A DNA Vaccine Against Proadrenomedullin N-Terminal 20 Peptide (PAMP) Reduces Angiogenesis and Increases Lymphocyte and Macrophage Infiltration but Has No Effect on Tumor Burden in a Mouse Model of Lung Metastasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Bacterial Strain
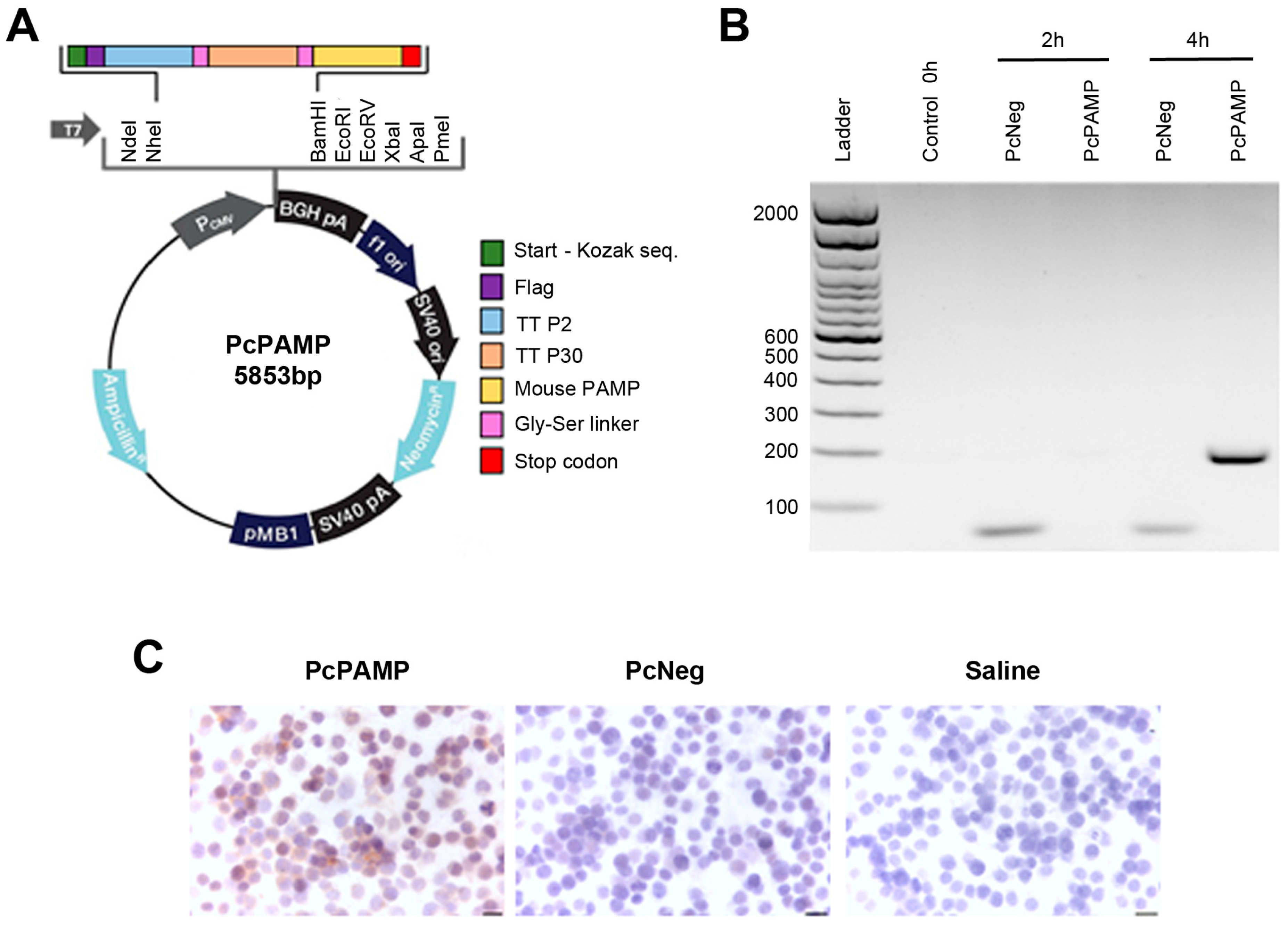
2.2. Plasmid Construction
2.3. Preparation of Bacterial Ghosts
2.4. Cell Transfection and Transcript Expression (RT-PCR and Immunocytochemistry)
2.5. Immunocytochemistry
2.6. Immunization Protocol and Lung Metastasis Model
2.7. Tissue Collection and Preparation
2.8. Mouse ELISA
2.9. Immunostaining Analyses
2.10. Splenocyte Isolation and Flow Cytometry Analysis
2.11. PAMP Stimulation Assay and Secretome Analysis
2.12. Statistical Analysis
3. Results
3.1. Plasmid Generation and Transcript Expression
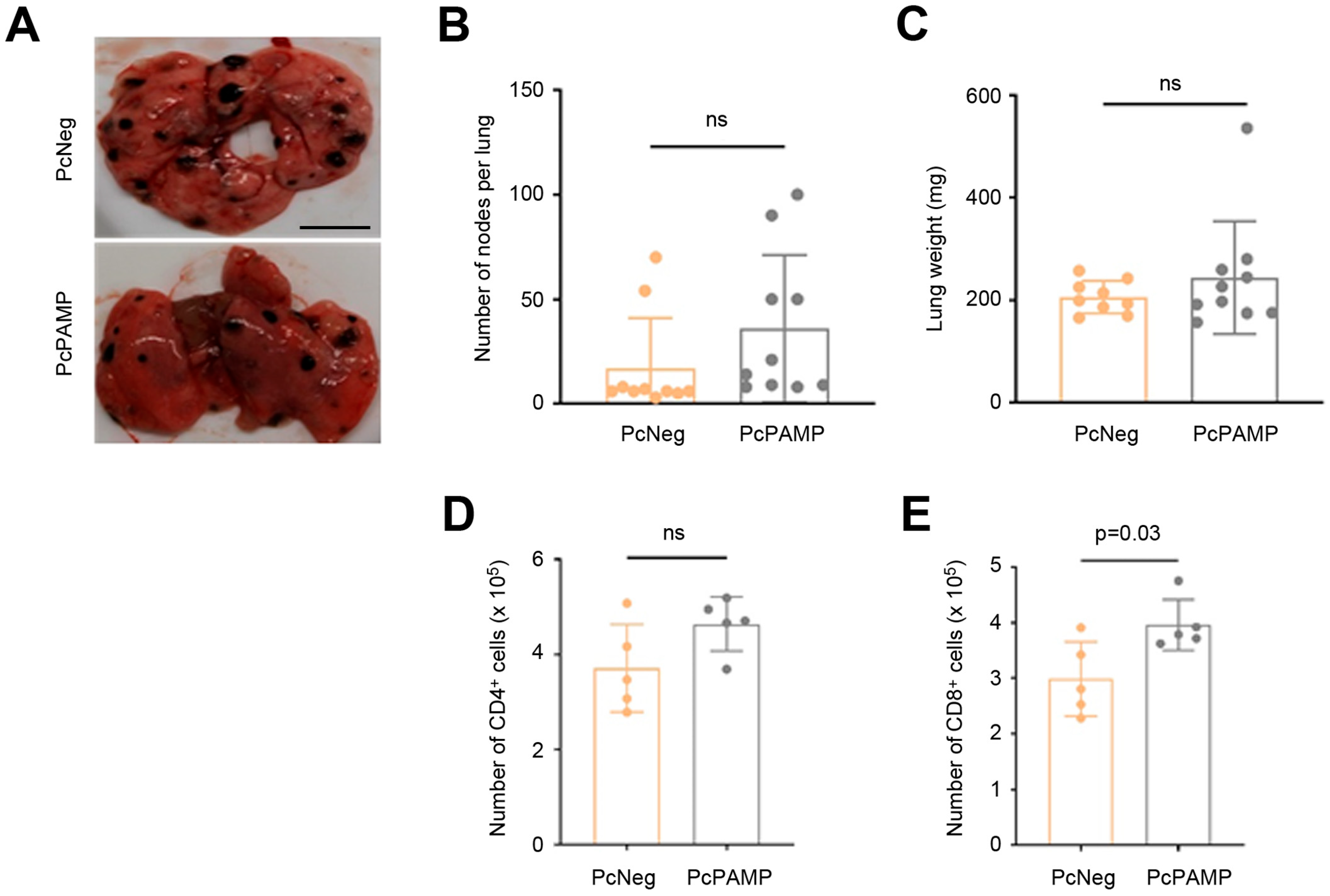
3.2. Immunization Campaign and Tumor Challenge
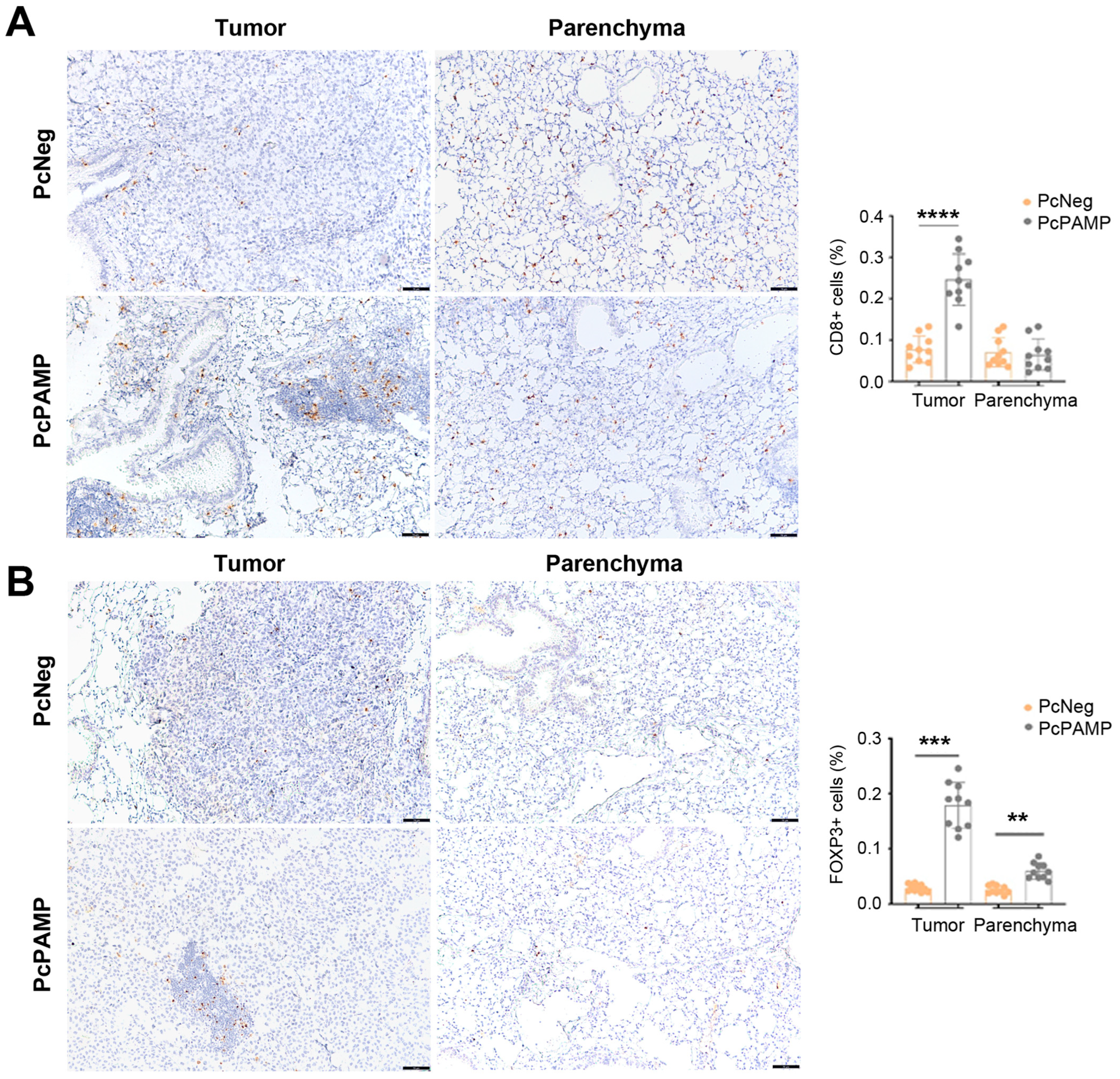
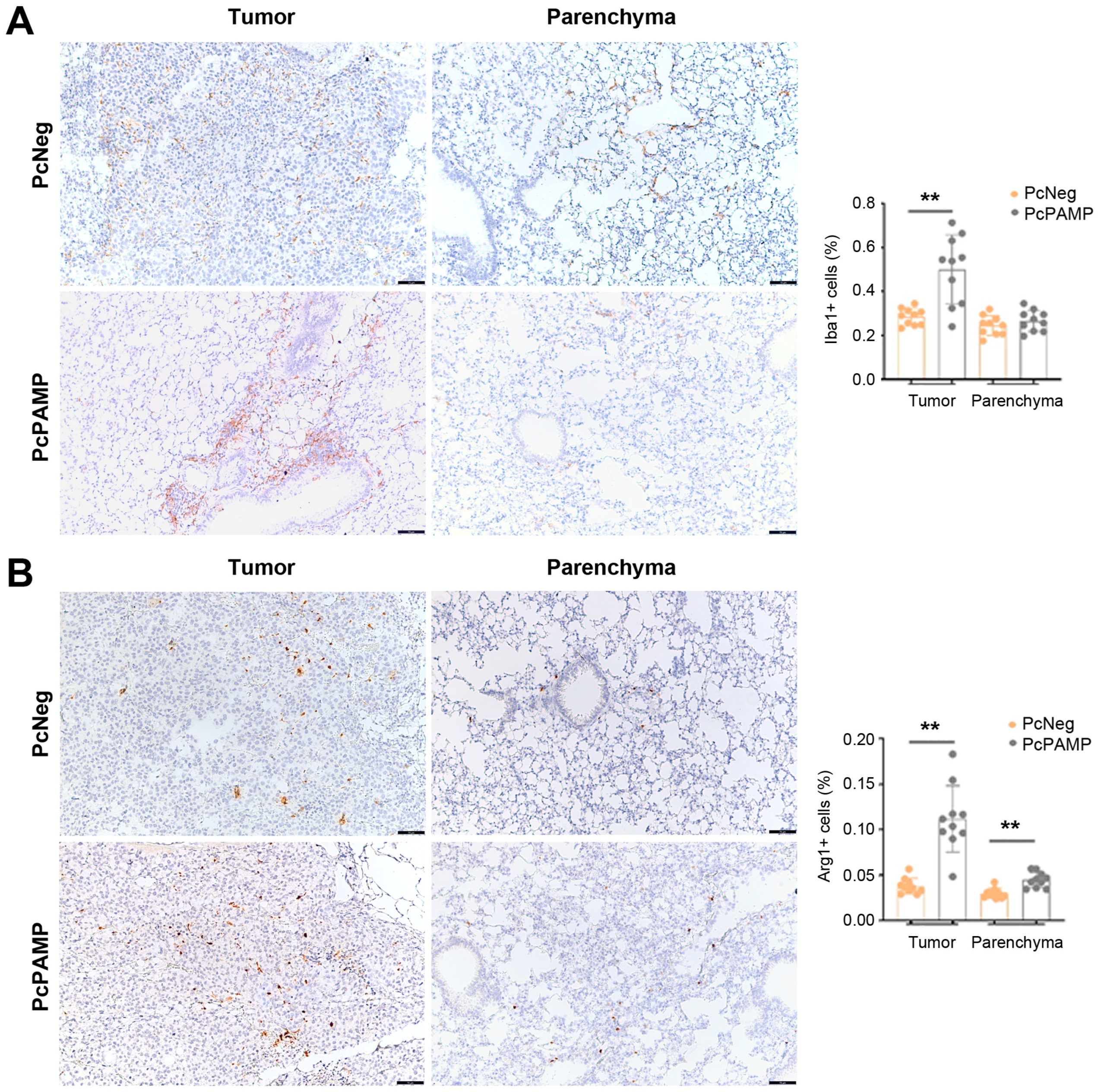
3.3. Lung Metastasis Quantification and Characterization of Cellular Immune Response
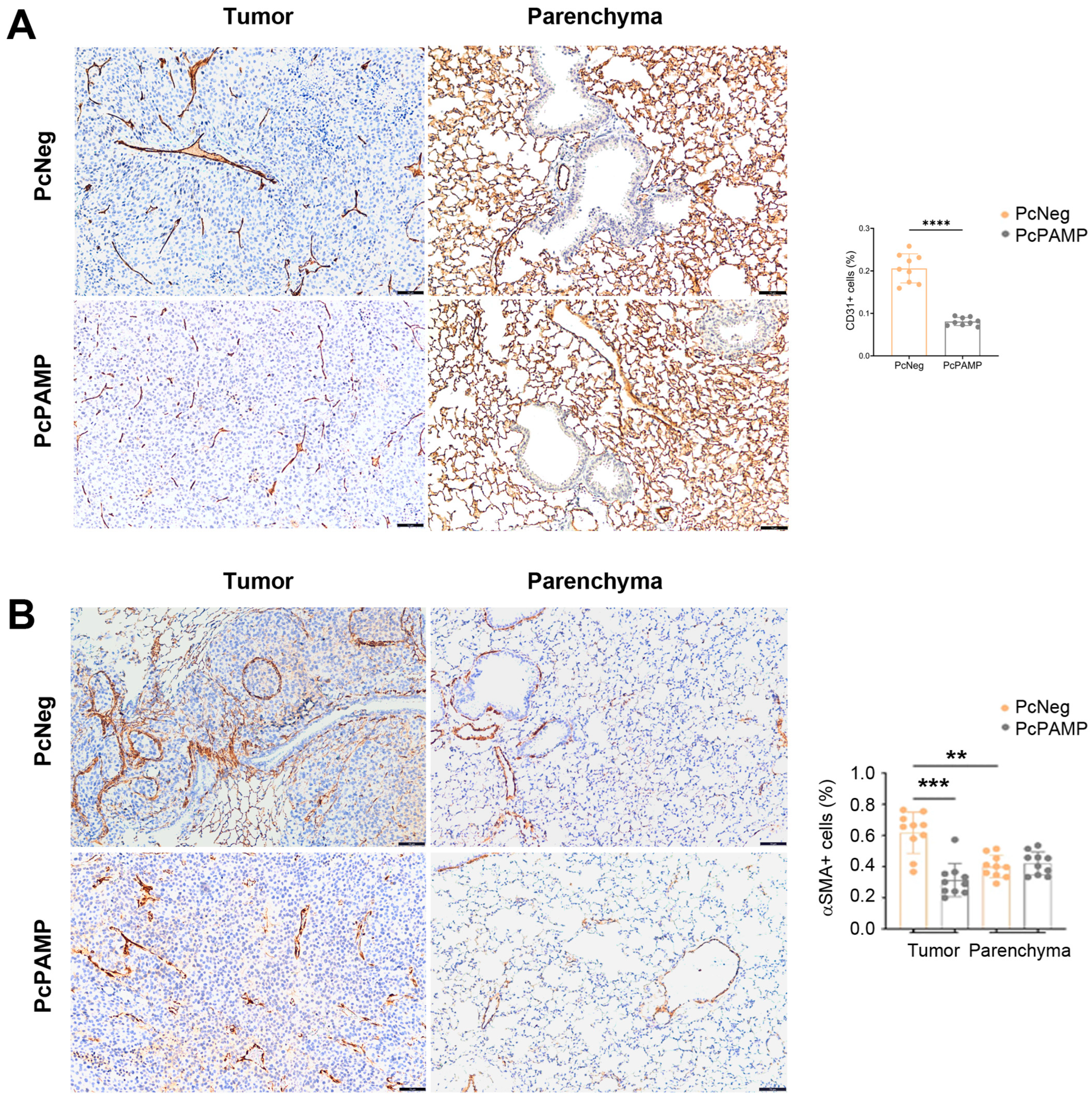
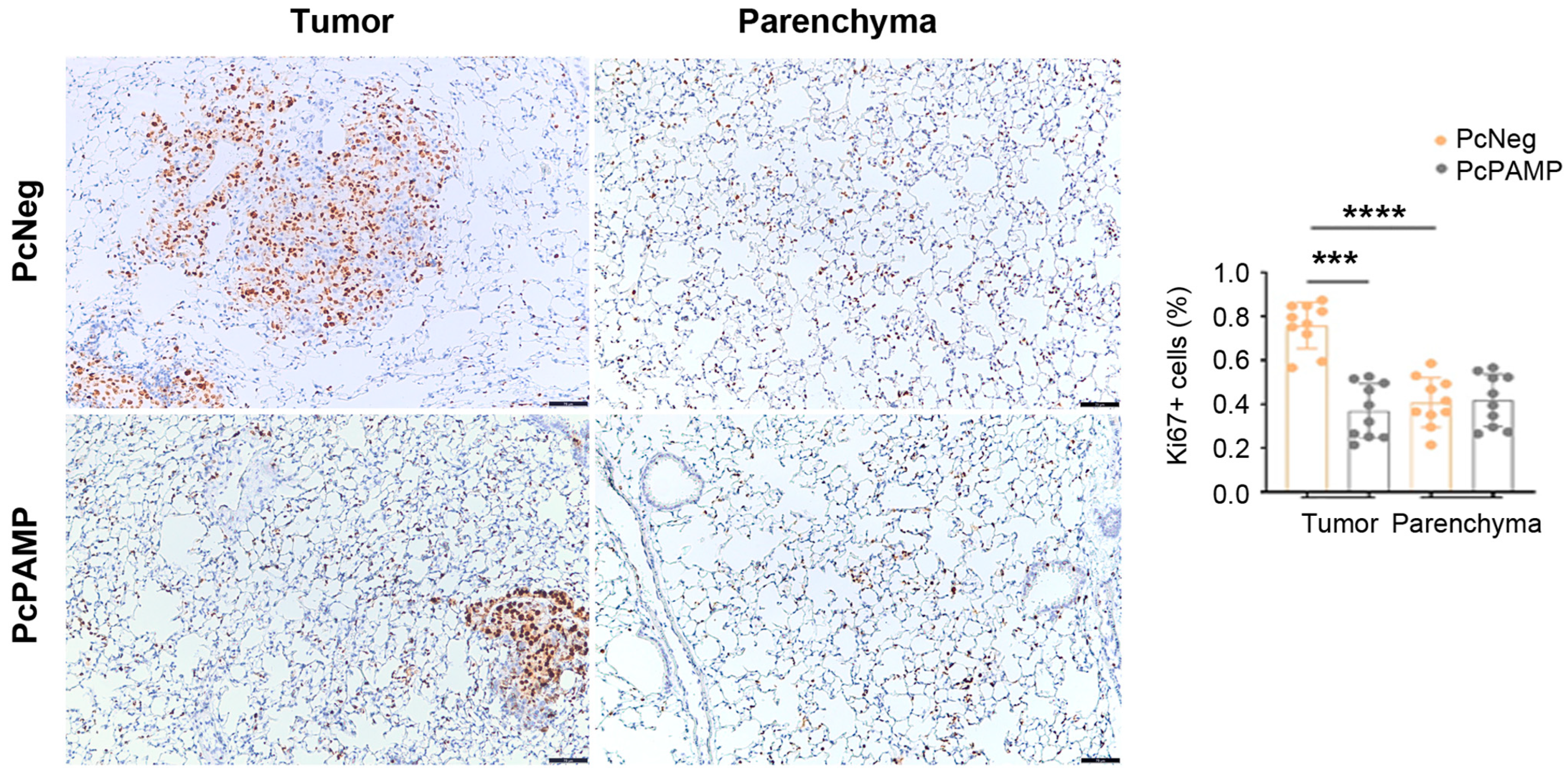
3.4. PAMP Vaccination Results in Less Angiogenesis and a Lower Number of Proliferating Tumor Cells in the Lung Metastases
3.5. PAMP Vaccination Results in a Massive Infiltration of Lymphocytes into the Lung Metastases
3.6. PAMP Vaccination Results in a Higher Infiltration of Macrophages into the Lung Metastases
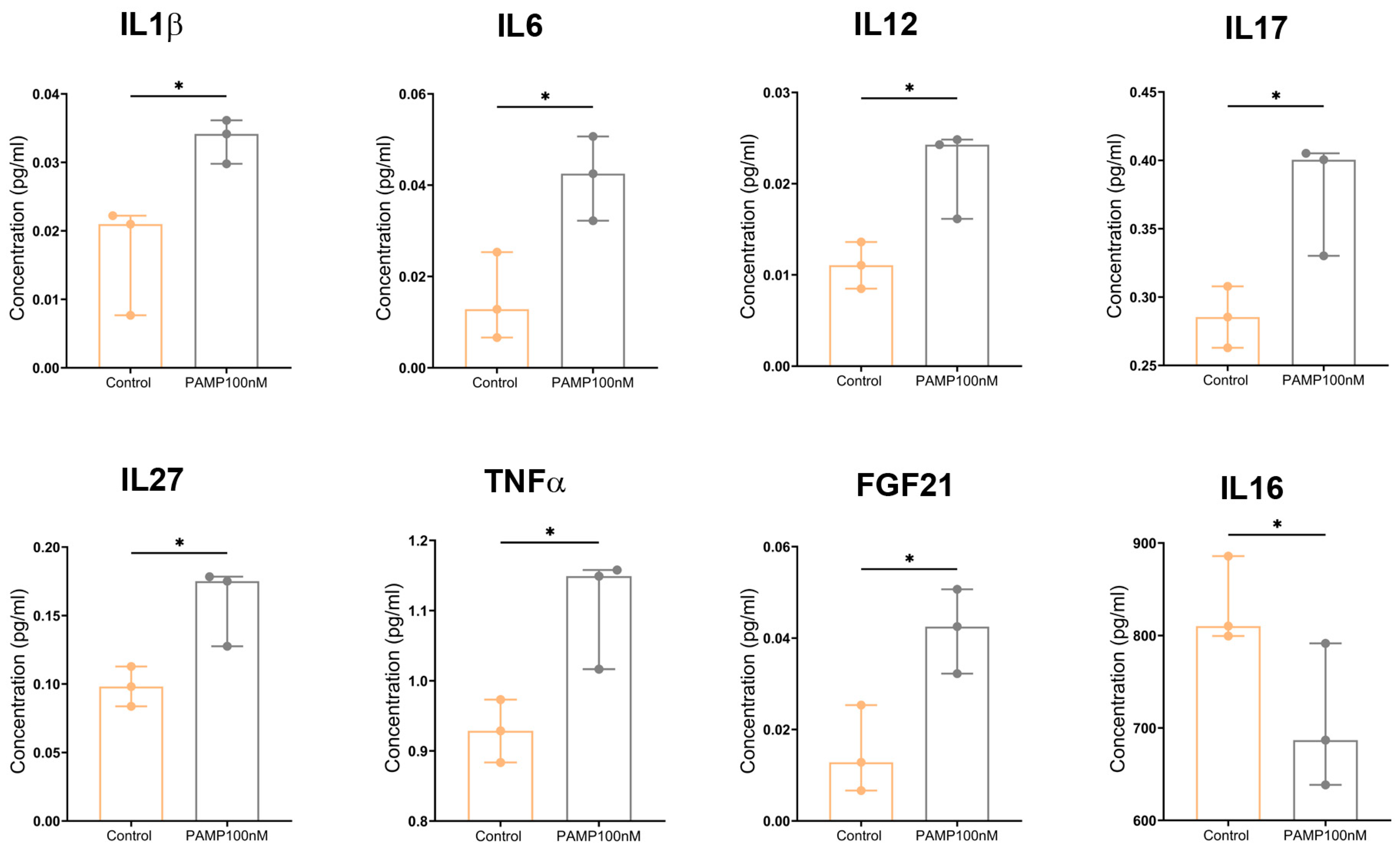
3.7. PAMP Modulates Cytokine Secretion in Mouse CD4+ T Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Adm | Proadrenomedullin gene |
| AM | Adrenomedullin |
| ACKR3 | Atypical chemokine receptor 3 |
| Arg1 | Arginase 1 |
| BSA | Bovine serum albumin |
| DAB | Diaminobenzidine |
| EC | Endothelial cells |
| HIF | Hypoxia-inducible factor |
| HRE | Hypoxia response element |
| HRP | Horseradish peroxidase |
| Iba1 | Ionized calcium-binding adapter molecule 1 |
| MrgX2 | Mas-related G protein-coupled receptor member X2 |
| PAMP | Proadrenomedullin N-terminal 20 peptide |
| SMA | Smooth muscle actin |
| TAM | Tumor-associated macrophage |
| TT | Tetanus toxin |
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Lacal, P.M.; Graziani, G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol. Res. 2018, 136, 97–107. [Google Scholar] [CrossRef]
- Jha, S.; Gupta, S.; Rani, S.; Arora, P.; Choudhary, N.; Kumar, S. Advances in VEGFR Inhibitors: A Comprehensive Review of Novel Anticancer Agents. Anti-Cancer Agents Med. Chem. 2025, 25, 663–687. [Google Scholar] [CrossRef] [PubMed]
- Wenger, R.H.; Stiehl, D.P.; Camenisch, G. Integration of oxygen signaling at the consensus HRE. Sci. STKE 2005, 2005, re12. [Google Scholar] [CrossRef]
- Martinez, A. A new family of angiogenic factors. Cancer Lett. 2006, 236, 157–163. [Google Scholar] [CrossRef]
- Schito, L.; Rey, S. Hypoxia: Turning vessels into vassals of cancer immunotolerance. Cancer Lett. 2020, 487, 74–84. [Google Scholar] [CrossRef]
- Okamoto, T.; Usuda, H.; Tanaka, T.; Wada, K.; Shimaoka, M. The Functional Implications of Endothelial Gap Junctions and Cellular Mechanics in Vascular Angiogenesis. Cancers 2019, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Martinez, A. Cell and molecular biology of the multifunctional peptide, adrenomedullin. Int. Rev. Cytol. 2002, 221, 1–92. [Google Scholar] [CrossRef]
- Lucyk, S.; Taha, H.; Yamamoto, H.; Miskolzie, M.; Kotovych, G. NMR conformational analysis of proadrenomedullin N-terminal 20 peptide, a proangiogenic factor involved in tumor growth. Biopolymers 2006, 81, 295–308. [Google Scholar] [CrossRef]
- Kitamura, K.; Kangawa, K.; Eto, T. Adrenomedullin and PAMP: Discovery, structures, and cardiovascular functions. Microsc. Res. Tech. 2002, 57, 3–13. [Google Scholar] [CrossRef]
- Kanazawa, H.; Kawaguchi, T.; Fujii, T.; Kudoh, S.; Hirata, K.; Kurihara, N.; Takeda, T. Comparison of bronchodilator responses to adrenomedullin and proadrenomedullin N-terminal 20 peptide. Life Sci. 1995, 57, L241–L245. [Google Scholar] [CrossRef]
- Shimosawa, T.; Ando, K.; Fujita, T. A newly identified peptide, proadrenomedullin N-terminal 20 peptide, induces hypotensive action via pertussis toxin-sensitive mechanisms. Hypertension 1997, 30, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Belloni, A.S.; Albertin, G.; Forneris, M.L.; Nussdorfer, G.G. Proadrenomedullin-derived peptides as autocrine-paracrine regulators of cell growth. Histol. Histopathol. 2001, 16, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Belloni, A.S.; Rossi, G.P.; Andreis, P.G.; Aragona, F.; Champion, H.C.; Kadowitz, P.J.; Murphy, W.A.; Coy, D.H.; Nussdorfer, G.G. Proadrenomedullin N-terminal 20 peptide (PAMP), acting through PAMP(12-20)-sensitive receptors, inhibits Ca2+-dependent, agonist-stimulated secretion of human adrenal glands. Hypertension 1999, 33, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Fernández De Arcaya, I.; Lostao, M.P.; Martinez, A.; Berjon, A.; Barber, A. Effect of adrenomedullin and proadrenomedullin N-terminal 20 peptide on sugar transport in the rat intestine. Regul. Pept. 2005, 129, 147–154. [Google Scholar] [CrossRef]
- Martinez, A.; Bengoechea, J.A.; Cuttitta, F. Molecular evolution of proadrenomedullin N-terminal 20 peptide (PAMP): Evidence for gene co-option. Endocrinology 2006, 147, 3457–3461. [Google Scholar] [CrossRef][Green Version]
- Martínez, A.; Zudaire, E.; Portal-Núñez, S.; Guédez, L.; Libutti, S.K.; Stetler-Stevenson, W.G.; Cuttitta, F. Proadrenomedullin NH2-terminal 20 peptide is a potent angiogenic factor, and its inhibition results in reduction of tumor growth. Cancer Res. 2004, 64, 6489–6494. [Google Scholar] [CrossRef]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef]
- Kamohara, M.; Matsuo, A.; Takasaki, J.; Kohda, M.; Matsumoto, M.; Matsumoto, S.-I.; Soga, T.; Hiyama, H.; Kobori, M.; Katou, M. Identification of MrgX2 as a human G-protein-coupled receptor for proadrenomedullin N-terminal peptides. Biochem. Biophys. Res. Commun. 2005, 330, 1146–1152. [Google Scholar] [CrossRef]
- Meyrath, M.; Palmer, C.B.; Reynders, N.; Vanderplasschen, A.; Ollert, M.; Bouvier, M.; Szpakowska, M.; Chevigné, A. Proadrenomedullin N-Terminal 20 Peptides (PAMPs) Are Agonists of the Chemokine Scavenger Receptor ACKR3/CXCR7. ACS Pharmacol. Transl. Sci. 2021, 4, 813–823. [Google Scholar] [CrossRef]
- Sackett, D.L.; Ozbun, L.; Zudaire, E.; Wessner, L.; Chirgwin, J.M.; Cuttitta, F.; Martínez, A. Intracellular proadrenomedullin-derived peptides decorate the microtubules and contribute to cytoskeleton function. Endocrinology 2008, 149, 2888–2898. [Google Scholar] [CrossRef] [PubMed]
- Larrayoz, I.M.; Martinez, A. Proadrenomedullin N-terminal 20 peptide increases kinesin’s velocity both in vitro and in vivo. Endocrinology 2012, 153, 1734–1742. [Google Scholar] [CrossRef]
- Larrayoz, I.M.; Martinez-Herrero, S.; Ochoa-Callejero, L.; Garcia-Sanmartin, J.; Martinez, A. Is the cytoskeleton an intracellular receptor for adrenomedullin and PAMP? Curr. Protein Pept. Sci. 2013, 14, 429–443. [Google Scholar] [CrossRef]
- Saleh, R.O.; Ibrahim, F.M.; Pallathadka, H.; Kaur, I.; Ahmad, I.; Ali, S.H.J.; Redhee, A.H.; Ghildiyal, P.; Jawad, M.A.; Alsaadi, S.B. Nucleic acid vaccines-based therapy for triple-negative breast cancer: A new paradigm in tumor immunotherapy arena. Cell Biochem. Funct. 2024, 42, e3992. [Google Scholar] [CrossRef]
- Zhang, X.; Goedegebuure, S.P.; Chen, M.Y.; Mishra, R.; Zhang, F.; Yu, Y.Y.; Singhal, K.; Li, L.; Gao, F.; Myers, N.B.; et al. Neoantigen DNA vaccines are safe, feasible, and induce neoantigen-specific immune responses in triple-negative breast cancer patients. Genome Med. 2024, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dixit, S.; Srinivasan, K.M.D.; Vincent, P.M.D.R. Personalized cancer vaccine design using AI-powered technologies. Front. Immunol. 2024, 15, 1357217. [Google Scholar] [CrossRef]
- Kozak, M.; Hu, J. DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines 2024, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Tadic, S.; Martinez, A. Nucleic acid cancer vaccines targeting tumor related angiogenesis. Could mRNA vaccines constitute a game changer? Front. Immunol. 2024, 15, 1433185. [Google Scholar] [CrossRef]
- Schmitz-Winnenthal, F.H.; Hohmann, N.; Niethammer, A.G.; Friedrich, T.; Lubenau, H.; Springer, M.; Breiner, K.M.; Mikus, G.; Weitz, J.; Ulrich, A.; et al. Anti-angiogenic activity of VXM01, an oral T-cell vaccine against VEGF receptor 2, in patients with advanced pancreatic cancer: A randomized, placebo-controlled, phase 1 trial. Oncoimmunology 2015, 4, e1001217. [Google Scholar] [CrossRef]
- Schmitz-Winnenthal, F.H.; Hohmann, N.; Schmidt, T.; Podola, L.; Friedrich, T.; Lubenau, H.; Springer, M.; Wieckowski, S.; Breiner, K.M.; Mikus, G.; et al. A phase 1 trial extension to assess immunologic efficacy and safety of prime-boost vaccination with VXM01, an oral T cell vaccine against VEGFR2, in patients with advanced pancreatic cancer. Oncoimmunology 2018, 7, e1303584. [Google Scholar] [CrossRef]
- Wick, W.; Wick, A.; Sahm, F.; Riehl, D.; von Deimling, A.; Bendszus, M.; Kickingereder, P.; Beckhove, P.; Winnenthal, F.H.S.; Jungk, C.; et al. ATIM-35, VXM01 phase 1 study in patients with progressive glioblastoma. Final results. Neuro Oncol. 2018, 20, vi9. [Google Scholar] [CrossRef]
- Wick, W.; Wick, A.; Chinot, O.L.; Bent, M.J.V.D.; De Vos, F.Y.F.L.; Mansour, M.; Podola, L.; Lubenau, H.; Platten, M. Oral DNA vaccination targeting VEGFR2 combined with anti-PDL1 avelumab in patients with progressive glioblastoma: Safety run-in results—NCT03750071. J. Clin. Oncol. 2020, 38, 3001. [Google Scholar] [CrossRef]
- Chambers, R.S.; Johnston, S.A. High-level generation of polyclonal antibodies by genetic immunization. Nat. Biotechnol. 2003, 21, 1088–1092. [Google Scholar] [CrossRef]
- Trinh, R.; Gurbaxani, B.; Morrison, S.L.; Seyfzadeh, M. Optimization of codon pair use within the (GGGGS)3 linker sequence results in enhanced protein expression. Mol. Immunol. 2004, 40, 717–722. [Google Scholar] [CrossRef]
- Rabea, S.; Salem-Bekhit, M.M.; Alanazi, F.K.; Yassin, A.S.; Moneib, N.A.; Hashem, A.E.M. A novel protocol for bacterial ghosts’ preparation using tween 80. Saudi Pharm. J. 2018, 26, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Callejero, L.; Pozo-Rodrigalvarez, A.; Martinez-Murillo, R.; Martinez, A. Lack of adrenomedullin in mouse endothelial cells results in defective angiogenesis, enhanced vascular permeability, less metastasis, and more brain damage. Sci. Rep. 2016, 6, 33495. [Google Scholar] [CrossRef] [PubMed]
- Montuenga, L.M.; Martinez, A.; Miller, M.J.; Unsworth, E.J.; Cuttitta, F. Expression of adrenomedullin and its receptor during embryogenesis suggests autocrine or paracrine modes of action. Endocrinology 1997, 138, 440–451. [Google Scholar] [CrossRef]
- Tadic, S.; Ochoa-Callejero, L.; Narro-Íñiguez, J.; Garcia-Sanmartin, J.; Martinez, A. An RNA vaccine against adrenomedullin reduces angiogenesis and tumor burden in a syngeneic metastatic melanoma mouse model. Front. Immunol. 2025, 16, 1604156. [Google Scholar]
- Smahel, M.; Duskova, M.; Polakova, I.; Musil, J. Enhancement of DNA vaccine potency against legumain. J. Immunother. 2014, 37, 293–303. [Google Scholar] [CrossRef]
- Pierini, S.; Tanyi, J.L.; Simpkins, F.; George, E.; Uribe-Herranz, M.; Drapkin, R.; Burger, R.; Morgan, M.A.; Facciabene, A. Ovarian granulosa cell tumor characterization identifies FOXL2 as an immunotherapeutic target. JCI Insight 2020, 5, e136773. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Yoon, J.; Shin, Y.; Kang, S.; Kim, S.Y.; Woo, S.; Song, J.; Jon, S. Neoantigen-Displaying Protein Nanoparticles as a Therapeutic Cancer Vaccine Against Melanoma. Adv. Healthc. Mater. 2025, 14, e2404316. [Google Scholar] [CrossRef] [PubMed]
- Strieth, S.; Eichhorn, M.E.; Sutter, A.; Jonczyk, A.; Berghaus, A.; Dellian, M. Antiangiogenic combination tumor therapy blocking αv-integrins and VEGF-receptor-2 increases therapeutic effects in vivo. Int. J. Cancer 2006, 119, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Larrayoz, I.M.; Martinez-Herrero, S.; Garcia-Sanmartin, J.; Ochoa-Callejero, L.; Martinez, A. Adrenomedullin and tumour microenvironment. J. Transl. Med. 2014, 12, 339. [Google Scholar] [CrossRef]
- Nakayama, A.; Roquid, K.A.; Iring, A.; Strilic, B.; Günther, S.; Chen, M.; Weinstein, L.S.; Offermanns, S. Suppression of CCL2 angiocrine function by adrenomedullin promotes tumor growth. J. Exp. Med. 2023, 220, e20211628. [Google Scholar] [CrossRef]
- Ando, K.; Omi, N.; Shimosawa, T.; Fujita, T. Proadrenomedullin N-terminal 20 peptide (PAMP) inhibits proliferation of human neuroblastoma TGW cells. FEBS Lett. 1997, 413, 462–466. [Google Scholar] [CrossRef]
- Moody, T.W.; Coy, D.; Cuttitta, F.; Montuenga, L.M. Proadrenomedullin NH2-terminal 20 peptide (PAMP) and adrenomedullin bind to teratocarcinoma cells. Peptides 2000, 21, 101–107. [Google Scholar] [CrossRef]
- Maibach, F.; Sadozai, H.; Seyed Jafari, S.M.; Hunger, R.E.; Schenk, M. Tumor-Infiltrating Lymphocytes and Their Prognostic Value in Cutaneous Melanoma. Front. Immunol. 2020, 11, 2105. [Google Scholar] [CrossRef] [PubMed]
- Mihm, M.C., Jr.; Mule, J.J. Reflections on the Histopathology of Tumor-Infiltrating Lymphocytes in Melanoma and the Host Immune Response. Cancer Immunol. Res. 2015, 3, 827–835. [Google Scholar] [CrossRef]
- Li, L.; Yu, R.; Cai, T.; Chen, Z.; Lan, M.; Zou, T.; Wang, B.; Wang, Q.; Zhao, Y.; Cai, Y. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int. Immunopharmacol. 2020, 88, 106939. [Google Scholar] [CrossRef]
- Giavina-Bianchi, M.; Giavina-Bianchi, P.; Sotto, M.N.; Muzikansky, A.; Kalil, J.; Festa-Neto, C.; Duncan, L.M. Increased NY-ESO-1 expression and reduced infiltrating CD3+ T cells in cutaneous melanoma. J. Immunol. Res. 2015, 2015, 761378. [Google Scholar] [CrossRef]
- Oliveira, G.; Stromhaug, K.; Cieri, N.; Iorgulescu, J.B.; Klaeger, S.; Wolff, J.O.; Rachimi, S.; Chea, V.; Krause, K.; Freeman, S.S.; et al. Landscape of helper and regulatory antitumour CD4+ T cells in melanoma. Nature 2022, 605, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.A.; Amir, E. HYPE or HOPE: The prognostic value of infiltrating immune cells in cancer. Br. J. Cancer 2017, 117, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; van der Leun, A.M.; Yofe, I.; Lubling, Y.; Gelbard-Solodkin, D.; van Akkooi, A.C.; Braber, M.v.D.; Rozeman, E.A.; Haanen, J.B.; Blank, C.U.; et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell 2019, 176, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef] [PubMed]
- Pierezan, F.; Mansell, J.; Ambrus, A.; Rodrigues, H.A. Immunohistochemical expression of ionized calcium binding adapter molecule 1 in cutaneous histiocytic proliferative, neoplastic and inflammatory disorders of dogs and cats. J. Comp. Pathol. 2014, 151, 347–351. [Google Scholar] [CrossRef]
- Arlauckas, S.P.; Garren, S.B.; Garris, C.S.; Kohler, R.H.; Oh, J.; Pittet, M.J.; Weissleder, R. Arg1 expression defines immunosuppressive subsets of tumor-associated macrophages. Theranostics 2018, 8, 5842–5854. [Google Scholar] [CrossRef]
- Forconi, F.; King, C.A.; Sahota, S.S.; Kennaway, C.K.; Russell, N.H.; Stevenson, F.K. Insight into the potential for DNA idiotypic fusion vaccines designed for patients by analysing xenogeneic anti-idiotypic antibody responses. Immunology 2002, 107, 39–45. [Google Scholar] [CrossRef]
- Radcliffe, J.N.; Roddick, J.S.; Friedmann, P.S.; Stevenson, F.K.; Thirdborough, S.M. Prime-boost with alternating DNA vaccines designed to engage different antigen presentation pathways generates high frequencies of peptide-specific CD8+ T cells. J. Immunol. 2006, 177, 6626–6633. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Mei, Q.; Zhao, B.; Chu, Q.; Dai, Z.; Wu, K. Exploiting innate immunity for cancer immunotherapy. Mol. Cancer 2023, 22, 187. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Taga, T.; Nakano, N.; Yasukawa, K.; Kashiwamura, S.; Shimizu, K.; Nakajima, K.; Pyun, K.H.; Kishimoto, T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc. Natl. Acad. Sci. USA 1985, 82, 5490–5494. [Google Scholar] [CrossRef] [PubMed]
- Center, D.M.; Cruikshank, W. Modulation of lymphocyte migration by human lymphokines. I. Identification and characterization of chemoattractant activity for lymphocytes from mitogen-stimulated mononuclear cells. J. Immunol. 1982, 128, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Ouaked, N.; Mantel, P.-Y.; Bassin, C.; Burgler, S.; Siegmund, K.; Akdis, C.A.; Schmidt-Weber, C.B. Regulation of the foxp3 gene by the Th1 cytokines: The role of IL-27-induced STAT1. J. Immunol. 2009, 182, 1041–1049. [Google Scholar] [CrossRef]


| Gene Name | Sequence |
|---|---|
| TT P2 epitope | AAGCAAAAATATTTTAATGCAGTATATAAAAGCAAATTCTAAATTTATAGGTATAACTGAACTAAAAAAATTAGAATCAAA |
| TT P30 epitope | ATATTGAATATAATGATATGTTTAATAATTTTACCGTTAGCTTTTGGTTGAGGGTTCCTAAAGTATCTGCTAGTCATTTAGAACAATATGGCACAAATGAG |
| Mouse PAMP | GCAGGGCCAGATACTCCTTCGCAGTTCCGAAAGAAGTGGAATAAGTGGGCGCTAAGTCGTGCAGGGCCAGATACTCCTTCGCAGTTCCGAAAGAAGTGGAATAAGTGGGCGCTAAGTCGTGGGAAGCGA |
| Linkers | AGCGGCGGCGGCAGCGGCGGCGGCAGCGGCGGCGGCAGC |
| Primary Antibody | Reference | RRID | Dilution | Second Layer | Dilution of Second Layer |
|---|---|---|---|---|---|
| PAMP | 16072 (Proteogenix, Schiltigheim, France) | AB_3694067 | 1:4000 | 7074 HRP goat anti-rabbit IgG (Cell Signaling, Danvers, MA, USA) | 1:2000 |
| CD31 | ab281583 (Abcam, Cambridge, UK) | AB_3096925 | 1:4000 | Novolink rabbit detection system | Prediluted |
| α-SMA | sc-53015 (Santa Cruz) | AB_628683 | 1:15,000 | Immpress mouse detection system | Prediluted |
| Ki67 | 12202 (Cell Signaling) | AB_2620142 | 1:1000 | Novolink rabbit detection system | Prediluted |
| CD3 | A0452 (Dako, Santa Clara, CA, USA) | AB_2335677 | 1:1000 | Novolink rabbit detection system | Prediluted |
| CD4 | ab288724 (Abcam, Cambridge, UK) | AB_2941893 | 1:2000 | Novolink rabbit detection system | Prediluted |
| CD8 | ab217344 (Abcam) | AB_2890649 | 1:1000 | Novolink rabbit detection system | Prediluted |
| FOXP3 | 14-5773-82 (Thermo Fisher) | AB_467576 | 1:100 | 112-035-003 HRP goat anti-rat (Jackson Immunoresearch, West Grove, PA, USA) | 1:200 |
| Iba1 | sc-32725 (Santa Cruz, Dallas, TX, USA) | AB_667733 | 1:8000 | Novolink mouse detection system | Prediluted |
| Arg1 | 93668 (Cell Signaling) | AB_2800207 | 1:1000 | Novolink rabbit detection system | Prediluted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, T.K.; Tadic, S.; Garrido, P.; Ochoa-Callejero, L.; Narro-Íñiguez, J.; García-Sanmartín, J.; Martínez, A. A DNA Vaccine Against Proadrenomedullin N-Terminal 20 Peptide (PAMP) Reduces Angiogenesis and Increases Lymphocyte and Macrophage Infiltration but Has No Effect on Tumor Burden in a Mouse Model of Lung Metastasis. Vaccines 2025, 13, 586. https://doi.org/10.3390/vaccines13060586
Raju TK, Tadic S, Garrido P, Ochoa-Callejero L, Narro-Íñiguez J, García-Sanmartín J, Martínez A. A DNA Vaccine Against Proadrenomedullin N-Terminal 20 Peptide (PAMP) Reduces Angiogenesis and Increases Lymphocyte and Macrophage Infiltration but Has No Effect on Tumor Burden in a Mouse Model of Lung Metastasis. Vaccines. 2025; 13(6):586. https://doi.org/10.3390/vaccines13060586
Chicago/Turabian StyleRaju, Tom Kalathil, Srdan Tadic, Pablo Garrido, Laura Ochoa-Callejero, Judit Narro-Íñiguez, Josune García-Sanmartín, and Alfredo Martínez. 2025. "A DNA Vaccine Against Proadrenomedullin N-Terminal 20 Peptide (PAMP) Reduces Angiogenesis and Increases Lymphocyte and Macrophage Infiltration but Has No Effect on Tumor Burden in a Mouse Model of Lung Metastasis" Vaccines 13, no. 6: 586. https://doi.org/10.3390/vaccines13060586
APA StyleRaju, T. K., Tadic, S., Garrido, P., Ochoa-Callejero, L., Narro-Íñiguez, J., García-Sanmartín, J., & Martínez, A. (2025). A DNA Vaccine Against Proadrenomedullin N-Terminal 20 Peptide (PAMP) Reduces Angiogenesis and Increases Lymphocyte and Macrophage Infiltration but Has No Effect on Tumor Burden in a Mouse Model of Lung Metastasis. Vaccines, 13(6), 586. https://doi.org/10.3390/vaccines13060586








