Abstract
Objectives: This study aims to assess the adjuvant effects of lentinan and its combination with Mn(J), a manganese-based colloidal adjuvant, on the BG (fusion protein BfrB-GrpE of Mycobacterium tuberculosis) subunit vaccine. Methods: A rabbit skin infection model was established to evaluate the immune protection conferred by the BG–lentinan vaccine, the BG–lentinan/Mn(J) vaccine, and the Bacillus Calmette-Guérin (BCG) vaccine against tuberculosis. Rabbits were vaccinated at weeks 0, 2, and 4. Six weeks post-vaccination, antigen-specific IgG levels were measured, followed by a BCG skin challenge. Results: Both the BG–lentinan and BG–lentinan/Mn(J) vaccines significantly increased antigen-specific IgG levels against BfrB and GrpE in rabbits (p < 0.05). Furthermore, these vaccines accelerated the pathological process following BCG infection. The bacterial load in nodules was notably reduced, with the BG–lentinan vaccine group exhibiting the lowest levels (p < 0.01). Conclusions: Lentinan and its combined adjuvant, lentinan/Mn(J), significantly enhance the immune response elicited by the BG tuberculosis subunit vaccine, providing effective protection.
1. Introduction
Tuberculosis, caused by Mycobacterium tuberculosis, is a severe respiratory disease that poses a significant challenge to global public health [1]. The Bacillus Calmette-Guérin (BCG) vaccine is the only widely used vaccine for tuberculosis; however, it offers limited protection in adults [2]. Consequently, the development of new tuberculosis vaccines has become a top priority. Several novel vaccines, including recombinant BCG, live attenuated, viral vector, recombinant protein subunit, and DNA vaccines, are currently in clinical or preclinical development [3]. Among these, protein subunit vaccines are considered highly promising due to their well-defined composition, high safety profile, and ability to enhance the immune protection provided by BCG [4]. Adjuvants are essential components of protein subunit vaccines, as they enhance the immunogenicity of protein antigens, improve protective effects, and reduce the required antigen dosage [5]. Currently, the adjuvants utilized in tuberculosis subunit vaccine research include ASO1E, IC31, GLA-SE, CpG ODN, and BC02 [6]. While these adjuvants have demonstrated progress in clinical trials, they still encounter certain limitations [7]. Therefore, the development of novel adjuvants is critical for advancing the field of tuberculosis subunit vaccine development.
Traditional Chinese medicine adjuvants are considered ideal biological response modifiers because of their diverse biologically active ingredients and broad immune activities [8]. Compared to conventional adjuvants, they have fewer toxic side effects and a lower risk of dependence. As vaccine technologies rapidly advance, researchers are focusing more on developing efficient adjuvant strategies. Given the difficulty of a single adjuvant meeting all ideal immune response criteria, combined adjuvant systems have become a key research focus. These systems combine two or more adjuvants to enhance the immune response through synergistic effects [9]. Various combined adjuvant systems have shown promise in tuberculosis subunit vaccine research, opening new avenues for vaccine adjuvant development [10]. Combined adjuvants are considered optimal for eliciting a more comprehensive immune response. Lentinan (LNT), a (1-3)-β-glucan extracted from the fruiting body of Lentinus edodes, exhibits immunomodulatory, anti-tumor, antioxidant, and other beneficial effects [11,12,13]. Studies have shown that lentinan enhances TNF-α and IL-12 production and promotes the generation of Listeria monocytogenes-specific CD8 + T cells [14]. As an adjuvant in the rabies vaccine, lentinan amplifies the immune response, achieving the efficacy of multiple injections with a single dose [15]. Additionally, lentinan has demonstrated potential as an adjuvant in vaccines for Newcastle disease, H5N1, and ovalbumin [15,16,17]. Although lentinan has well-established immunomodulatory properties, its potential as an adjuvant in tuberculosis subunit vaccines remains largely unexplored. Therefore, based on the previously constructed tuberculosis multi-antigen recombinant protein BG [18], this study evaluated the adjuvant effect of lentinan on the tuberculosis subunit vaccine.
Manganese ions (Mn2+) have been shown to enhance antigen uptake and presentation by activating the cGAS-STING pathway, thereby promoting both humoral and cellular immune responses with minimal side effects [19]. Their adjuvant potential has been confirmed in vaccines targeting SARS-CoV-2, rabies, and influenza [20,21]. Our previous studies demonstrated that the Mn(J) adjuvant enhances both cellular and humoral immunity induced by BG subunit vaccines, significantly reducing bacterial load and accelerating the healing of immunopathological lesions in vaccinated mice and rabbits [22]. In this study, we further compared the adjuvant effects of lentinan alone and in combination with Mn(J).
2. Materials and Methods
2.1. Animals
Female New Zealand White rabbits (ordinary grade), weighing 2.0–3.0 kg, were sourced from Yuhang Kelian Rabbit Industry. The rabbits were housed at the Animal Experimental Research Center of Zhejiang University of Traditional Chinese Medicine. During the experiment, the rabbits had ad libitum access to food and water, and bedding materials were changed regularly. The experiment began following a 1-week acclimatization period. The study was approved by the Animal Ethics Committee of Zhejiang University of Chinese Medicine (approval number: IACUC-20231113-04).
2.2. Reagent and Instrument
The Bacille Calmette–Guérin (BCG) strain from Denmark was generously provided by the Lanzhou Biological Products Research Institute. Lentinan, a polysaccharide derived from shiitake mushrooms, was obtained from Chengdu Manste Biotechnology Co., Ltd. (Chengdu, Sichuan, China). Mn(J) was supplied by Jiangsu MnStarter Biotechnology Company (Qidong, Jiangsu, China). The goat anti-rabbit IgG antibody (AB6721), conjugated with horseradish peroxidase (HRP), was purchased from Abcam (Cambridge, UK). The acid-fast staining kit was obtained from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China), while pathological staining reagents were provided by the Pathological Laboratory of Zhejiang Chinese Medical University.
2.3. Preparation of Fusion Protein BG and Single Antigens BfrB and GrpE
Fusion protein BG and single antigens BfrB and GrpE were prepared as described previously [18]. Briefly, the fusion protein BG and single antigens BfrB and GrpE were overexpressed in E. coli BL21 (DE3 pLysS) induced with 0.5 mM IPTG, followed by cell disruption via sonication. Since the fusion protein BG lacks a purification tag, it was purified using ammonium sulfate precipitation and hydrophobic interaction chromatography (HIC). In contrast, His-tagged BfrB and GrpE proteins were purified by Ni-NTA affinity chromatography.
2.4. Vaccine Preparation and Immunization Procedures
For the BG–lentinan vaccine, 375 μL of the fusion protein (0.4 mg/mL) was mixed with 750 μL of lentinan (0.1 mg/mL), followed by dilution with PBS to a final volume of 1500 μL. For the candidate vaccine containing lentinan/Mn(J), 375 μL of the fusion protein was mixed with 750 μL of lentinan, followed by the addition of 150 μL of a suspension containing Mn(J). The final volume was adjusted to 1500 μL with PBS. BCG strains were cultured on Lowenstein–Jensen medium. After incubation, the bacterial cells were harvested and adjusted to a concentration of 5 × 107 CFU/mL for subsequent use.
After one week of acclimation, the rabbits were randomly divided into four groups (n = 3 per group). Each group was balanced and assigned a distinct immunization method. The first group received a PBS vaccination, the second group was vaccinated with the BCG Denmark strain and served as the control group, the third group received the BG–lentinan vaccine, and the fourth group was immunized with the BG–lentinan/Mn(J) vaccine. The PBS group received an injection of 1000 μL of PBS. The BCG group received 1000 μL (1 × 107 CFU). The BG–lentinan group was vaccinated with 1000 μL containing 100 μg of fusion protein and 500 μg of lentinan. The BG–lentinan/Mn(J) group was inoculated with 1000 μL containing 100 μg of fusion protein, 500 μg of lentinan, and 500 μg of Mn(J). All animals received subcutaneous injections at weeks 0, 2, and 4, except the BCG group, which was injected only once at week 0. The Mn(J) dosage was selected based on our previous studies [22]. The lentinan dosage was informed by studies of other polysaccharide adjuvants, including those from Astragalus and Coriolus versicolor, used in subunit vaccines [23,24].
2.5. Detection of Antigen-Specific Antibody Levels in Rabbit Serum
Six weeks after the final immunization, serum samples were collected from rabbits. IgG antibody levels specific to the individual BfrB and GrpE antigens were quantified using ELISA to assess the immune response to each component. Firstly, 96-well plates were coated with 5 μg/mL of BfrB or GrpE and incubated overnight at 4 °C. The plates were then washed with PBS containing 0.05% Tween 20 (PBST) and blocked with 5% BSA at 37 °C for 1 h. Serum samples were initially diluted 1:100, and subsequently serially diluted to a maximum of 1:409,600 or 1:819,200. Anti-BfrB and anti-GrpE antibodies were then added at a dilution of 1:12,000, resulting in a final volume of 100 μL per well. The plates were incubated at 37 °C for 1 h. After washing with PBST, a color developer was added to each well. After a 15-min incubation at room temperature, 100 μL of stop solution was added, and the optical density (OD) was measured at 450 nm.
2.6. Rabbit Skin Liquefaction Model and Evaluation of Vaccine Protective Effects
Six weeks after the final vaccination, a 100 μL suspension of Mycobacterium bovis containing 5 × 106 CFU was intradermally injected into the subcutaneous tissue on both sides of the rabbits’ backs to replicate the pathological changes seen in the lungs of tuberculosis patients [25,26]. Each rabbit was injected at four points, spaced 2 to 3 cm apart. Pathological changes at the injection sites were observed and recorded daily, including tuberculous granuloma, liquefaction, ulceration, and healing. The criteria for identifying these typical pathological features are as follows: (1) Tuberculous granuloma: The nodules are distinctly red, swollen, and hard in texture. (2) Liquefaction: The nodules begin to soften. Gentle insertion of a sterile syringe needle reveals liquefaction when liquid appears on the needle upon withdrawal, or when gentle pressure is applied, causing the substance to flow out. The peak of liquefaction occurs when the nodule reaches maximum volume, at which point a significant amount of caseous liquid is expelled. (3) Ulceration: The center of the nodule ruptures, releasing a small amount of caseous liquid. (4) Healing: The nodule volume decreases to 60% of its maximum size, and scabbing begins at the rupture site. Complete healing is indicated when the nodule volume reduces to 25% of its maximum size, with visible epithelial formation. Additionally, the width, length, and height of the nodule were measured, and its volume was calculated by multiplying these three dimensions.
2.7. Quantification of Bacterial Load in the Skin Nodules
At the peak of liquefaction, a syringe was used to collect as many liquid samples as possible from the lesions. At the end of the healing period, the rabbit was euthanized, and the nodules were excised for homogenization. The collected caseous liquid samples and nodules from the healing period were weighed under sterile conditions, and tissue CFU counts were determined after homogenization. For quantitative CFU analysis, the homogenate was serially diluted and inoculated on Lowenstein–Jensen medium, where it was incubated for three weeks. Finally, the presence of bacteria in the colonies was confirmed by acid-fast staining.
2.8. Pathological Examination of Tuberculosis Nodule Sections
Throughout the healing period, nodules and adjacent lesional skin tissue were collected for pathological analysis. Firstly, the dissected nodule tissue was immersed in a 10% tissue fixative solution for 24 to 48 h. After fixation, the tissue was dehydrated with increasing ethanol concentrations, cleared with xylene, paraffin-embedded, and blocked. Next, tissue sections were cut to a thickness of 4–5 microns using an ultra-thin semi-automatic rotary slicer and mounted onto slides. Finally, all slides were stained with hematoxylin and eosin and analyzed using a pathological section scanner.
2.9. Statistics and Analysis
Data were analyzed using Microsoft Excel 2019 and GraphPad Prism 8, with the mean ± standard deviation (SD) used to describe the data. A one-way analysis of variance (ANOVA) was performed to compare the mean levels between groups, with Tukey’s post hoc test used for multiple comparisons. Statistical significance was considered at p < 0.05.
3. Results
3.1. Both the BG–Lentinan Vaccine and BG–Lentinan/Mn(J) Vaccine Effectively Induce High Levels of IgG Antibodies in Rabbits
To evaluate the adjuvant effect of lentinan on the tuberculosis fusion protein BG, we combined fusion protein BG with lentinan or the complex adjuvant lentinan and Mn(J) to prepare the BG–lentinan and BG–lentinan/Mn(J) vaccines. Both vaccines were administered to New Zealand rabbits at weeks 0, 2, and 4 (Figure 1).
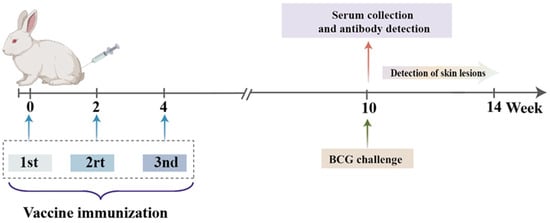
Figure 1.
Vaccination procedure for rabbits. The BCG immunization group received a single vaccination at week 0, while the other groups were vaccinated three times at weeks 0, 2, and 4. The antigen-specific immune response was evaluated six weeks after the final immunization. All rabbits received subcutaneous injections.
Six weeks after the final immunization, serum samples were collected from each rabbit (n = 3 per group), and IgG antibody levels specific to BfrB and GrpE were measured by ELISA. As shown in Figure 2, rabbits immunized with BG–lentinan or BG–lentinan/Mn(J) vaccines produced significantly higher IgG antibody levels compared to the PBS and BCG control groups, especially for GrpE-specific responses (Figure 2B). Among the subunit vaccine groups, BG–lentinan/Mn(J) induced higher GrpE and BfrB-specific IgG levels than BG–lentinan alone, although the difference was not statistically significant. The higher IgG levels in the BG–lentinan/Mn(J) group suggest that the combination with Mn(J) can enhance the adjuvant effect of lentinan to some extent. These findings demonstrate that both BG–lentinan and BG–lentinan/Mn(J) vaccines effectively elicit strong humoral immune responses, with the combined adjuvant showing a better effect.
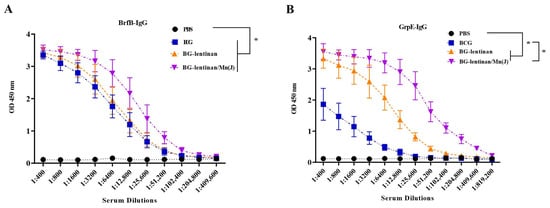
Figure 2.
Antigen-specific IgG levels in immunized rabbits. Serum samples were collected six weeks after the final immunization to assess anti-BfrB and anti-GrpE IgG levels. (A) BfrB-specific IgG levels. (B) GrpE-specific IgG levels. * p < 0.05. Data are presented as mean ± standard deviation. Each data point represents an individual animal (n = 3 per group).
3.2. Both BG–Lentinan and BG–Lentinan/Mn(J) Vaccines Significantly Shorten the Immunopathological Process Following M. bovis BCG Challenge
To further evaluate the protective effect of vaccination, we infected the dorsal skin of rabbits with attenuated M. bovis BCG six weeks after the final immunization. This approach simulates M. tuberculosis infection in human lungs. To evaluate the impact of the BG–lentinan and BG–lentinan/Mn(J) vaccines on the immunopathological process following tuberculosis infection in the rabbit skin model, we monitored pathological changes at the injection site daily after M. bovis BCG infection. We also recorded the volume of skin lesions and the time points at which typical pathological symptoms of tuberculosis appeared (Figure 3, Table 1).
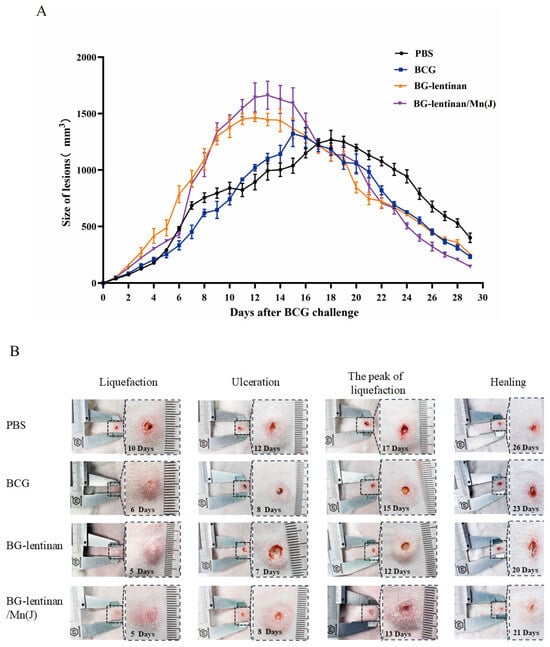
Figure 3.
The dynamic progression of skin lesions following M. bovis BCG challenge. Six weeks after the final vaccine immunization, 5 × 106 CFU of BCG was injected intradermally at multiple sites on both sides of the rabbit’s skin. The volume of the resulting nodules was measured daily by evaluating their width, length, and thickness. (A) Volumes of the skin lesions in different vaccine groups. (B) Photographic representation of various pathological stages of tubercles. The figure was generated by the authors. Data represent means of 3 lesions per group.

Table 1.
Immunization groups and vaccination protocols.
Following the M. bovis BCG challenge, the maximum volume of tuberculosis immunopathological lesions varied significantly across different groups (Table 2). Rabbits vaccinated with the BG–lentinan and combined adjuvant vaccines exhibited larger volumes of tuberculosis granulomas. During the peak liquefaction period, the granuloma volumes in the BG–lentinan and BG–lentinan/Mn(J) groups were 1465.43 ± 79.31 mm3 and 1662.64 ± 241 mm3, respectively, both significantly higher than those in the BCG and PBS groups (Figure 3A). Furthermore, there were significant differences in the onset times of typical pathological symptoms, such as tuberculous granuloma formation, liquefaction, ulceration, and healing. The results showed that these symptoms appeared earlier in the vaccine groups than in the PBS group. Among the vaccine groups, rabbits in the BG–lentinan group showed the earliest liquefaction, ulceration, peak liquefaction, and healing times, occurring on days 5, 7, 12, and 20 post-challenge, respectively. However, the healing process in this group was slower, beginning on day 20 and ending on day 26. In contrast, rabbits vaccinated with BG–lentinan/Mn(J) or BCG exhibited similar liquefaction and ulceration times, occurring on days 5/6 and 8 post-challenge, respectively. Notably, rabbits in the combined adjuvant group healed the earliest, starting on day 21 and completing on day 25, resulting in the shortest total healing duration of 4 days among all groups. Rabbits in the BCG group met the healing criteria on day 23, but full recovery was not achieved until day 28. Overall, both subunit vaccines accelerated the pathological process compared to the PBS group.

Table 2.
Effects of different vaccine candidates on M. bovis BCG-induced rabbit skin lesions.
3.3. Both the BG–Lentinan and BG–Lentinan/Mn(J) Vaccines Significantly Reduce the Bacterial Load at the Tuberculosis Infection Site
Reducing the bacterial load at infected sites is the most direct indicator of the protective effect provided by vaccines and is considered the gold standard. To evaluate the protective efficacy of the BG–lentinan and BG–lentinan/Mn(J) vaccines, we assessed liquefied samples at the peak of liquefaction and tissue from healing following M. bovis BCG infection in the skin (Figure 4 and Figure 5). The results showed that during the peak liquefaction period, the Mycobacterium load in the PBS group was the highest, at 2.0 × 107 CFU/g, while the BG–lentinan vaccine group had the lowest bacterial load, at 5.45 × 105 CFU/g. The BG–lentinan/Mn(J) and BCG groups had bacterial loads of 1.39 × 106 CFU/g and 1.47 × 106 CFU/g, respectively. Notably, the bacterial load in the nodules during the healing period decreased significantly compared to the peak liquefaction period, following a trend similar to that observed during peak liquefaction. Among the groups, the PBS group had the highest bacterial load (5.68 × 103 CFU/g), while the BG–lentinan vaccine group had the lowest (4.27 × 102 CFU/g), followed by the combined adjuvant group (2.07 × 103 CFU/g) and the BCG group (1.68 × 103 CFU/g). To rule out other bacterial contamination, we performed acid-fast staining of liquefied necrotic substances before counting CFU, confirming that all samples contained mycobacteria. A comparison of bacterial loads across groups revealed that both the BG–lentinan and BG–lentinan/Mn(J) vaccines significantly reduced bacterial loads in tuberculous nodules, with the BG–lentinan vaccine showing the most effective protective effect. The protective effect of the BG–lentinan/Mn(J) vaccine was slightly inferior to that of the BG–lentinan vaccine but comparable to that of BCG.
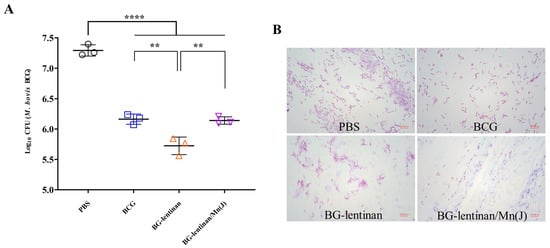
Figure 4.
The bacterial load in the caseum necrosis at the peak of liquefaction. Liquefied caseous samples were collected at the peak of liquefaction to assess bacterial load following intradermal BCG challenge. Samples were obtained from rabbits in four groups: PBS (negative control), BCG (positive control), BG–lentinan, and BG–lentinan/Mn(J) vaccine groups. (A) The number of tuberculosis bacilli per gram of caseous liquefied sample. (B) Acid-fast bacterial staining of cultured caseous liquefied substances. ** p < 0.01, **** p < 0.0001. Data represent means of 3 lesions per group.
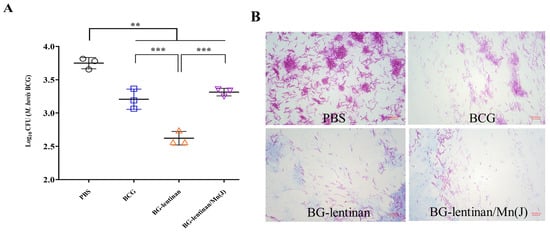
Figure 5.
Bacterial load in healing skin nodules. At the complete healing stage, the rabbits were euthanized, and tissue samples from the lesion sites and surrounding skin were collected for hematoxylin and eosin (H&E) staining. Samples were obtained from rabbits in four groups: PBS (negative control), BCG (positive control), BG–lentinan, and BG–lentinan/Mn(J) vaccine groups. (A) The number of tuberculosis bacilli per gram of skin nodules. (B) Acid-fast bacterial staining of cultured skin nodule homogenates. ** p < 0.01, *** p < 0.001. Data represent means of 3 lesions per group.
Pathological examinations of the skin lesion site were performed during both the peak liquefaction and the complete healing period. Hematoxylin–eosin (HE) staining revealed that pathological changes in skin lesions across all groups were consistent with mycobacterial infection, characterized by inflammatory cells, lymphocyte infiltration, liquefied necrotic tissue, and Langhans giant cells (Figure 6). These findings further substantiate the efficacy of the tuberculosis replacement rabbit model. Notably, no significant differences in pathological changes were observed among the groups during the healing period.
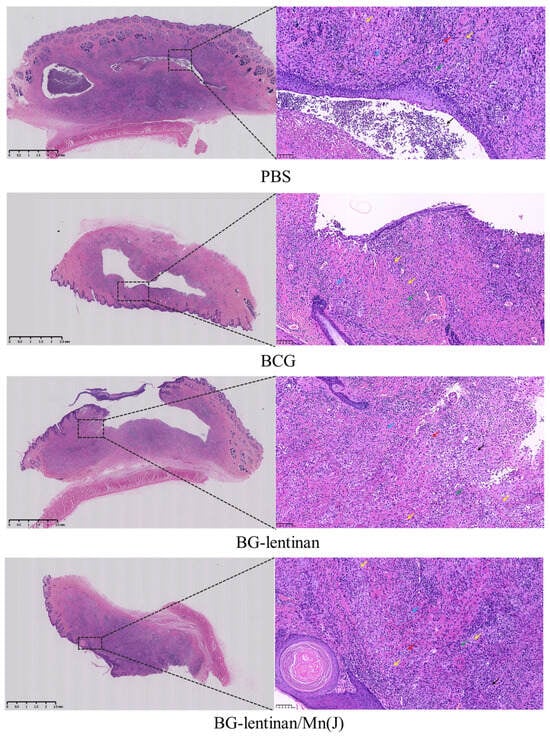
Figure 6.
Histopathology of skin lesions after M. bovis BCG challenge. The rabbits were euthanized at the end of the complete healing period, and both the lesion site and adjacent skin tissue were collected for HE staining. The results include the original scan at 25× magnification and a localized magnification at 200× of the nodules. Various cells and areas of necrosis in the enlarged pathological sections are indicated with arrows of different colors: red arrows denote eosinophils, green arrows represent lymphocytes, yellow arrows indicate Langhans giant cells, black arrows mark the necrotic areas or abscesses, and blue arrows highlight plasma cells.
4. Discussion
Traditional Chinese medicine adjuvants, such as polysaccharides, saponins, and flavonoids, are known for their high safety and low dependency. These adjuvants effectively reduce the required antigen dose, extend immune protection duration, and significantly enhance both cellular and humoral immune responses, highlighting their substantial developmental potential [7,27]. This study evaluated the adjuvant effect of lentinan, both alone and in combination with Mn(J), in a tuberculosis subunit vaccine. The results showed that both adjuvants significantly enhanced the antibody response of rabbits to BfrB and GrpE antigens, while also accelerating the pathological process after infection with the attenuated M. bovis BCG strain in the skin, reducing bacterial load in the nodules. As a result, lentinan show promising potential for use in the development of tuberculosis vaccines.
Selecting an appropriate animal model is crucial for the development of tuberculosis vaccines [28]. While mice, guinea pigs, and primates offer distinct research advantages, they have limitations in accurately simulating the pathology of human tuberculosis [29,30,31]. In contrast, rabbits exhibit delayed hypersensitivity reactions, liquefaction, cavity formation, and tuberculous granulomas following M. tuberculosis infection, closely resembling human tuberculosis manifestations and facilitating experimental manipulation [32]. However, the need for Animal Biosafety Level 3 conditions for tuberculosis infection, coupled with the larger size of rabbits, limits the broader application of this model in vaccine research. Our previous study found no significant difference in the liquefaction of skin tuberculosis caused by infection with attenuated M. bovis BCG compared to that induced by virulent strains [33]. Building on this foundation, the present study assessed the effects of BG–lentinan and BG–lentinan/Mn(J) vaccines in a rabbit skin BCG infection model.
This study compares the immunogenicity and protective efficacy of the BG–lentinan and BG–lentinan/Mn(J) vaccines in rabbits, highlighting the potential of both single and complex adjuvants in tuberculosis vaccine development. The results show that both the BG–lentinan and BG–lentinan/Mn(J) vaccines significantly enhance IgG antibody levels against BfrB and GrpE antigens in rabbit serum, emphasizing the immune-boosting effects of traditional Chinese medicine adjuvants. Notably, the BG–lentinan/Mn(J) vaccine exhibits a greater capacity to elevate GrpE-specific IgG antibody levels. In terms of protective efficacy, the BG–lentinan group showed the lowest bacterial load during both the peak and healing period of lesion liquefaction, indicating a strong anti-tuberculosis response. In contrast, the BG–lentinan/Mn(J) group exhibited a faster healing rate. Moreover, no additional bacterial contamination was detected through acid-fast staining and microscopy, confirming that the bacteria in the lesion liquefaction were mycobacteria. Histopathological examinations showed that the pathological changes in skin lesions across all groups were consistent with mycobacterial infection, supporting the use of rabbits as a suitable alternative animal model for tuberculosis research.
Although M. tuberculosis primarily combats infection through T-cell-mediated immunity [34], studies have shown that antibody production is equally crucial in preventing tuberculosis [35]. Pre-treatment with specific antibodies or vaccination can significantly reduce the M. tuberculosis load and prolong the survival of infected animals [36,37]. Other studies have shown that vaccine-induced antibody levels are strongly correlated with the reduction of M. tuberculosis [38,39]. This study further confirms that lentinan, especially when combined with Mn(J), significantly enhances the production of antigen-specific antibodies, reduces bacterial load, and accelerates healing at infected sites. These findings substantiate the correlation between vaccine-induced specific antibodies and their protective effects, providing a solid foundation for the design and improvement of tuberculosis vaccines.
Our evaluation of protective efficacy showed that all subunit vaccinations promote liquefaction and healing at the lesion site, while also reducing bacterial load in liquefaction pathology. This effect may be attributed to the vaccine’s ability to trigger a robust immune response at the infection site, similar to the mechanism described in the Koch phenomenon. Notably, the BG–lentinan/Mn(J) vaccine excels at enhancing IgG antibody levels and accelerating healing, suggesting that the combined adjuvant may further amplify the immune response through synergistic effects. However, the specific mechanisms through which different adjuvant components contribute to the immune response require further investigation. We speculate that the combination of the two adjuvant components may activate additional signaling pathways and cellular responses, leading to a stronger immune response compared to lentinan used alone.
Lentinan, a fungal-derived β-glucan, has shown strong immunoenhancing effects, good safety, and low dependency in vaccine studies involving rabies, Newcastle disease, H5N1 influenza, and ovalbumin. In this study, we found that lentinan exhibits a certain adjuvant effect in tuberculosis subunit vaccines. Mechanistically, it might bind to Dectin-1 receptors on dendritic cells (DCs) and macrophages [40], activating the Syk-Card9-Bcl10-MALT1 axis, which triggers NF-κB and MAPK (p38, ERK, JNK) pathways [41]. This promotes DC maturation, upregulates co-stimulatory molecules (CD80, CD86), enhances antigen presentation, and drives CD4+ T cell differentiation, particularly toward Th1 and Th17 subsets [42]. Lentinan also stimulates macrophage phagocytosis, antigen processing, and secretion of cytokines such as IL-12, TNF-α, and IL-6, thereby linking innate and adaptive immunity [43]. Beyond enhancing primary immune responses, lentinan may also support the induction and maintenance of immunological memory. Lentinan has been shown to promote T follicular helper (Tfh) cell differentiation, facilitating germinal center formation and the generation of memory B cells and long-lived plasma cells [44,45,46]. Moreover, lentinan-based adjuvants such as GO-LNT/OVA have been shown to prolong antigen presentation and immune activation relative to GO/OVA, thereby supporting sustained humoral responses [47]. Due to the limited availability of rabbit-specific reagents, immune memory responses were not assessed in this study. Future research will focus on assessing the role of lentinan in the induction, maintenance, and functional activation of memory responses to better clarify its potential as a long-term immunostimulatory adjuvant for tuberculosis subunit vaccines.
In summary, this study assessed the adjuvant effect of lentinan and its combination with Mn(J) in a tuberculosis subunit vaccine using a rabbit model. The findings indicate that these two adjuvants significantly enhanced the immune response in rabbits against BfrB and GrpE. The specific immune response generated effectively reduces bacterial load following mycobacterial infection and accelerates the healing process at the infected site. Furthermore, as a vaccine adjuvant, lentinan has demonstrated high efficiency, safety, and low dependency in vaccines for rabies, Newcastle disease in chickens, H5N1 influenza, and ovalbumin [9,15,16,17]. This suggests that lentinan has the potential to serve as an adjuvant in tuberculosis vaccines. Certainly, in subsequent studies, it is necessary to use virulent tuberculosis strains to further verify the adjuvant effect of lentinan and to determine its optimal dosage.
Author Contributions
H.N. conceived the work, designed the study, analyzed the data, and wrote the manuscript; S.Z. mainly performed the experiments, analyzed the data, and wrote the manuscript; Y.H., X.Z., Z.L., Q.H. and X.Y. participated in the evaluation of vaccines in animal models. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (LY24H290004), the Zhejiang Provincial Medical and Health Science and Technology Plan (2025KY950), and the National Natural Science Foundation of China Youth Foundation (81701969).
Institutional Review Board Statement
Animal experiments were performed in compliance with the guidelines of the China Council on Animal Care and Use. The protocols were approved by the Institutional Animal Care and Use Committee of Zhejiang University of Chinese Medicine (approval number: IACUC-20231113-04).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in the published article. Additional datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bagcchi, S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe 2023, 4, E20. [Google Scholar] [CrossRef] [PubMed]
- Nguipdop-Djomo, P.; Heldal, E.; Rodrigues, L.C.; Abubakar, I.; Mangtani, P. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: A retrospective population-based cohort study. Lancet Infect. Dis. 2016, 16, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.J. Recent advances in the development of vaccines for tuberculosis. Ther. Adv. Vaccines 2015, 3, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.P.; Liang, Y.; Wu, X.Q. The current status, challenges, and future developments of new tuberculosis vaccines. Hum. Vaccines Immunother. 2018, 14, 1697–1716. [Google Scholar] [CrossRef]
- Duong, V.T.; Skwarczynski, M.; Toth, I. Towards the development of subunit vaccines against tuberculosis: The key role of adjuvant. Tuberculosis 2023, 139, 102307. [Google Scholar] [CrossRef]
- Schrager, L.K.; Vekemens, J.; Drager, N.; Lewinsohn, D.M.; Olesen, O.F. The status of tuberculosis vaccine development. Lancet Infect. Dis. 2020, 20, E28–E37. [Google Scholar] [CrossRef]
- Wan, X.H.; Yin, Y.M.; Zhou, C.Z.; Hou, L.; Cui, Q.H.; Zhang, X.P.; Cai, X.Q.; Wang, Y.L.; Wang, L.Z.; Tian, J.Z. Polysaccharides derived from Chinese medicinal herbs: A promising choice of vaccine adjuvants. Carbohydr. Polym. 2022, 276, 118739. [Google Scholar] [CrossRef]
- Zhao, D.P.; Chen, X.H.; Wang, L.Y.; Zhang, J.J.; Zhao, Z.P.; Yue, N.; Zhu, Y.L.; Fei, W.T.; Li, X.Y.; Tan, L.Y.; et al. Bidirectional and persistent immunomodulation of polysaccharide as an adjuvant of influenza and recombinant SARS-CoV-2 vaccine. Int. J. Biol. Macromol. 2023, 234, 123635. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, L.; Gu, P.; Bo, R.; Wusiman, A.; Liu, J.; Hu, Y.; Wang, D. Preparation of lentinan-calcium carbonate microspheres and their application as vaccine adjuvants. Carbohydr. Polym. 2020, 245, 116520. [Google Scholar] [CrossRef]
- Han, B.; Tang, S.; Zhu, D.; Li, H.; Feng, Y.; Su, C.; Xu, Y.; Leng, H.; Wang, Y.; Zhang, Y.; et al. Research Progress on Novel Vaccine Adjuvants. China Anim. Husb. Vet. Med. 2023, 50, 2460–2467. [Google Scholar]
- Wu, X.; Zheng, Z.; Guo, T.; Wang, K.; Zhang, Y. Molecular dynamics simulation of lentinan and its interaction with the innate receptor dectin-1. Int. J. Biol. Macromol. 2021, 171, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Zhang, L.; Tian, Q. Mushroom polysaccharide lentinan for treating different types of cancers: A review of 12 years clinical studies in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 297–328. [Google Scholar] [PubMed]
- He, W. Natural Human Immune Modulator—Lentinan. Guangxi Light Ind. 2000, 1, 28–30. [Google Scholar]
- Kupfahl, C.; Geginat, G.; Hof, H. Lentinan has a stimulatory effect on innate and adaptive immunity against murine Listeria monocytogenes infection. Int. Immunopharmacol. 2006, 6, 686–696. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, H.Z.; Zhang, J.C.; Guan, Y.; Zhang, Y.J. Single-injection subunit vaccine for rabies prevention using lentinan as adjuvant. Int. J. Biol. Macromol. 2024, 254, 128118. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, Y.; Wang, D.; Ma, X.; Zhao, X.; Zhao, B.; Wang, J.; Liu, P. Sulfated modification can enhance the adjuvanticity of lentinan and improve the immune effect of ND vaccine. Vaccine 2009, 27, 660–665. [Google Scholar] [CrossRef]
- He, J.; Liu, Z.; Jiang, W.; Zhu, T.; Wusiman, A.; Gu, P.; Liu, J.; Wang, D. Immune-adjuvant activity of lentinan-modified calcium carbonate microparticles on a H5N1 vaccine. Int. J. Biol. Macromol. 2020, 163, 1384–1392. [Google Scholar] [CrossRef]
- Niu, H.; Cao, Q.; Zhang, T.; Du, Y.; He, P.; Jiao, L.; Wang, B.; Zhu, B.; Hu, L.; Zhang, Y. Construction and evaluation of a novel multi-antigenic Mycobacterium tuberculosis subunit vaccine candidate BfrB-GrpE/DPC. Int. Immunopharmacol. 2023, 124 Pt B, 111060. [Google Scholar] [CrossRef]
- Wang, C.; Guan, Y.; Lv, M.; Zhang, R.; Guo, Z.; Wei, X.; Du, X.; Yang, J.; Li, T.; Wan, Y.; et al. Manganese Increases the Sensitivity of the cGAS-STING Pathway for Double-Stranded DNA and Is Required for the Host Defense against DNA Viruses. Immunity 2018, 48, 675–687.e7. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, Y.; Chen, C.; Zhang, C.; Huang, F.; Zhou, M.; Chen, H.; Fu, Z.F.; Zhao, L. Colloidal Manganese Salt Improves the Efficacy of Rabies Vaccines in Mice, Cats, and Dogs. J. Virol. 2021, 95, e0141421. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, Z.; Lin, X.; Wang, L.; Zhu, H.; Su, Z.; Zhang, S. In situ bio-mineralized Mn nanoadjuvant enhances anti-influenza immunity of recombinant virus-like particle vaccines. J. Control. Release 2024, 368, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cao, Q.; Zhang, Z.; Du, Y.; Hou, Y.; Zhang, X.; Xie, Z.; Zhou, Y.; Zhu, B.; Zhang, Y.; et al. The adjuvant effect of manganese on tuberculosis subunit vaccine Bfrb-GrpE. NPJ Vaccines 2024, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi, G.; Yeung, K.S.; Leung, P.C.; Lee, M.; Lau, C.B.; Vickers, A.; Hood, C.; Deng, G.; Cheung, N.K.; Cassileth, B.; et al. Evaluation of widely consumed botanicals as immunological adjuvants. Vaccine 2008, 26, 4860–4865. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chen, X.; Zhao, B.; Lv, Y.; Zhang, H.; Liu, H.; Chen, Z.; Chen, Y.; Zeng, X. Astragalus polysaccharides enhance the humoral and cellular immune responses of hepatitis B surface antigen vaccination through inhibiting the expression of transforming growth factor β and the frequency of regulatory T cells. FEMS Immunol. Med. Microbiol. 2011, 63, 228–235. [Google Scholar] [CrossRef]
- Ando, M.; Dannenberg, A.M., Jr. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions: IV. Macrophage turnover, lysosomal enzymes, and division in healing lesions. Lab. Investig. J. Tech. Methods Pathol. 1972, 27, 466–472. [Google Scholar]
- Chandrasekhar, S.; Shima, K.; Dannenberg, A.M.; Kambara, T.; Fabrikant, J.I.; Roessler, W.G. Radiation, Infection, and Macrophage Function IV. Effect of Radiation on the Proliferative Abilities of Mononuclear Phagocytes in Tuberculous Lesions of Rabbits. Infect. Immun. 1971, 3, 254–259. [Google Scholar] [CrossRef]
- Shuai, Z.; Hongxia, N. Research Progress on Adjuvants for Tuberculosis Protein Subunit Vaccines. Chin. J. Microbiol. Immunol. 2024, 44, 489–500. [Google Scholar]
- Meyer, J.; McShane, H. The next 10 years for tuberculosis vaccines: Do we have the right plans in place? Expert Rev. Vaccines 2013, 12, 443–451. [Google Scholar] [CrossRef]
- Das, G.; Vohra, H.; Saha, B.; Agrewala, J.N.; Mishra, G.C. Apoptosis of Th1-like cells in experimental tuberculosis (TB). Clin. Exp. Immunol. 1999, 115, 324–328. [Google Scholar] [CrossRef]
- Fratazzi, C.; Arbeit, R.D.; Carini, C.; Remold, H.G. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J. Immunol. 1997, 158, 4320–4327. [Google Scholar] [CrossRef]
- Gutierrez, M.G.; Master, S.S.; Singh, S.B.; Taylor, G.A.; Colombo, M.I.; Deretic, V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004, 119, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, A.M. Liquefaction and cavity formation in pulmonary TB: A simple method in rabbit skin to test inhibitors. Tuberculosis 2009, 89, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.P.; Zhu, B.D.; Shi, W.L.; Wang, M.Z.; Da, Z.J.; Zhang, Y. Evaluation of mycobacterial virulence using rabbit skin liquefaction model. Virulence 2010, 1, 156–163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lewinsohn, D.A.; Lewinsohn, D.M.; Scriba, T.J. Polyfunctional CD4+ T Cells As Targets for Tuberculosis Vaccination. Front. Immunol. 2017, 8, 1262. [Google Scholar] [CrossRef]
- Abebe, F. Synergy between Th1 and Th2 responses during Mycobacterium tuberculosis infection: A review of current understanding. Int. Rev. Immunol. 2019, 38, 172–179. [Google Scholar] [CrossRef]
- Hamasur, B.; Haile, M.; Pawlowski, A.; Schroder, U.; Kallenius, G.; Svenson, S.B. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab’)2 fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin. Exp. Immunol. 2004, 138, 30–38. [Google Scholar] [CrossRef]
- Teitelbaum, R.; Glatman-Freedman, A.; Chen, B.; Robbins, J.B.; Unanue, E.; Casadevall, A.; Bloom, B.R. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. USA 1998, 95, 15688–15693. [Google Scholar] [CrossRef]
- Huygen, K.; Content, J.; Denis, O.; Montgomery, D.L.; Yawman, A.M.; Deck, R.R.; DeWitt, C.M.; Orme, I.M.; Baldwin, S.; D’Souza, C.; et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 1996, 2, 893–898. [Google Scholar] [CrossRef]
- Niu, H.; Hu, L.; Li, Q.; Da, Z.; Wang, B.; Tang, K.; Xin, Q.; Yu, H.; Zhang, Y.; Wang, Y.; et al. Construction and evaluation of a multistage Mycobacterium tuberculosis subunit vaccine candidate Mtb10.4-HspX. Vaccine 2011, 29, 9451–9458. [Google Scholar] [CrossRef]
- Coco, C.; Zannoni, G.F.; Caredda, E.; Sioletic, S.; Boninsegna, A.; Migaldi, M.; Rizzo, G.; Bonetti, L.R.; Genovese, G.; Stigliano, E.; et al. Increased expression of CD133 and reduced dystroglycan expression are strong predictors of poor outcome in colon cancer patients. J. Exp. Clin. Cancer Res. 2012, 31, 71. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Nunes, F.M.; Rzeski, W. Branched mannans from the mushroom Cantharellus cibarius enhance the anticancer activity of natural killer cells against human cancers of lung and colon. Food Funct. 2019, 10, 5816–5826. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Meng, Y.; Shan, F. Effects of Lentinan on the Phenotype and Function of Mouse Bone Marrow Dendritic Cells. J. Microbiol. 2014, 34, 54–58. [Google Scholar]
- Liu, Q.; Dong, L.; Li, H.; Yuan, J.; Peng, Y.; Dai, S. Lentinan mitigates therarubicin-induced myelosuppression by activating bone marrow-derived macrophages in an MAPK/NF-κB-dependent manner. Oncol. Rep. 2016, 36, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Construction and Evaluation of SARS-CoV-2 N Protein Vaccine and the Adjuvant Effect of Lentinan. Master’s Thesis, Guangdong Medical University, Zhanjiang, China, 2022. [Google Scholar]
- Hao, H.; Nakayamada, S.; Yamagata, K.; Ohkubo, N.; Iwata, S.; Inoue, Y.; Zhang, M.; Zhang, T.; Kanda Satoh, Y.; Shan, Y.; et al. Conversion of T Follicular Helper Cells to T Follicular Regulatory Cells by Interleukin-2 Through Transcriptional Regulation in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2021, 73, 132–142. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef]
- Liu, Z.; He, J.; Zhu, T.; Hu, C.; Bo, R.; Wusiman, A.; Hu, Y.; Wang, D. Lentinan-Functionalized Graphene Oxide Is an Effective Antigen Delivery System That Modulates Innate Immunity and Improves Adaptive Immunity. ACS Appl. Mater. Interfaces 2020, 12, 39014–39023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).