Immunotherapeutic Blockade of CD47 Increases Virus Neutralization Antibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Ethical Approval
2.2. Virus
2.3. Anti-CD47 Monoclonal Antibodies
2.4. Neutralization Antibody Assay
2.5. Flow Cytometry
2.6. Intracellular Cytokine Staining
2.7. Statistical Analysis
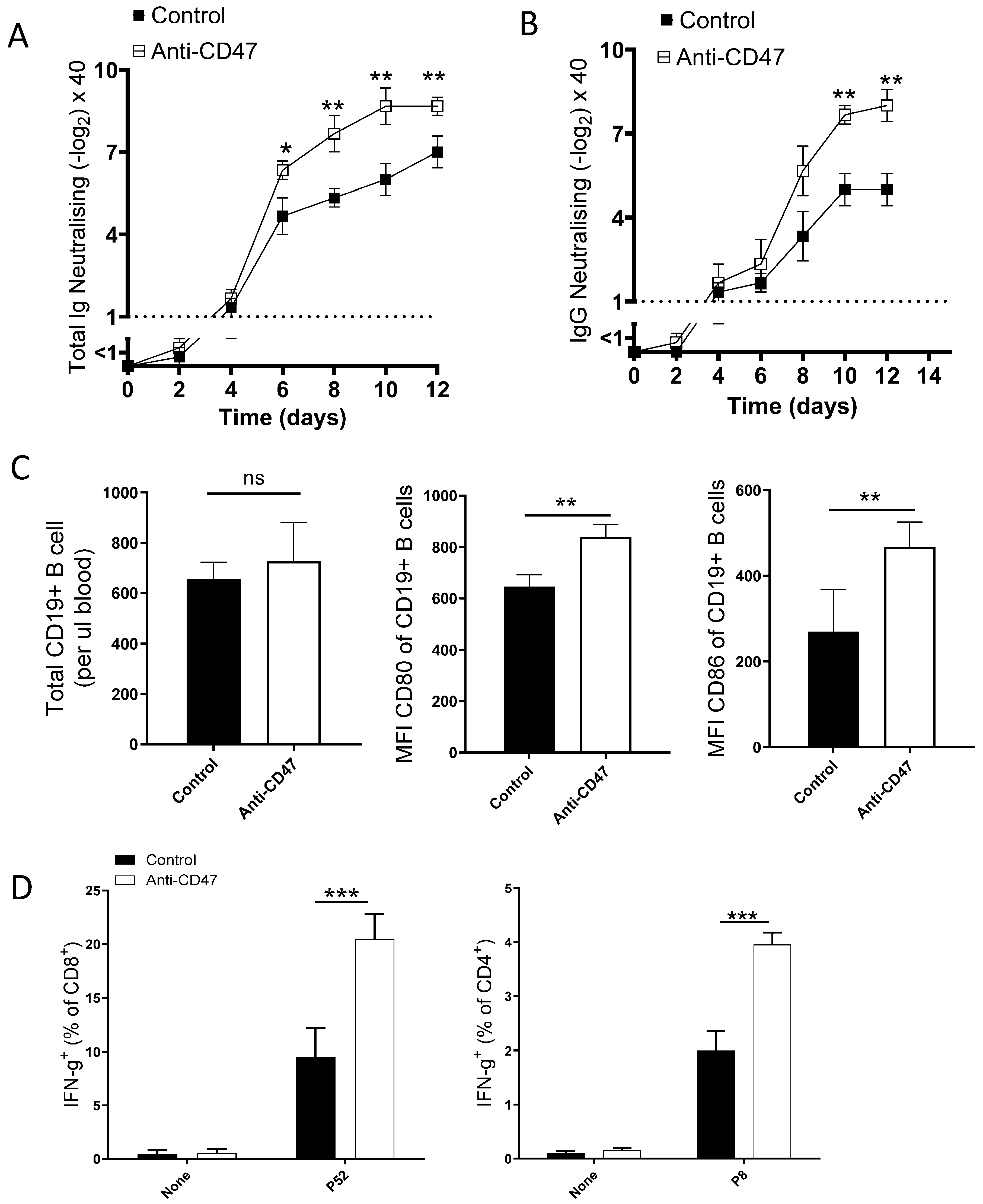
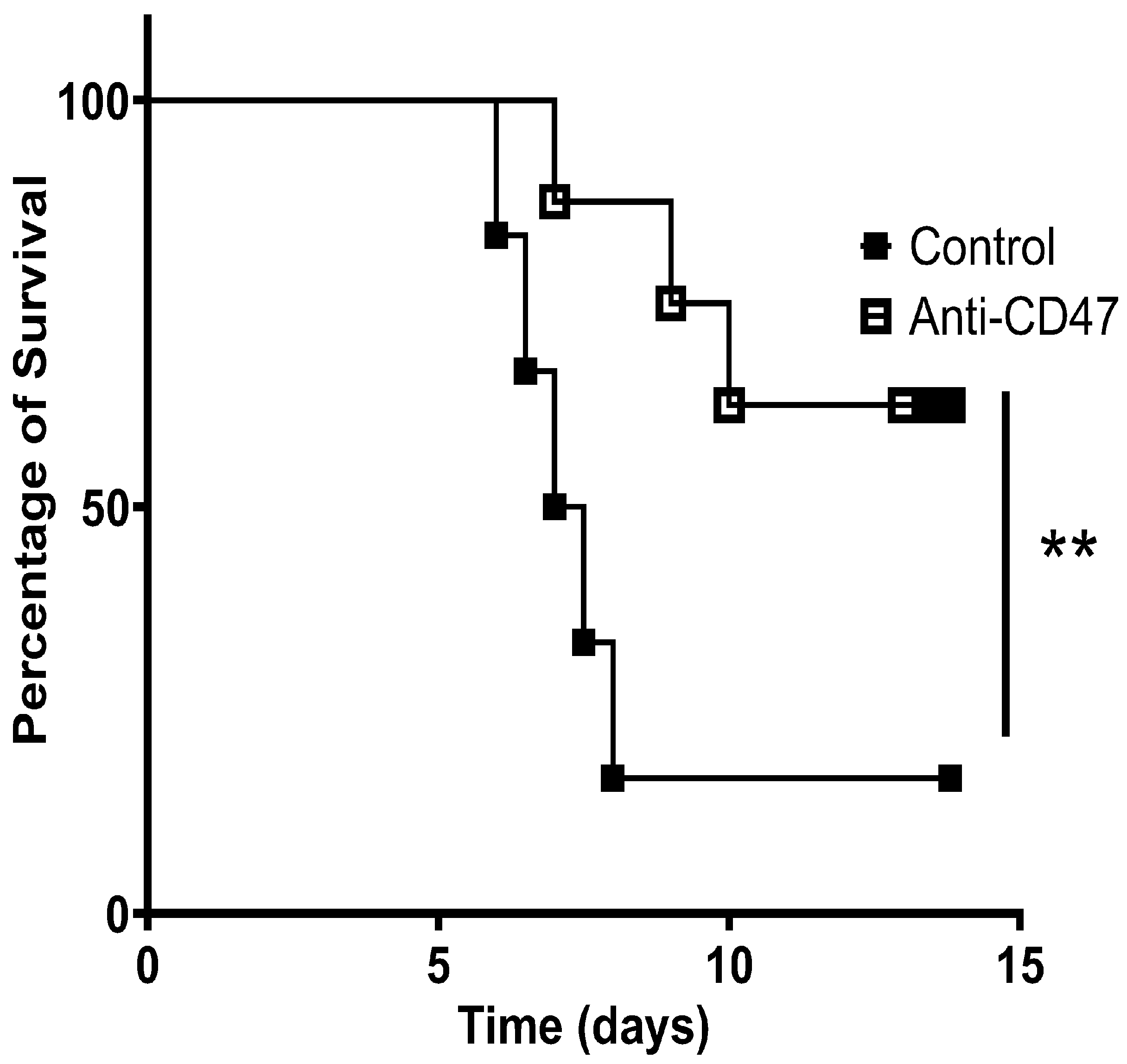
3. Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, P.A. CD47: A Cell Surface Glycoprotein Which Regulates Multiple Functions of Hematopoietic Cells in Health and Disease. ISRN Hematol. 2013, 2013, 614619. [Google Scholar] [CrossRef] [PubMed]
- Cham, L.B.; Torrez Dulgeroff, L.B.; Tal, M.C.; Adomati, T.; Li, F.; Bhat, H.; Huang, A.; Lang, P.A.; Moreno, M.E.; Rivera, J.M.; et al. Immunotherapeutic Blockade of CD47 Inhibitory Signaling Enhances Innate and Adaptive Immune Responses to Viral Infection. Cell Rep. 2020, 31, 107494. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Ye, Z.H.; Huang, M.Y.; Lu, J.J. Regulation of CD47 expression in cancer cells. Transl. Oncol. 2020, 13, 100862. [Google Scholar] [CrossRef]
- Cham, L.B.; Rosas-Umbert, M.; Lin, L.; Tolstrup, M.; Sogaard, O.S. Single-Cell Analysis Reveals That CD47 mRNA Expression Correlates with Immune Cell Activation, Antiviral Isgs, and Cytotoxicity. Cell Physiol. Biochem. 2024, 58, 322–335. [Google Scholar] [CrossRef]
- Huang, C.; Wang, X.; Wang, Y.; Feng, Y.; Wang, X.; Chen, S.; Yan, P.; Liao, J.; Zhang, Q.; Mao, C.; et al. Sirpalpha on tumor-associated myeloid cells restrains antitumor immunity in colorectal cancer independent of its interaction with CD47. Nat. Cancer 2024, 5, 500–516. [Google Scholar] [CrossRef]
- Myers, L.M.; Tal, M.C.; Torrez Dulgeroff, L.B.; Carmody, A.B.; Messer, R.J.; Gulati, G.; Yiu, Y.Y.; Staron, M.M.; Angel, C.L.; Sinha, R.; et al. A functional subset of CD8(+) T cells during chronic exhaustion is defined by SIRPalpha expression. Nat. Commun. 2019, 10, 794. [Google Scholar] [CrossRef]
- Yilmaz, L.; Oztuzcu, S.; Eronat, O.; Ugur, B.K.; Gumus, M.; Guler, S.; Tasdemir, D.; Bozgeyik, E.; Caska, H.; Bozgeyik, I. Exploring NK cell dynamics and the CD47-SIRPalpha axis in colon cancer. Hum. Immunol. 2025, 86, 111254. [Google Scholar] [CrossRef]
- Willingham, S.B.; Volkmer, J.P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef]
- Barclay, A.N.; Van den Berg, T.K. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: Structure, function, and therapeutic target. Annu. Rev. Immunol. 2014, 32, 25–50. [Google Scholar] [CrossRef]
- Polara, R.; Ganesan, R.; Pitson, S.M.; Robinson, N. Cell autonomous functions of CD47 in regulating cellular plasticity and metabolic plasticity. Cell Death Differ. 2024, 31, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Kaur, S.; Ivins-O’Keefe, K.; Roberts, D.D. Thrombospondin-1 is a CD47-dependent endogenous inhibitor of hydrogen sulfide signaling in T cell activation. Matrix Biol. 2013, 32, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Rogers, N.M.; Ghimire, K. Thrombospondin-1 CD47 Signalling: From Mechanisms to Medicine. Int. J. Mol. Sci. 2021, 22, 4062. [Google Scholar] [CrossRef] [PubMed]
- Cham, L.B.; Adomati, T.; Li, F.; Ali, M.; Lang, K.S. CD47 as a Potential Target to Therapy for Infectious Diseases. Antibodies 2020, 9, 44. [Google Scholar] [CrossRef]
- Yamada-Hunter, S.A.; Theruvath, J.; McIntosh, B.J.; Freitas, K.A.; Lin, F.; Radosevich, M.T.; Leruste, A.; Dhingra, S.; Martinez-Velez, N.; Xu, P.; et al. Engineered CD47 protects T cells for enhanced antitumour immunity. Nature 2024, 630, 457–465. [Google Scholar] [CrossRef]
- Tseng, D.; Volkmer, J.P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108. [Google Scholar] [CrossRef]
- Collins, J.M.; Redman, J.M.; Gulley, J.L. Combining vaccines and immune checkpoint inhibitors to prime, expand, and facilitate effective tumor immunotherapy. Expert. Rev. Vaccines 2018, 17, 697–705. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Hassouneh, F.; Alvarez-Heredia, P.; Pera, A.; Solana, R. Immune Checkpoint Inhibitors for Vaccine Improvements: Current Status and New Approaches. Pharmaceutics 2022, 14, 1721. [Google Scholar] [CrossRef]
- Shahin, M.; Fadavi, P.; Ramandi, M.M.A.; Shahrokh, S.; Taghizadeh-Hesary, F. De novo myasthenia gravis in a patient with malignant melanoma after concurrent SARS-CoV-2 vaccination and immune checkpoint inhibitor therapy: Case report and literature review. eNeurologicalSci 2024, 37, 100534. [Google Scholar] [CrossRef]
- Lao, Y.; Shen, D.; Zhang, W.; He, R.; Jiang, M. Immune Checkpoint Inhibitors in Cancer Therapy-How to Overcome Drug Resistance? Cancers 2022, 14, 3575. [Google Scholar] [CrossRef]
- New, J.; Shenton, L.; Ksayer, R.; Wang, J.; Zakharia, K.; Nicholson, L.J.; Pandey, A.C. Immune Checkpoint Inhibitors and Vaccination: Assessing Safety, Efficacy, and Synergistic Potential. Vaccines 2024, 12, 1270. [Google Scholar] [CrossRef] [PubMed]
- Antoun, E.; Peng, Y.; Dong, T. Vaccine-induced CD8(+) T cells are key to protection from SARS-CoV-2. Nat. Immunol. 2023, 24, 1594–1596. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Ko, E.J.; Lee, Y.; Lee, Y.N.; Bian, Z.; Liu, Y.; Kang, S.M. CD47 Plays a Role as a Negative Regulator in Inducing Protective Immune Responses to Vaccination against Influenza Virus. J. Virol. 2016, 90, 6746–6758. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Han, S.; Jang, I.H.; Ryu, J.; Rha, M.S.; Cho, H.J.; Yoon, S.S.; Nam, K.T.; Kim, C.H.; Park, M.S.; et al. Airway epithelial CD47 plays a critical role in inducing influenza virus-mediated bacterial super-infection. Nat. Commun. 2024, 15, 3666. [Google Scholar] [CrossRef]
- Yiu, Y.Y.; Hansen, P.S.; Torrez Dulgeroff, L.B.; Blacker, G.; Myers, L.; Galloway, S.; Gars, E.; Colace, O.; Mansfield, P.; Hasenkrug, K.J.; et al. CD47 Blockade Leads to Chemokine-Dependent Monocyte Infiltration and Loss of B Cells from the Splenic Marginal Zone. J. Immunol. 2022, 208, 1371–1377. [Google Scholar] [CrossRef]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B cells as antigen presenting cells. Front. Immunol. 2022, 13, 954936. [Google Scholar] [CrossRef]
- Kleponis, J.; Skelton, R.; Zheng, L. Fueling the engine and releasing the break: Combinational therapy of cancer vaccines and immune checkpoint inhibitors. Cancer Biol. Med. 2015, 12, 201–208. [Google Scholar] [CrossRef]
- Strauss, J.; Madan, R.A.; Gulley, J.L. Considerations for the combination of anticancer vaccines and immune checkpoint inhibitors. Expert. Opin. Biol. Ther. 2016, 16, 895–901. [Google Scholar] [CrossRef]
- Salewski, I.; Kuntoff, S.; Kuemmel, A.; Feldtmann, R.; Felix, S.B.; Henze, L.; Junghanss, C.; Maletzki, C. Combined vaccine-immune-checkpoint inhibition constitutes a promising strategy for treatment of dMMR tumors. Cancer Immunol. Immunother. 2021, 70, 3405–3419. [Google Scholar] [CrossRef]
- Kim, C.G.; Sang, Y.B.; Lee, J.H.; Chon, H.J. Combining Cancer Vaccines with Immunotherapy: Establishing a New Immunological Approach. Int. J. Mol. Sci. 2021, 22, 8035. [Google Scholar] [CrossRef]
- Chong, C.R.; Park, V.J.; Cohen, B.; Postow, M.A.; Wolchok, J.D.; Kamboj, M. Safety of Inactivated Influenza Vaccine in Cancer Patients Receiving Immune Checkpoint Inhibitors. Clin. Infect. Dis. 2020, 70, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.I.; Lopez-Olivo, M.A.; Geng, Y.; Suarez-Almazor, M.E. COVID-19 vaccination in patients with cancer receiving immune checkpoint inhibitors: A systematic review and meta-analysis. J. Immunother. Cancer 2023, 11, e006246. [Google Scholar] [CrossRef] [PubMed]
- Gwynn, M.E.; DeRemer, D.L.; Saunders, K.M.; Parikh, J.; Bollag, R.J.; Clemmons, A.B. Immune-mediated adverse events following influenza vaccine in cancer patients receiving immune checkpoint inhibitors. J. Oncol. Pharm. Pract. 2020, 26, 647–654. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cham, L.B.; Hamdan, T.A.; Bhat, H.; Sirajo, B.; Ali, M.; Tabbara, K.S.; Farid, E.; Barbouche, M.-R.; Adomati, T. Immunotherapeutic Blockade of CD47 Increases Virus Neutralization Antibodies. Vaccines 2025, 13, 602. https://doi.org/10.3390/vaccines13060602
Cham LB, Hamdan TA, Bhat H, Sirajo B, Ali M, Tabbara KS, Farid E, Barbouche M-R, Adomati T. Immunotherapeutic Blockade of CD47 Increases Virus Neutralization Antibodies. Vaccines. 2025; 13(6):602. https://doi.org/10.3390/vaccines13060602
Chicago/Turabian StyleCham, Lamin B., Thamer A. Hamdan, Hilal Bhat, Bello Sirajo, Murtaza Ali, Khaled Saeed Tabbara, Eman Farid, Mohamed-Ridha Barbouche, and Tom Adomati. 2025. "Immunotherapeutic Blockade of CD47 Increases Virus Neutralization Antibodies" Vaccines 13, no. 6: 602. https://doi.org/10.3390/vaccines13060602
APA StyleCham, L. B., Hamdan, T. A., Bhat, H., Sirajo, B., Ali, M., Tabbara, K. S., Farid, E., Barbouche, M.-R., & Adomati, T. (2025). Immunotherapeutic Blockade of CD47 Increases Virus Neutralization Antibodies. Vaccines, 13(6), 602. https://doi.org/10.3390/vaccines13060602






