mRNA Vaccine Technology Beyond COVID-19
Abstract
:1. Introduction
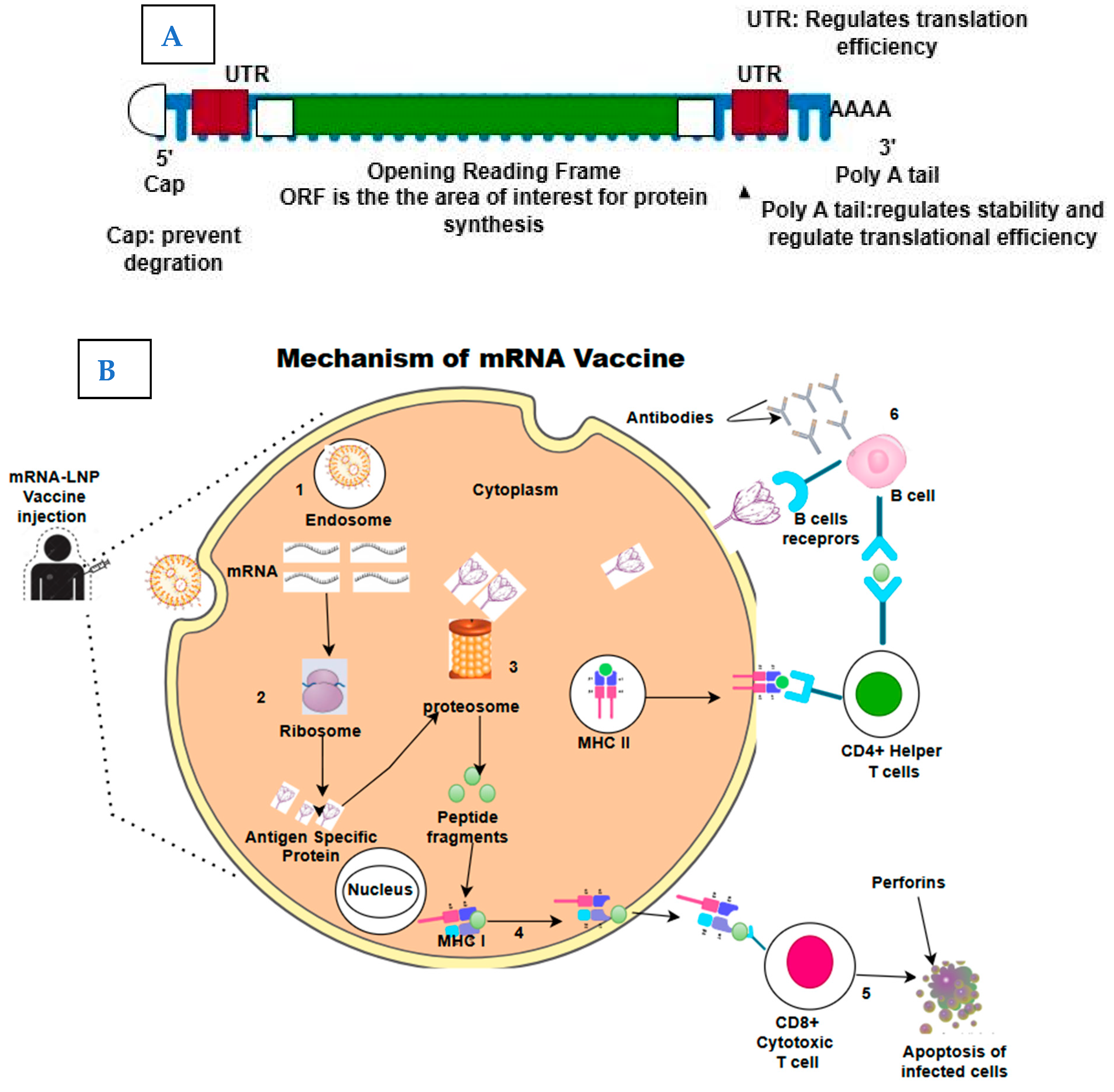
2. The Structure and Mechanism of Action of mRNA Vaccines
Key Advantages over Traditional Vaccine Platforms
3. Lipid Nanoparticle (LNP) Delivery Systems
3.1. Advances in Lipid Nanoparticle (LNP) Delivery Systems
3.2. Types of Lipid Nanoparticles (LNPs)
3.3. Improvement in Delivery Systems
3.4. Strategies for Enhancing Stability and Immunogenicity
4. Applications Beyond COVID-19
4.1. Infectious Diseases
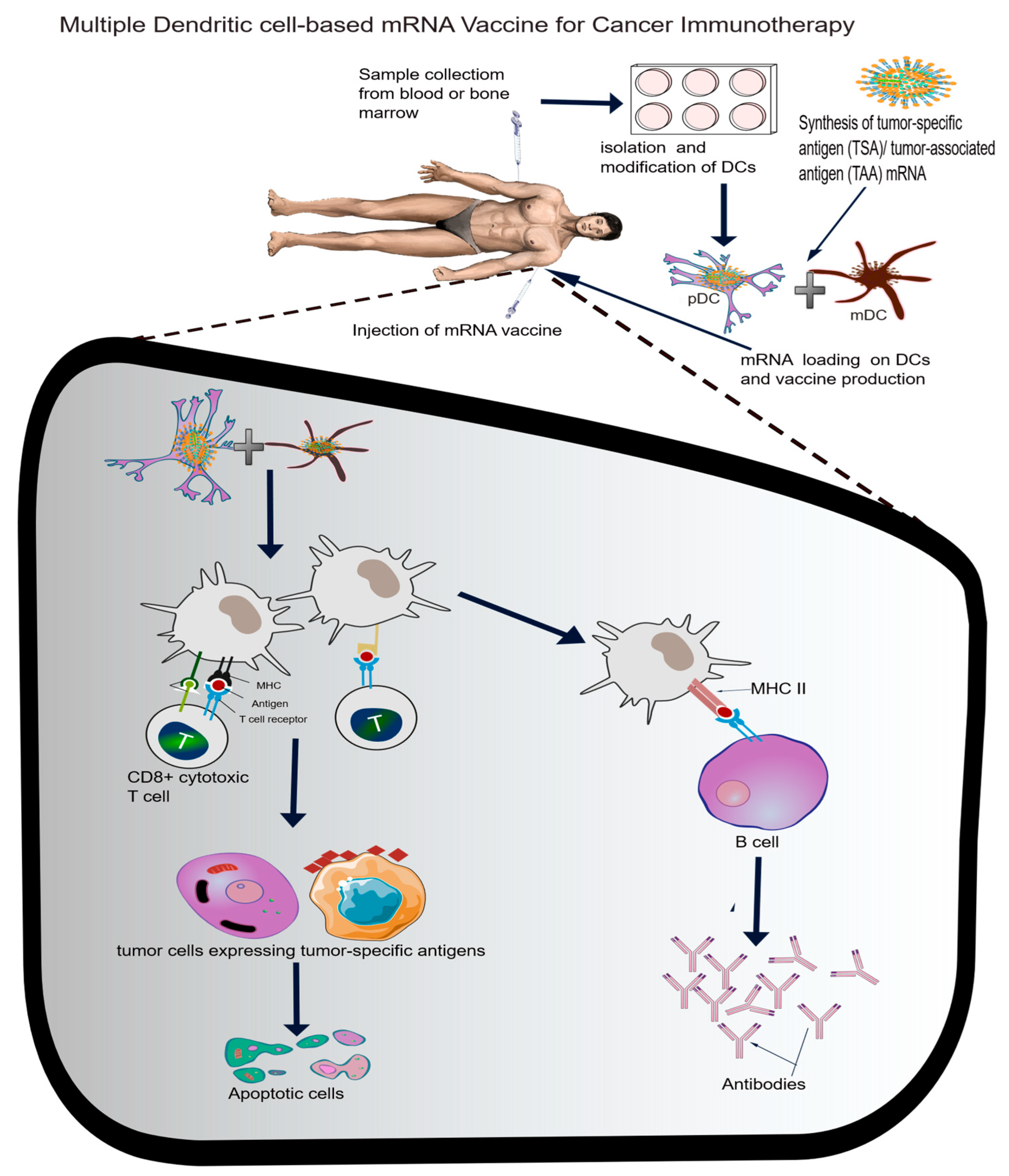
4.2. Cancer Immunology
4.3. Personalized Medicine
5. Current Challenges
5.1. Manufacturing and Scale-Up
5.1.1. Challenges in Production and Supply Chain Scalability
5.1.2. Addressing Regional Manufacturing Gaps
5.2. Cost and Accessibility
5.2.1. High Costs of mRNA Technology Compared to Traditional Vaccines
5.2.2. Strategies for Reducing Costs and Ensuring Global Equity
5.3. Safety Concerns and Public Acceptance
5.3.1. Addressing Adverse Effects (e.g., Myocarditis)
5.3.2. Public Scepticism in the Wake of Misinformation
5.4. Regulatory and Ethical Considerations
5.4.1. Regulatory Landscape for mRNA Vaccines Beyond COVID
5.4.2. Ethical Concerns Related to Personalized mRNA Vaccines
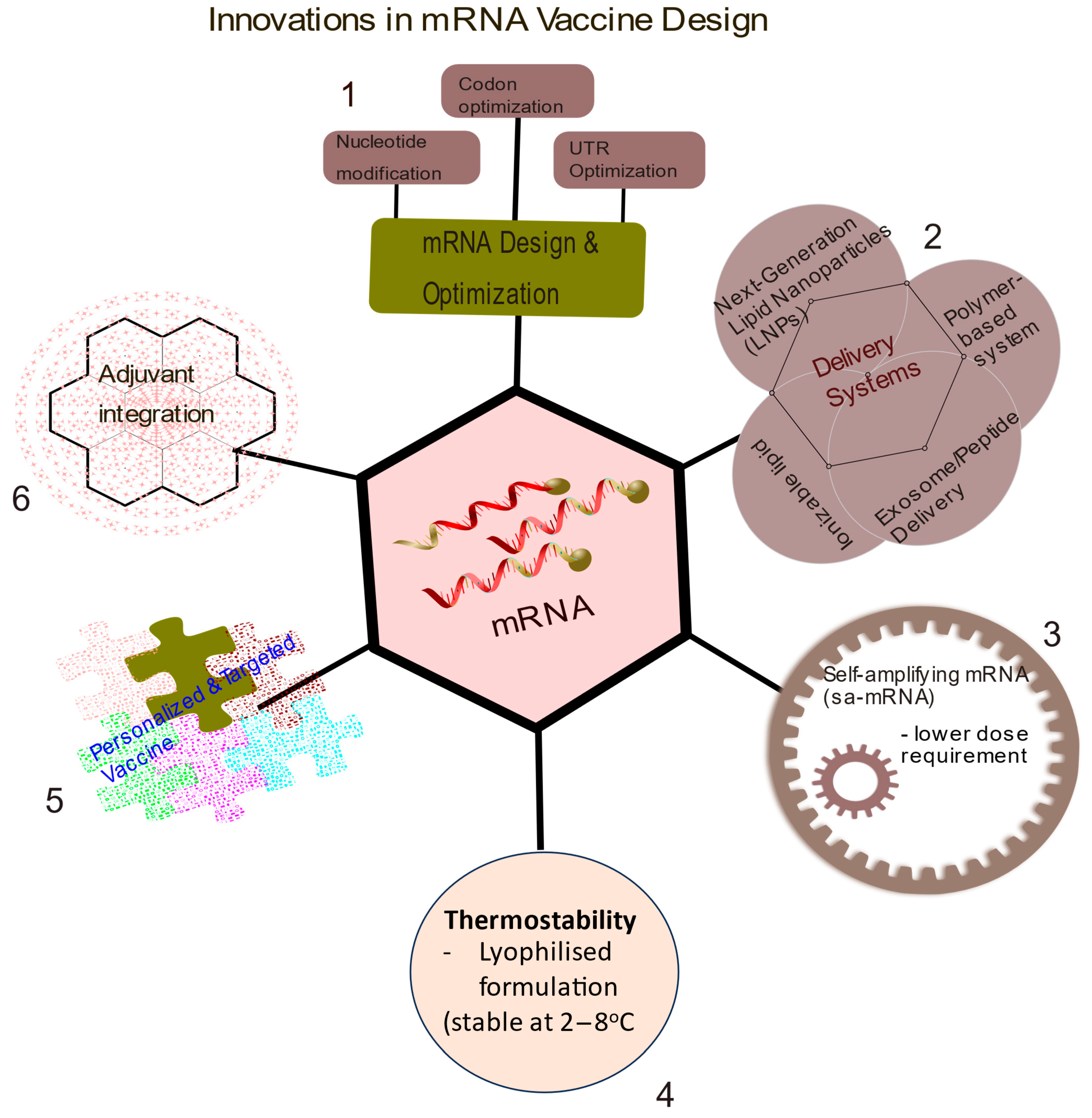
6. Innovations in mRNA Vaccine Designs
6.1. Multivalent Vaccine Designs for Multiple Pathogens or Cancer Antigens
6.2. Future Direction
7. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Hsu, F.J.; Benike, C.; Fagnoni, F.; Liles, T.M.; Czerwinski, D.; Taidi, B.; Engleman, E.G.; Levy, R. Vaccination of patients with B–cell lymphoma using autologous antigen–pulsed dendritic cells. Nat. Med. 1996, 2, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Igyártó, B.Z.; Qin, Z. The mRNA-LNP vaccines–the good, the bad and the ugly? Front. Immunol 2024, 15, 1336906. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Rehman, K.; Mahmood, A.; Shabbir, M.; Liang, Y.; Duan, L.; Zeng, H. Exosome for mRNA delivery: Strategies and therapeutic applications. J. Nanobiotechnol. 2024, 22, 395. [Google Scholar] [CrossRef]
- Han, X.; Zhang, H.; Butowska, K.; Swingle, K.L.; Alameh, M.-G.; Weissman, D.; Mitchell, M.J. An ionizable lipid toolbox for RNA delivery. Nat. Commun. 2021, 12, 7233. [Google Scholar] [CrossRef]
- Hannani, D.; Leplus, E.; Laurin, D.; Caulier, B.; Aspord, C.; Madelon, N.; Bourova-Flin, E.; Brambilla, C.; Brambilla, E.; Toffart, A.-C.; et al. A New Plasmacytoid Dendritic Cell-Based Vaccine in Combination with Anti-PD-1 Expands the Tumor-Specific CD8+ T Cells of Lung Cancer Patients. Int. J. Mol. Sci. 2023, 24, 1897. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.; Chaperot, L.; Hannani, D.; Costa, J.B.; Templier, I.; Trabelsi, S.; Gil, H.; Moisan, A.; Persoons, V.; Hegelhofer, H.; et al. An innovative plasmacytoid dendritic cell line-based cancer vaccine primes and expands antitumor T-cells in melanoma patients in a first-in-human trial. OncoImmunology 2020, 9, 1738812. [Google Scholar] [CrossRef]
- Lenogue, K.; Walencik, A.; Laulagnier, K.; Molens, J.-P.; Benlalam, H.; Dreno, B.; Coulie, P.; Pule, M.; Chaperot, L.; Plumas, J. Engineering a Human Plasmacytoid Dendritic Cell-Based Vaccine to Prime and Expand Multispecific Viral and Tumor Antigen-Specific T-Cells. Vaccines 2021, 9, 141. [Google Scholar] [CrossRef]
- Perez, C.R.; De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.-J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhou, Y.; Zhang, S.; Chen, S.-J. mRNA vaccine sequence and structure design and optimization: Advances and challenges. J. Biol. Chem. 2025, 301, 108015. [Google Scholar] [CrossRef]
- Jain, S.; Venkataraman, A.; Wechsler, M.E.; Peppas, N.A. Messenger RNA-based vaccines: Past, present, and future directions in the context of the COVID-19 pandemic. Adv. Drug Deliv. Rev. 2021, 179, 114000. [Google Scholar] [CrossRef]
- Iavarone, C.; O’hagan, D.T.; Yu, D.; Delahaye, N.F.; Ulmer, J.B. Mechanism of action of mRNA-based vaccines. Expert. Rev. Vaccines 2017, 16, 871–881. [Google Scholar] [CrossRef]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- Cagigi, A.; Loré, K. Immune Responses Induced by mRNA Vaccination in Mice, Monkeys and Humans. Vaccines 2021, 9, 61. [Google Scholar] [CrossRef]
- Gergen, J.; Petsch, B. mrna-Based Vaccines and Mode of Action. In MRNA Vaccines; Yu, D., Petsch, B., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 440, pp. 1–30. [Google Scholar] [CrossRef]
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery Strategies for mRNA Vaccines. Pharm. Med. 2022, 36, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Armbruster, N.; Jasny, E.; Petsch, B. Advances in RNA Vaccines for Preventive Indications: A Case Study of a Vaccine against Rabies. Vaccines 2019, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent Advances in the Lipid Nanoparticle-Mediated Delivery of mRNA Vaccines. Vaccines 2023, 11, 658. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Parhi, R.; Suresh, P. Preparation and Characterization of Solid Lipid Nanoparticles-A Review. Curr. Drug Discov. Technol. 2012, 9, 2–16. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gong, L.; Wang, P.; Zhao, X.; Zhao, F.; Zhang, Z.; Li, Y.; Huang, W. Recent Advances in Lipid Nanoparticles for Delivery of mRNA. Pharmaceutics 2022, 14, 2682. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Chong, K.; Cui, M.; Cao, Z.; Tang, C.; Tian, Z.; Hu, Y.; Zhao, Y. Recent Advances in Lipid Nanoparticles and Their Safety Concerns for mRNA Delivery. Vaccines 2024, 12, 1148. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Ahmadi, F.; Hashemi, S.M.H.; Babaei, A.; Yaddollahi, S.; Rostamkalaei, S.S.; Asare-Addo, K.; Nokhodchi, A. Solid lipid nanoparticles and nanostructured lipid carriers: A review of the methods of manufacture and routes of administration. Pharm. Dev. Technol. 2022, 27, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Park, J.W.; Yoon, I.-S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): Recent advances in drug delivery. J. Pharm. Investig. 2013, 43, 353–362. [Google Scholar] [CrossRef]
- Kremsner, P.G.; Guerrero, R.A.A.; Arana-Arri, E.; Martinez, G.J.A.; Bonten, M.; Chandler, R.; Corral, G.; de Block, E.J.L.; Ecker, L.; Gabor, J.J.; et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): A randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect. Dis. 2022, 22, 329–340. [Google Scholar] [CrossRef]
- Huang, P.; Deng, H.; Zhou, Y.; Chen, X. The roles of polymers in mRNA delivery. Matter 2022, 5, 1670–1699. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for mRNA Vaccines. In MRNA Vaccines; Yu, D., Petsch, B., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 440, pp. 71–110. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Ahmadieh-Yazdi, A.; Heidari, R.; Chamanara, M.; Akbari, M.; Poondla, N.; Yang, P.; Malih, S.; Manoochehri, H.; Tanzadehpanah, H.; et al. Revolutionizing cancer treatment: The power of dendritic cell-based vaccines in immunotherapy. Biomed. Pharmacother. 2025, 184, 117858. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, F.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Stewart-Jones, G.B.E.; Elbashir, S.M.; Wu, K.; Lee, D.; Renzi, I.; Ying, B.; Koch, M.; Sein, C.E.; Choi, A.; Whitener, B.; et al. Development of SARS-CoV-2 mRNA vaccines encoding spike N-terminal and receptor binding domains. Biorxiv 2022. [CrossRef]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Klepac, P.; Funk, S.; Hollingsworth, T.D.; Metcalf, C.J.E.; Hampson, K. Six challenges in the eradication of infectious diseases. Epidemics 2015, 10, 97–101. [Google Scholar] [CrossRef]
- Parhiz, H.; Atochina-Vasserman, E.N.; Weissman, D. mRNA-based therapeutics: Looking beyond COVID-19 vaccines. Lancet 2024, 403, 1192–1204. [Google Scholar] [CrossRef]
- Keddy, K.H.; Gobena, T. The continuing challenge of infectious diseases. Lancet Infect. Dis. 2024, 24, 800–801. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Khormi, A.H.I.; Qohal, R.M.M.; Masrai, A.Y.A.; Hakami, K.H.H.; Ogdy, J.A.; Almarshad, A.A.; Merai, A.M.A.; Harrisi, H.S.; Alotaibi, M.M.; Alotaibi, A.M.; et al. Emerging Trends in mRNA Vaccine Technology: Beyond Infectious Diseases. Egypt. J. Chem. 2024, 67, 1567–1574. [Google Scholar] [CrossRef]
- Tripathi, T. Advances in vaccines: Revolutionizing disease prevention. Sci. Rep. 2023, 13, 11748. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Weissman, D. Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 2020, 65, 14–20. [Google Scholar] [CrossRef]
- Overmars, I.; Au-Yeung, G.; Nolan, T.M.; Steer, A.C. mRNA vaccines: A transformative technology with applications beyond COVID -19. Med. J. Aust. 2022, 217, 71–75. [Google Scholar] [CrossRef]
- Szabó, G.T.; Mahiny, A.J.; Vlatkovic, I. COVID-19 mRNA vaccines: Platforms and current developments. Mol. Ther. 2022, 30, 1850–1868. [Google Scholar] [CrossRef]
- Medina-Magües, L.G.; Gergen, J.; Jasny, E.; Petsch, B.; Lopera-Madrid, J.; Medina-Magües, E.S.; Salas-Quinchucua, C.; Osorio, J.E. mRNA Vaccine Protects against Zika Virus. Vaccines 2021, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Ma, X.; Sheng, Z.; Qi, L.; Liu, C.; Wang, D.; Huang, B.; Li, F.; Song, M. Identification of Goose-Origin Parvovirus as a Cause of Newly Emerging Beak Atrophy and Dwarfism Syndrome in Ducklings. J. Clin. Microbiol. 2016, 54, 1999–2007. [Google Scholar] [CrossRef]
- Bollman, B.; Nunna, N.; Bahl, K.; Hsiao, C.J.; Bennett, H.; Butler, S.; Foreman, B.; Burgomaster, K.E.; Aleshnick, M.; Kong, W.-P.; et al. An optimized messenger RNA vaccine candidate protects non-human primates from Zika virus infection. Npj Vaccines 2023, 8, 58. [Google Scholar] [CrossRef]
- Berkley, S.F.; Koff, W.C. Scientific and policy challenges to development of an AIDS vaccine. Lancet 2007, 370, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Morris, L. mRNA vaccines offer hope for HIV. Nat. Med. 2021, 27, 2082–2084. [Google Scholar] [CrossRef]
- Ahmed, Y.; Tian, M.; Gao, Y. Development of an anti-HIV vaccine eliciting broadly neutralizing antibodies. AIDS Res. Ther. 2017, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H. Challenges in the development of an HIV-1 vaccine. Nature 2008, 455, 613–619. [Google Scholar] [CrossRef]
- Boomgarden, A.C.; Upadhyay, C. Progress and Challenges in HIV-1 Vaccine Research: A Comprehensive Overview. Vaccines 2025, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Herschhorn, A. mRNA-based HIV-1 vaccines. Clin. Microbiol. Rev. 2024, 37, e00041-24. [Google Scholar] [CrossRef]
- Khalid, K.; Padda, J.; Khedr, A.; Ismail, D.; Zubair, U.; Al-Ewaidat, O.A.; Padda, S.; Cooper, A.C.; Jean-Charles, G. HIV and Messenger RNA Vaccine. Cureus 2021. [CrossRef]
- Moderna. Moderna Announces First Participant Dosed in Phase 1 Study of its HIV Trimer mRNA Vaccine. n.d. Available online: https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-First-Participant-Dosed-in-Phase-1-Study-of-its-HIV-Trimer-mRNA-Vaccine/default.aspx (accessed on 21 May 2025).
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef]
- Gouma, S.; Anderson, E.M.; Hensley, S.E. Challenges of Making Effective Influenza Vaccines. Annu. Rev. Virol. 2020, 7, 495–512. [Google Scholar] [CrossRef]
- Becker, T.; Elbahesh, H.; Reperant, L.A.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E. Influenza Vaccines: Successes and Continuing Challenges. J. Infect. Dis. 2021, 224, S405–S419. [Google Scholar] [CrossRef] [PubMed]
- Fleeton, M.N.; Chen, M.; Berglund, P.; Rhodes, G.; Parker, S.E.; Murphy, M.; Atkins, G.J.; Liljeström, P. Self-Replicative RNA Vaccines Elicit Protection against Influenza A Virus, Respiratory Syncytial Virus, and a Tickborne Encephalitis Virus. J. Infect. Dis. 2001, 183, 1395–1398. [Google Scholar] [CrossRef]
- Mazunina, E.P.; Gushchin, V.A.; Kleymenov, D.A.; Siniavin, A.E.; Burtseva, E.I.; Shmarov, M.M.; Mukasheva, E.A.; Bykonia, E.N.; Kozlova, S.R.; Evgrafova, E.A.; et al. Trivalent mRNA vaccine-candidate against seasonal flu with cross-specific humoral immune response. Front. Immunol. 2024, 15, 1381508. [Google Scholar] [CrossRef]
- Leonard, A.; Weiss, M.J. Hematopoietic stem cell collection for sickle cell disease gene therapy. Curr. Opin. Hematol. 2024, 31, 104–114. [Google Scholar] [CrossRef]
- Hatta, M.; Hatta, Y.; Choi, A.; Hossain, J.; Feng, C.; Keller, M.W.; Ritter, J.M.; Huang, Y.; Fang, E.; Pusch, E.A.; et al. An influenza mRNA vaccine protects ferrets from lethal infection with highly pathogenic avian influenza A(H5N1) virus. Sci. Transl. Med. 2024, 16, eads1273. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, A.V.; Khan, M.M.; Zhen, X.; Tang, Y.; Tao, W. Clinical advances of mRNA vaccines for cancer immunotherapy. Med. 2025, 6, 100562. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Bidram, M.; Zhao, Y.; Shebardina, N.G.; Baldin, A.V.; Bazhin, A.V.; Ganjalikhany, M.R.; Zamyatnin, A.A.; Ganjalikhani-Hakemi, M. mRNA-Based Cancer Vaccines: A Therapeutic Strategy for the Treatment of Melanoma Patients. Vaccines 2021, 9, 1060. [Google Scholar] [CrossRef]
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. Recent advances in mRNA cancer vaccines: Meeting challenges and embracing opportunities. Front. Immunol. 2023, 14, 1246682. [Google Scholar] [CrossRef]
- Sharp, M.; Dohme, L.L.C. A Clinical Study of V940 Plus Pembrolizumab in People With High-Risk Melanoma (V940-001). n.d. Available online: https://clinicaltrials.gov/study/NCT05933577?cond=mRNA-4157%20(V940)&rank=1 (accessed on 6 April 2025).
- Seraphin, T.P.; Joko-Fru, W.Y.; Kamaté, B.; Chokunonga, E.; Wabinga, H.; Somdyala, N.I.M.; Manraj, S.S.; Ogunbiyi, O.J.; Dzamalala, C.P.; Finesse, A.; et al. Rising Prostate Cancer Incidence in Sub-Saharan Africa: A Trend Analysis of Data from the African Cancer Registry Network. Cancer Epidemiol. Biomarkers Prev. 2021, 30, 158–165. [Google Scholar] [CrossRef]
- Kübler, H.; Scheel, B.; Gnad-Vogt, U.; Miller, K.; Schultze-Seemann, W.; Vom Dorp, F.; Parmiani, G.; Hampel, C.; Wedel, S.; Trojan, L.; et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: A first-in-man phase I/IIa study. J. Immunother. Cancer 2015, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, G.L.; Gupta, V.K.; Prasad, K.; Kim, E.; Tej, M.B.; Mohanty, P.; Verma, H.K.; Raju, G.S.R.; Bhaskar, L.; Huh, Y.S. Recent advances and future perspectives in the therapeutics of prostate cancer. Exp. Hematol. Oncol. 2023, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Elkashif, A.; Saha, C.; Coulter, J.A.; Dunne, N.J.; McCarthy, H.O. Key considerations for a prostate cancer mRNA vaccine. Crit. Rev. Oncol. Hematol. 2025, 208, 104643. [Google Scholar] [CrossRef]
- Carrasco-Ramiro, F.; Peiró-Pastor, R.; Aguado, B. Human genomics projects and precision medicine. Gene Ther. 2017, 24, 551–561. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Lin, E.Z.; Anekoji, M.; Ichim, T.E.; Hu, J.; Marincola, F.M.; Jones, L.D.; Kesari, S.; Ashili, S. Advancing personalized medicine in brain cancer: Exploring the role of mRNA vaccines. J. Transl. Med. 2023, 21, 830. [Google Scholar] [CrossRef]
- Fu, Q.; Zhao, X.; Hu, J.; Jiao, Y.; Yan, Y.; Pan, X.; Wang, X.; Jiao, F. mRNA vaccines in the context of cancer treatment: From concept to application. J. Transl. Med. 2025, 23, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, Q.; Zeng, Z.; Fan, C.; Xiong, W. Advances and prospects of mRNA vaccines in cancer immunotherapy. Biochim. Biophys. Acta BBA–Rev. Cancer 2024, 1879, 189068. [Google Scholar] [CrossRef]
- Hu, C.; Bai, Y.; Liu, J.; Wang, Y.; He, Q.; Zhang, X.; Cheng, F.; Xu, M.; Mao, Q.; Liang, Z. Research progress on the quality control of mRNA vaccines. Expert. Rev. Vaccines 2024, 23, 570–583. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Weichenthal, M.; Svane, I.M.; Mangana, J.; Leiter, U.; Meier, F.; Ruhlmann, C.; Ziogas, D.; Dummer, R.; Cerenzuela, P.; Manzano, J.L.; et al. Real-World efficiency of pembrolizumab in metastatic melanoma patients following adjuvant anti-PD1 treatment. EJC Skin. Cancer 2024, 2, 100271. [Google Scholar] [CrossRef]
- Stallard, J. In Early-Phase Pancreatic Cancer Clinical Trial, Investigational mRNA Vaccine Induces Sustained Immune Activity in Small Patient Group. 2025. Available online: https://www.mskcc.org/news/can-mrna-vaccines-fight-pancreatic-cancer-msk-clinical-researchers-are-trying-find-out. (accessed on 20 April 2025).
- Chitwood, H.; Myers, A. Use of Circulating Tumor DNA to Monitor Minimal Residual Disease Among Patients With Colorectal Cancer. Clin. J. Oncol. Nurs. 2023, 27, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Oosting, L.T.; Franke, K.; Martin, M.V.; Kloosterman, W.P.; Jamieson, J.A.; Glenn, L.A.; de Jager, M.W.; van Zanten, J.; Allersma, D.P.; Gareb, B. Development of a Personalized Tumor Neoantigen Based Vaccine Formulation (FRAME-001) for Use in a Phase II Trial for the Treatment of Advanced Non-Small Cell Lung Cancer. Pharmaceutics 2022, 14, 1515. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Rong, Z.; Liu, L.; Sang, Y.; Yang, J.; Wang, S. Streamlined and on-demand preparation of mRNA products on a universal integrated platform. Microsyst. Nanoeng. 2023, 9, 97. [Google Scholar] [CrossRef]
- Doua, J.; Ndembi, N.; Auerbach, J.; Kaseya, J.; Zumla, A. Advancing local manufacturing capacities for vaccines within Africa–Opportunities, priorities and challenges. Vaccine 2025, 50, 126829. [Google Scholar] [CrossRef]
- Davidopoulou, C.; Kouvelas, D.; Ouranidis, A. COMPARING vaccine manufacturing technologies recombinant DNA vs in vitro transcribed (IVT) mRNA. Sci. Rep. 2024, 14, 21742. [Google Scholar] [CrossRef]
- Berkley, S. Ramping up Africa’s vaccine manufacturing capability is good for everyone. Here’s why. World Econ. Forum. 2022, 17. Available online: https://www.weforum.org/stories/2022/10/ramping-up-africa-s-vaccine-manufacturing-capability-is-good-for-everyone-heres-why/ (accessed on 25 March 2025).
- Van De Pas, R.; Widdowson, M.-A.; Ravinetto, R.; NSrinivas, P.; Ochoa, T.J.; Fofana, T.O.; Van Damme, M. COVID-19 vaccine equity: A health systems and policy perspective. Expert. Rev. Vaccines 2022, 21, 25–36. [Google Scholar] [CrossRef]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef]
- Scholkmann, F.; May, C.-A. COVID-19, post-acute COVID-19 syndrome (PACS, “long COVID”) and post-COVID-19 vaccination syndrome (PCVS, “post-COVIDvac-syndrome”): Similarities and differences. Pathol.–Res. Pract. 2023, 246, 154497. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.J.; Li, S.; Amarasena, T.H.; Reynaldi, A.; Lee, W.S.; Leeming, M.G.; O’onnor, M.G.; Nguyen, J.; Kent, H.E.; Caruso, F.; et al. Blood Distribution of SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine in Humans. ACS Nano 2024, 18, 27077–27089. [Google Scholar] [CrossRef] [PubMed]
- Bitounis, D.; Jacquinet, E.; Rogers, M.A.; Amiji, M.M. Strategies to reduce the risks of mRNA drug and vaccine toxicity. Nat. Rev. Drug Discov. 2024, 23, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. FDA pauses all infant RSV vaccine trials after rise in severe illnesses. BMJ 2024, 387, q2852. [Google Scholar] [CrossRef]
- Meghana, G.V.R.; Chavali, D.P. Examining the Dynamics of COVID-19 Misinformation: Social Media Trends, Vaccine Discourse, and Public Sentiment. Cureus 2023, 15, e48239. [Google Scholar] [CrossRef]
- Bouderhem, R. Challenges Faced by States and the WHO in Efficiently Regulating the Use of mRNA Vaccines. Med. Sci. Forum 2024, 26, 1. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, S.; Srinivasan, K.M.D.; Vincent, P.M.D.R. Personalized cancer vaccine design using AI-powered technologies. Front. Immunol. 2024, 15, 1357217. [Google Scholar] [CrossRef]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Clemente, B.; Denis, M.; Silveira, C.P.; Schiavetti, F.; Brazzoli, M.; Stranges, D. Straight to the point: Targeted mRNA-delivery to immune cells for improved vaccine design. Front. Immunol. 2023, 14, 1294929. [Google Scholar] [CrossRef]
| Company/Sponsor | Candidate | Product Type | Disease(s) | Status | Reference (Accessed on 26 May 2025) |
|---|---|---|---|---|---|
| BioNTech, Germany/Roche, USA | BNT112 (autogene cevumeran) vaccine, neoantigen | mRNA vaccine | Malignant melanoma | Phase II | NCT03815058 (https://clinicaltrials.gov/study/NCT03815058) |
| Colorectal cancer | Phase II | NCT04486378 (https://clinicaltrials.gov/study/NCT04486378) | |||
| Other metastatic tumours | Phase II | NCT03289962 (https://clinicaltrials.gov/study/NCT03289962) | |||
| Pancreatic ductal adenocarcinoma (PDAC) | Phase II | NCT05968326 (https://clinicaltrials.gov/study/NCT05968326) | |||
| Muscle invasive urothelial carcinoma (MIUC) | Phase II | NCT06534983 (https://clinicaltrials.gov/study/NCT06534983) | |||
| Moderna/Merck and Co., USA. | mRNA-4157 vaccine/neoantigen | mRNA vaccine | Bladder cancer | Phase I/II | NCT06305767 (https://clinicaltrials.gov/study/NCT06305767) |
| non-small cell lung cancer (NSCLC) | Phase III | NCT06077760 (https://clinicaltrials.gov/study/NCT06077760) | |||
| Renal cell carcinoma | Phase II | NCT06307431 (https://clinicaltrials.gov/study/NCT06307431) | |||
| Malignant melanoma | Phase III | NCT05933577 (https://clinicaltrials.gov/study/NCT05933577) | |||
| Others | Phase II/III | NCT06295809 (https://clinicaltrials.gov/study/NCT06295809) | |||
| mRNA-1195 | mRNA vaccine | Multiple Sclerosis | Phase II | NCT06735248 (https://clinicaltrials.gov/study/NCT06735248?rank=1) | |
| mRNA-3927 | Propionic Acidemia | Phase I/II | NCT04159103 (https://clinicaltrials.gov/study/NCT04159103) | ||
| mRNA-3745 | Glycogen Storage Disease Type 1a (GSD1a) | Phase I/II | NCT05095727 (https://clinicaltrials.gov/study/NCT05095727?rank=1) | ||
| mRNA-3705 | methylmalonic acidemia (MMA) | Phase I/II | NCT05295433 (https://clinicaltrials.gov/study/NCT05295433?rank=1) | ||
| mRNA-1083 | COVID-19 + Influenza | Phase III | NCT06694389 (https://clinicaltrials.gov/study/NCT06694389?rank=1) | ||
| mRNA-1975, mRNA-1982 | Lyme Disease | Phase I/II | NCT05975099 (https://clinicaltrials.gov/study/NCT05975099?rank=1) | ||
| mRNA-1653 | hMPV + Parainfluenza Virus Type 3 (PIV3) | Phase I | NCT04144348 (https://clinicaltrials.gov/study/NCT04144348?rank=1) | ||
| mRNA-1045 | Influenza + RSV | Phase I | NCT05585632 (https://clinicaltrials.gov/study/NCT05585632?rank=1) | ||
| mRNA-1230 | Influenza + RSV + SARS-CoV-2 | Phase I | NCT05585632 (https://clinicaltrials.gov/study/NCT05585632?rank=1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oloruntimehin, S.; Akinyi, F.; Paul, M.; Ariyo, O. mRNA Vaccine Technology Beyond COVID-19. Vaccines 2025, 13, 601. https://doi.org/10.3390/vaccines13060601
Oloruntimehin S, Akinyi F, Paul M, Ariyo O. mRNA Vaccine Technology Beyond COVID-19. Vaccines. 2025; 13(6):601. https://doi.org/10.3390/vaccines13060601
Chicago/Turabian StyleOloruntimehin, Sola, Florence Akinyi, Michael Paul, and Olumuyiwa Ariyo. 2025. "mRNA Vaccine Technology Beyond COVID-19" Vaccines 13, no. 6: 601. https://doi.org/10.3390/vaccines13060601
APA StyleOloruntimehin, S., Akinyi, F., Paul, M., & Ariyo, O. (2025). mRNA Vaccine Technology Beyond COVID-19. Vaccines, 13(6), 601. https://doi.org/10.3390/vaccines13060601





