, D.; Azevedo, A.; Marques, M. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Huo, H.; Wang, J.; Li, C.; Xiao, S.; Wang, H.; Ge, J.; Zhong, G.; Wen, Z.; Wang, C.; Lang, Q.; et al. Safety and immunogenicity of a SARS-CoV-2 mRNA vaccine (SYS6006) in minks, cats, blue foxes, and raccoon dogs. Front. Cell. Infect. Microbiol. 2024, 14, 1468775. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, H.; Wang, H.; Liang, S.; Wang, Z.; Wang, J.; Wang, Q.; Li, C.; Tao, Y.; Ge, J.; Wen, Z.; et al. Safety and Immunogenicity of a Canine Distemper DNA Vaccine Formulated with Lipid Nanoparticles in Dogs, Foxes, and Raccoon Dogs. Vaccines 2025, 13, 614. https://doi.org/10.3390/vaccines13060614
Huo H, Wang H, Liang S, Wang Z, Wang J, Wang Q, Li C, Tao Y, Ge J, Wen Z, et al. Safety and Immunogenicity of a Canine Distemper DNA Vaccine Formulated with Lipid Nanoparticles in Dogs, Foxes, and Raccoon Dogs. Vaccines. 2025; 13(6):614. https://doi.org/10.3390/vaccines13060614
Chicago/Turabian StyleHuo, Hong, Han Wang, Shulin Liang, Zilong Wang, Jinming Wang, Qingzhu Wang, Chan Li, Yuting Tao, Jinying Ge, Zhiyuan Wen, and et al. 2025. "Safety and Immunogenicity of a Canine Distemper DNA Vaccine Formulated with Lipid Nanoparticles in Dogs, Foxes, and Raccoon Dogs" Vaccines 13, no. 6: 614. https://doi.org/10.3390/vaccines13060614
APA StyleHuo, H., Wang, H., Liang, S., Wang, Z., Wang, J., Wang, Q., Li, C., Tao, Y., Ge, J., Wen, Z., Wang, J., Chen, W., Wang, X., Shuai, L., & Bu, Z. (2025). Safety and Immunogenicity of a Canine Distemper DNA Vaccine Formulated with Lipid Nanoparticles in Dogs, Foxes, and Raccoon Dogs. Vaccines, 13(6), 614. https://doi.org/10.3390/vaccines13060614




