A Multiepitope Nanovaccine Candidate Adjuvanted with Porcine Ferritin Scaffold for African Swine Fever Virus
Abstract
1. Introduction
2. Materials and Methods
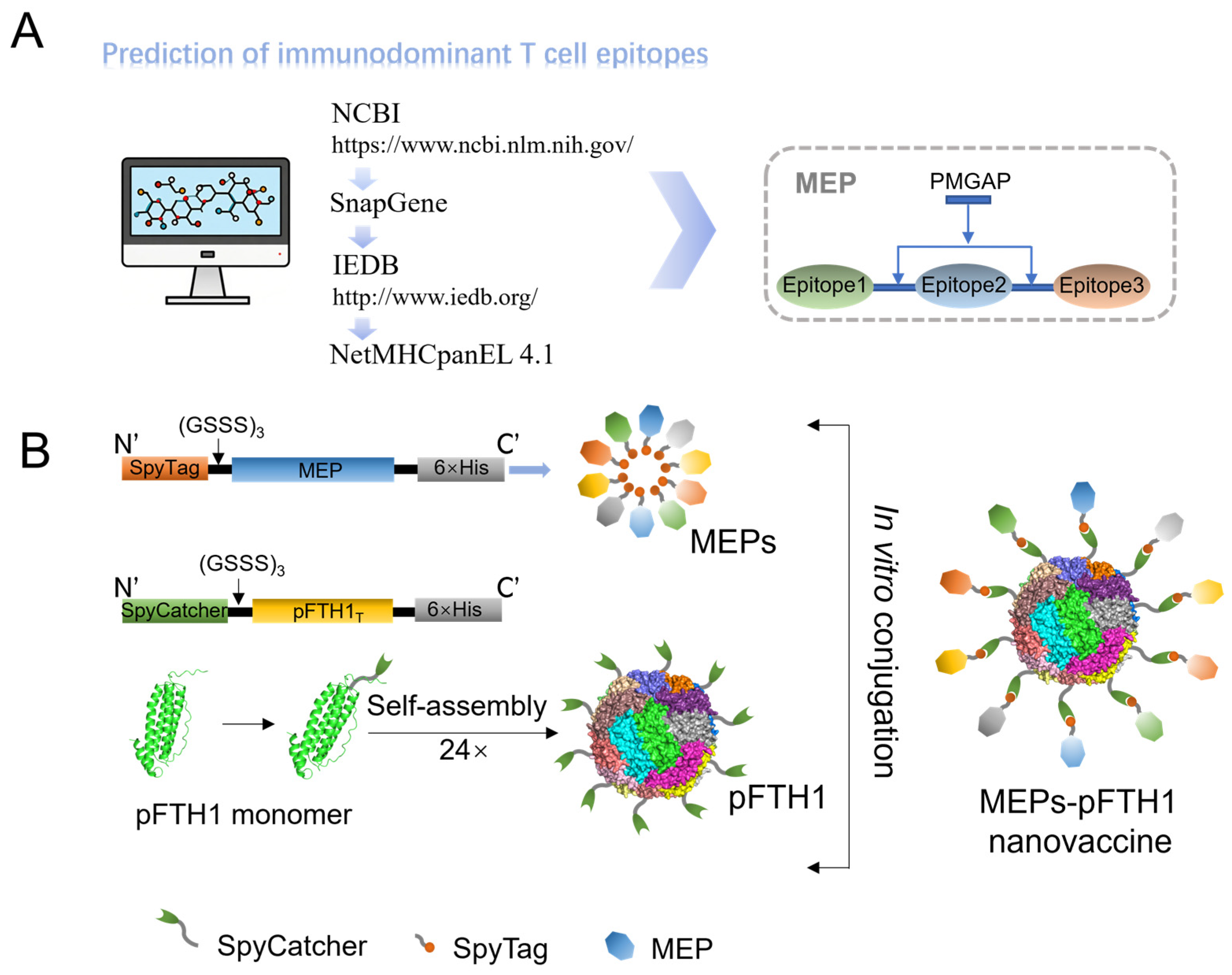
2.1. Prediction of ASFV TEPs and Screening of Immunodominant TEPs
2.2. Construction of Recombinant MEPs
2.3. Construction of pFTH1 Plasmids
2.4. Expression and Purification of Recombinant MEPs and pFTH1 Proteins
2.5. Preparation of MEPs-pFTH1 Nanoparticles
2.6. Western Blot
2.7. Transmission Electron Microscopy (TEM)
2.8. Immunization of Mice and Sample Collection
2.9. Immunization of Pigs and Sample Collection
2.10. Enzyme-Linked Immunosorbent Assay
2.11. Enzyme-Linked Immunospot Assay
2.12. Serum Virus Neutralization Assay
2.13. Quantitative PCR
2.14. Statistical Analysis
3. Results
3.1. Screening of Immunodominant TEPs and Construction of MEPs-pFTH1 Particles
3.2. Characterization of MEPs-pFTH1 Nanoparticles
3.3. pFTH1 Is Minimally Immunogenic in Pigs
3.4. MEPs-pFTH1 Nanoparticles Are Highly Biocompatible
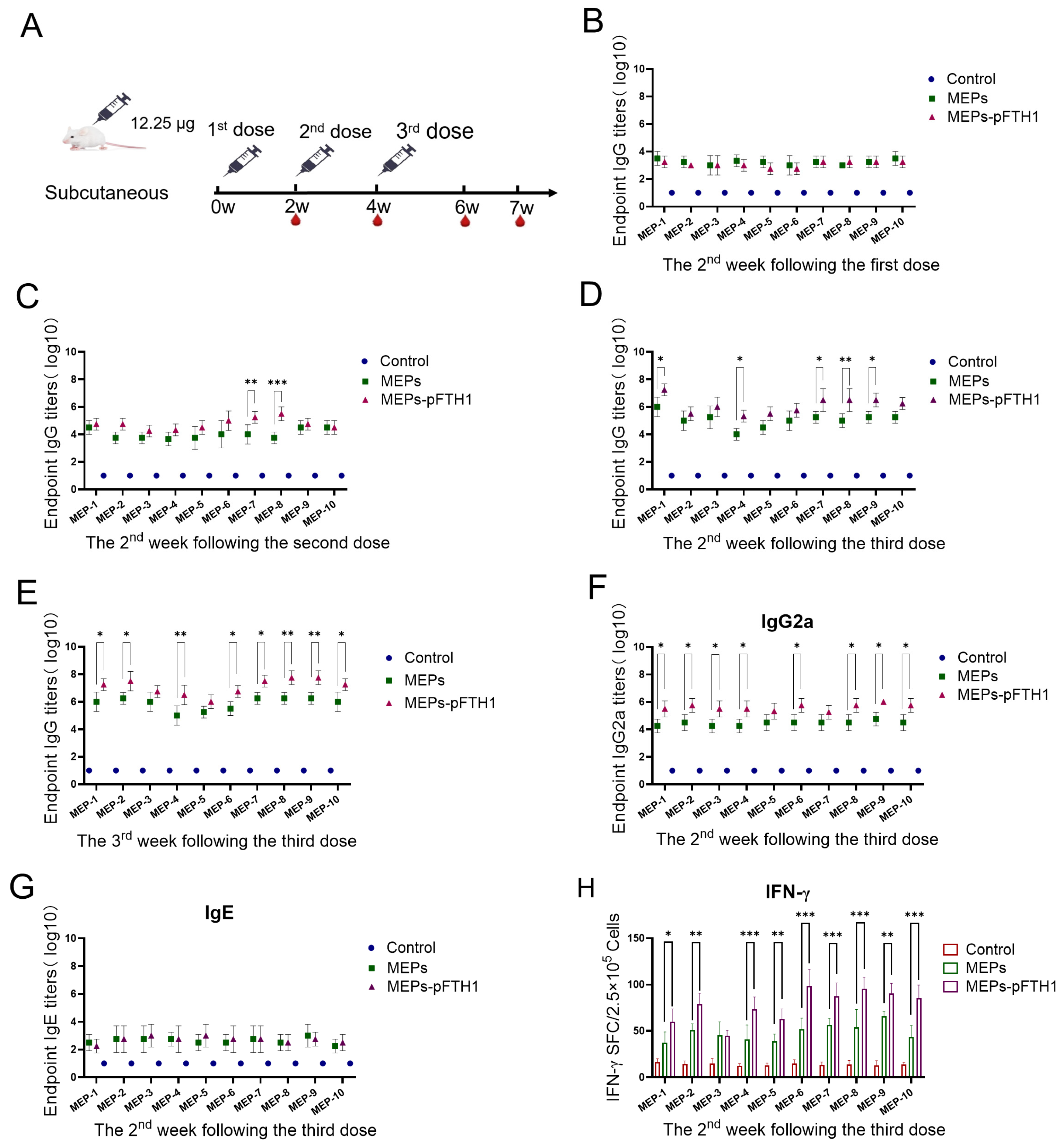
3.5. MEPs-pFTH1 Nanoparticles Induce Strong Humoral and Cellular Immune Responses in Mice
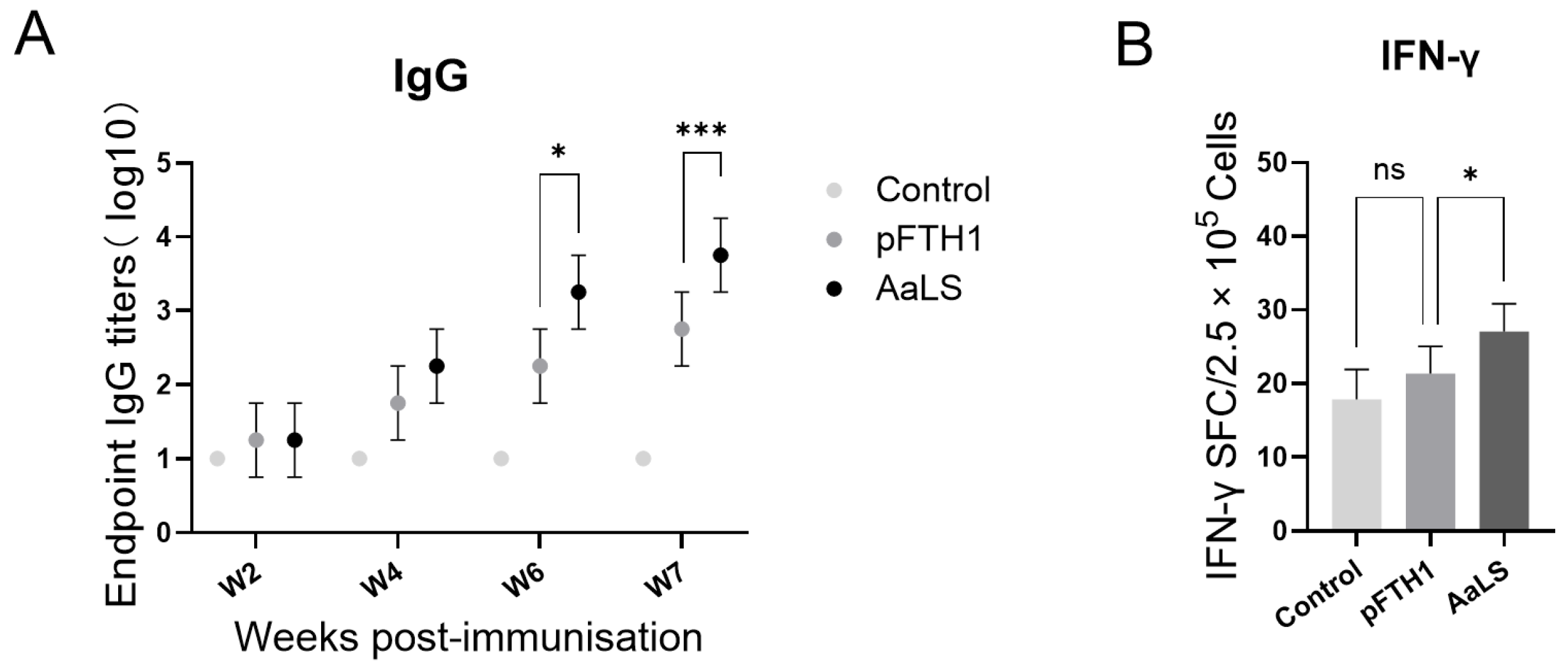
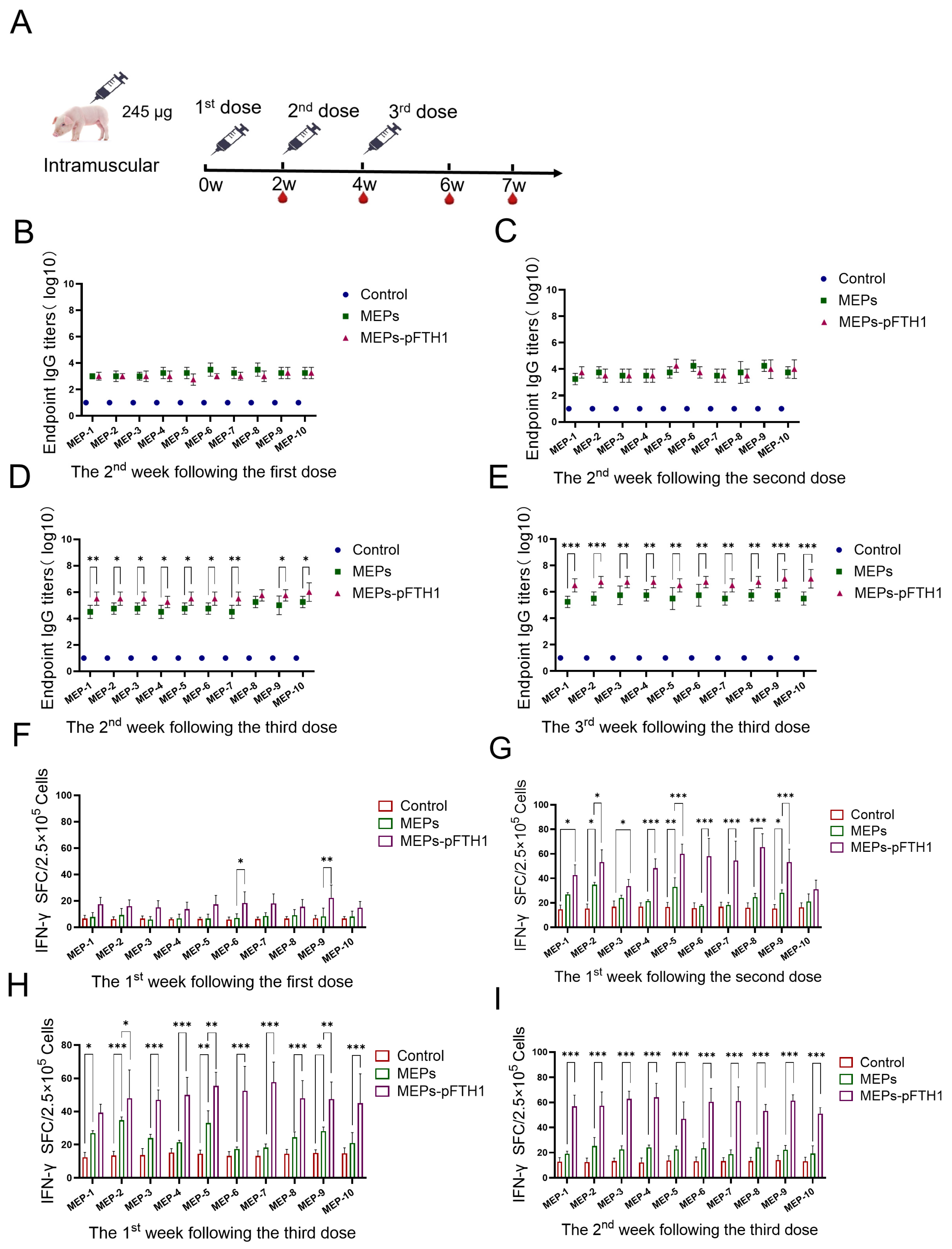
3.6. MEPs-pFTH1 Nanoparticles Induce Robust Humoral and Cellular Immune Responses in Pigs
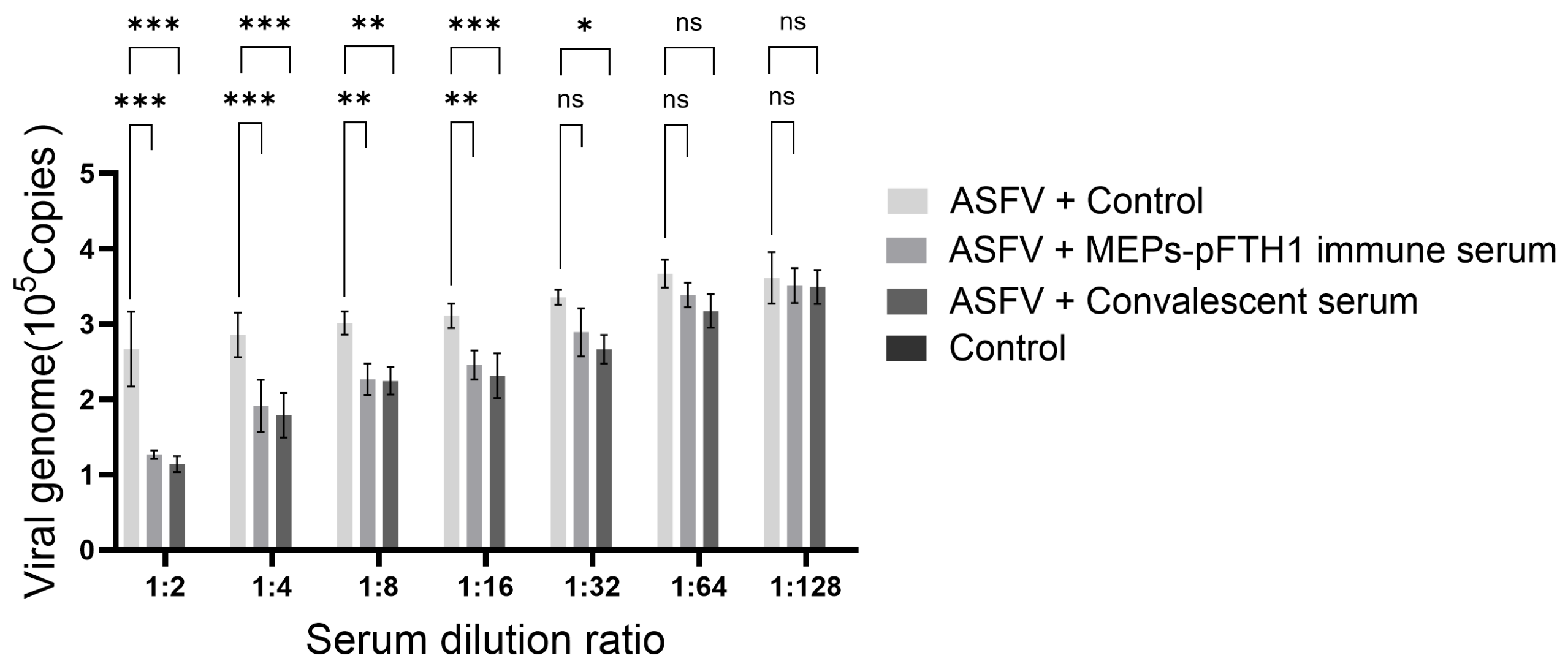
3.7. Immune Serum from Pigs Injected with the MEPs-pFTH1 Nanoparticles Significantly Inhibits ASFV Replication
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gabriel, C.; Blome, S.; Malogolovkin, A.; Parilov, S.; Kolbasov, D.; Teifke, J.P.; Beer, M. Characterization of African swine fever virus caucasus isolate in european wild boars. Emerg. Infect. Dis. 2011, 17, 2342–2345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef]
- Stone, S.S.; Hess, W.R. Antibody response to inactivated preparations of African swine fever virus in pigs. Am. J. Vet. Res. 1967, 28, 475–481. [Google Scholar]
- Blome, S.; Gabriel, C.; Beer, M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine 2014, 32, 3879–3882. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Richt, J.A. Subunit vaccine approaches for African swine fever virus. Vaccines 2019, 7, 56. [Google Scholar] [CrossRef]
- Rock, D.L. Challenges for African swine fever vaccine development—“perhaps the end of the beginning”. Vet. Microbiol. 2017, 206, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, B.; Díaz-Gil, G.; García-Gallo, M.; Quetglas, J.I.; Rodríguez-Crespo, I.; Dixon, L.; Dixon, J.M.; Alonso, C. The African swine fever virus dynein-binding protein p54 induces infected cell apoptosis. FEBS Lett. 2004, 569, 224–228. [Google Scholar] [CrossRef]
- Escribano, J.M.; Galindo, I.; Alonso, C. Antibody-mediated neutralization of African swine fever virus: Myths and facts. Virus Res. 2013, 173, 101–109. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pig. Sci. China (Life Sci.) 2020, 63, 623–634. [Google Scholar] [CrossRef]
- Tran, X.H.; Le, T.T.P.; Nguyen, Q.H.; Do, T.T.; Nguyen, V.D.; Gay, C.G.; Borca, M.V.; Gladue, D.P. African swine fever virus vaccine candidate ASFV-G-ΔI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transbound. Emerg. Dis. 2022, 69, e497. [Google Scholar] [CrossRef]
- Ding, M.; Dang, W.; Liu, H.; Xu, F.; Huang, H.; Sunkai, Y.; Li, T.; Pei, J.; Liu, X.; Zhang, Y.; et al. Combinational deletions of MGF360-9L and MGF505-7R attenuated highly virulent African swine fever virus and conferred protection against homologous challenge. J. Virol. 2022, 96, e0032922. [Google Scholar] [CrossRef] [PubMed]
- Bhatnager, R.; Bhasih, M.; Arora, J.; Dang, A.S. Epitope based peptide vaccine against SARS-CoV-2: An immune-informatics approach. J. Biomol. Struct. Dyn. 2021, 39, 5690–5705. [Google Scholar] [CrossRef] [PubMed]
- Nelde, A.; Rammensee, H.G.; Walz, J.S. The peptide vaccine of the future. Mol. Cell Proteom. 2021, 20, 100022. [Google Scholar] [CrossRef]
- Lamontagne, F.; Khatri, V.; St-Louis, P.; Bourgault, S.; Bourgault, D. Vaccination strategies based on bacterial self-assembling proteins as antigen delivery nanoscaffolds. Vaccines 2022, 10, 1920. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.J.; Zmolek, A.C.; Irvine, D.J. Beyond antigens and adjuvants: Formulating future vaccines. J. Clin. Investig. 2016, 126, 799–808. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Saudan, M.P. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Miller, N.E.; Michel, C.C.; Nanjee, M.N.; Olszewski, W.L.; Miller, I.P.; Hazell, M.; Hazell, M.; Olivecrona, G.; Sutton, P.; Humphreys, S.M.; et al. Secretion of adipokines by human adipose tissue in vivo: Partitioning between capillary and lymphatic transport. Am. J. Physiol. Endocrinol. Metab. 2011, 301, e659–e667. [Google Scholar] [CrossRef]
- Reddy, S.T.; Rehor, A.; Schmoekel, H.G.; Hubbell, J.A.; Swartz, M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 2006, 112, 26–34. [Google Scholar] [CrossRef]
- Roth, G.A.; Saouaf, O.M.; Smith, A.A.A.; Gale, E.C.; Hernández, M.A.; Idoyaga, J.; Appel, E. Prolonged codelivery of hemagglutinin and a TLR7/8 agonist in a supramolecular polymer-nanoparticle hydrogel enhances potency and breadth of influenza vaccination. ACS Biomater. Sci. Eng. 2021, 7, 1889–1899. [Google Scholar] [CrossRef]
- Supersaxo, A.; Hein, W.R.; Steffen, H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm. Res. 1990, 7, 167–169. [Google Scholar] [CrossRef]
- Al-Halifa, S.; Gauthier, L.; Arpin, D.; Bourgault, S.; Archambault, D. Nanoparticle-based vaccines against respiratory viruses. Front. Immunol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Zottig, X.; Cote-Cyr, M.; Arpin, D.; Archambault, D.; Bourgault, S. Protein supramolecular structures: From self-assembly to nanovaccine design. Nanomaterilas 2020, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Fries, C.N.; Curvino, E.J.; Chen, J.L.; Permar, S.R.; Fouda, G.G.; Fouda, J.H. Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nat. Nanotechnol. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Bennett, N.R.; Zwick, D.B.; Courtney, A.H.; Kiessling, L.L. Multivalent antigens for promoting B and T cell activation. ACS Chem. Biol. 2015, 10, 1817–1824. [Google Scholar] [CrossRef]
- Hong, S.; Zhang, Z.; Liu, H.; Tian, M.; Zhu, X.; Zhang, Z.; Wang, W.; Zhou, X.; Zhang, F.; Ge, Q.; et al. B Cells are the dominant antigen-presenting cells that activate naive CD4+ T cells upon immunization with a virus-derived nanoparticle antigen. Immunity 2018, 49, 695–708. [Google Scholar] [CrossRef]
- Pompano, R.R.; Chen, J.; Verbus, E.A.; Han, H.; Fridman, A.; McNeely, T.; Collier, J.H.; Chong, A.S. Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Adv. Health Mater. 2014, 3, 1898–1908. [Google Scholar] [CrossRef]
- Walls, A.C.; Fiala, B.; Schäfer, A.; Chong, S.; Pham, M.N.; Murphy, M.; Tse, L.V.; Shehata, L.; Shehata, M.A.; Chen, C. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell 2020, 183, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chao, C.W.; Tsybovsky, Y.; Abiona, O.M.; Hutchinson, G.B.; Hutchinson, J.I.; Olia, A.S.; Pegu, A.; Phung, E.; Phung, G.B.E.; et al. A platform incorporating trimeric antigens into self-assembling nanoparticles reveals SARS-CoV-2-spike nanoparticles to elicit substantially higher neutralizing responses than spike alone. Sci. Rep. 2020, 10, 18149. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Rohovie, M.J.; Nagasawa, M.; Swartz, J.R. Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery. Bioeng. Transl. Med. 2017, 2, 43–57. [Google Scholar] [CrossRef]
- Yan, D.; Wei, Y.; Guo, H.; Sun, S. The application of virus-like particles as vaccines and biological vehicles. Appl. Microbiol. Biotechnol. 2015, 99, 10415–10432. [Google Scholar] [CrossRef] [PubMed]
- Zabel, F.; Kündig, T.M.; Bachmann, M.F. Virus-induced humoral immunity: On how B cell responses are initiated. Curr. Opin. Virol. 2013, 3, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Lagoutte, P.; Mignon, C.; Stadthagen, G.; Potisopon, S.; Potisopon, S.; Mast, J.; Lugari, A.; Werle, B. Simultaneous surface display and cargo loading of encapsulin nanocompartments and their use for rational vaccine design. Vaccine 2018, 36, 3622–3628. [Google Scholar] [CrossRef] [PubMed]
- Salinas, N.D.; Ma, R.; McAleese, H.; Ouahes, T.; Long, C.A.; Miura, K.; Lambert, L.E.; Tolia, N.H. A self-assembling pfs230D1-ferritin nanoparticle vaccine has potent and durable malaria transmission-reducing activity. Vaccines 2024, 12, 546. [Google Scholar] [CrossRef]
- Ma, X.; Zou, F.; Yu, F.; Li, R.; Yuan, Y.; Zhang, Y.; Zhang, X.; Deng, J.; Chen, T.; Song, Z.; et al. Nanoparticle vaccines based on the receptor binding domain (RBD) and heptad repeat (HR) of SARS-CoV-2 elicit robust protective immune responses. Immunity 2020, 53, 1315–1330.e9. [Google Scholar] [CrossRef]
- Wang, W.; Huang, B.; Zhu, Y.; Tan, W.; Zhu, M. Ferritin nanoparticle-based SARS-CoV-2 RBD vaccine induces a persistent antibody response and long-term memory in mice. Cell. Mol. Immunol. 2021, 18, 749–751. [Google Scholar] [CrossRef]
- He, L.; Chaudhary, A.; Lin, X.; Sou, C.; Alkutkar, T.; Kumar, S.; Ngo, T.; Kosviner, E.; Ozorowski, G.; Stanfield, R.L.; et al. Single-component multilayered self-assembling nanoparticles presenting rationally designed glycoprotein trimers as Ebola virus vaccines. Nat. Commun. 2021, 12, 2633. [Google Scholar] [CrossRef]
- Tokatlian, T.; Read, B.J.; Jones, C.A.; Kulp, D.W.; Menis, S.M.; Chang, J.Y.H.; Steichen, J.M.; Kumari, S.; Allen, J.D.; Dane, E.L.; et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 2019, 363, 649–654. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Bian, Y.; Wang, S.; Wang, S.; Chai, Q.; Guo, Z.; Wang, Z.; Zhu, P.; Peng, H.; et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 2020, 15, 406–416. [Google Scholar] [CrossRef]
- Petithory, T.; Pieuchot, L.; Josien, L.; Ponche, A.; Anselme, K.; Vonna, L.; Ponche, A.; Anselme, K.; Vonna, L. Size-dependent internalization efficiency of macrophages from adsorbed nanoparticle-Based monolayers. Nanomaterials 2021, 11, 1963. [Google Scholar] [CrossRef]
- Karger, A.; Pérez-Núñez, D.; Urquiza, J.; Hinojar, P.; Alonso, C.; Freitas, F.B.; Revilla, Y.; Le Potier, M.F.; Montoya, M. An update on African swine fever virology. Viruses 2019, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Niu, J.; Zhang, J.; Peng, Y.; Feng, X.; Huang, F.; Liu, J.; Li, S.; Chen, Z. Thermostable T Cell Multiepitope Nanoparticle Antigens Inducing Potent Immune Responses against the Swine Fever Virus. ACS Infect. Dis. 2023, 9, 2358–2368. [Google Scholar] [CrossRef]
- Jurtz, V.; Paul, S.; Andreatta, M.; Marcatili, P.; Peters, B.; Nielsen, M. NetMHCpan-4.0: Improved peptide-MHC class I interaction predictions integrating eluted ligand and peptide binding affinity data. J. Immunol. 2017, 199, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Vijver, S.V.; Danklmaier, S.; Pipperger, L.; Gronauer, R.; Floriani, G.; Hackl, H.; Das, K.; Wollmann, G. Prediction and validation of murine MHC class I epitopes of the recombinant virus VSV-GP. Front. Immunol. 2023, 13, 1100730. [Google Scholar] [CrossRef] [PubMed]
- Foged, C. Subunit vaccines of the future: The need for safe, customized and optimized particulate delivery systems. Ther. Deliv. 2011, 8, 1057–1077. [Google Scholar] [CrossRef]
- Plotkin, S.A. Vaccination against the major infectious diseases. Comptes Rendus Acad. Sci. III 1999, 322, 943–951. [Google Scholar] [CrossRef]
- Karch, C.P.; Burkhard, P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharm. 2016, 120, 1–14. [Google Scholar] [CrossRef]
- Khoshnejad, M.; Parhiz, H.; Shuvaev, V.V.; Dmochowski, I.J.; Muzykantov, V.R. Ferritin-based Drug Delivery Systems: Hybrid Nanocarriers for Vascular Immunotargeting. J. Control. Release 2018, 282, 13–24. [Google Scholar] [CrossRef]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef]
- Song, J.; Wang, M.; Zhou, L.; Tian, P.; Sun, Z.; Sun, J.; Wang, X.; Zhuang, G.; Jiang, D.; Wu, Y.; et al. A candidate nanoparticle vaccine comprised of multiple epitopes of the African swine fever virus elicits a robust immune response. J. Nanobiotechnol. 2023, 21, 424. [Google Scholar] [CrossRef]
- Xu, Z.; Kulp, D.W. Protein engineering and particulate display of B-cell epitopes to facilitate development of novel vaccines. Curr. Opin. Immunol. 2019, 59, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, A.; Rassek, O.; Vrohlings, M.; Marrero-Nodarse, A.; Moehle, K.; Robinson, J.A.; Ghasparian, A. An epitope-specific chemically defined nanoparticle vaccine for respiratory syncytial virus. NPJ Vaccines 2021, 6, 85. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Control | pFTH1 | MEPs | MEPs-pFTH1 | Normal Reference Range |
|---|---|---|---|---|---|
| WBC (109/L) | 16.58 ± 1.09 | 16.96 ± 1.54 | 17.58 ± 0.95 | 18.55 ± 1.27 | 7.00–20.00 |
| Neu% | 39.93 ± 2.35 | 40.74 ± 1.09 | 38.70 ± 2.21 | 38.34 ± 1.17 | 23.00–64.00 |
| Lym (%) | 45.77 ± 3.42 | 45.20 ± 1.45 | 46.24 ± 1.82 | 46.66 ± 1.60 | 26.00–67.00 |
| Mon% | 7.03 ± 1.07 | 6.27 ± 0.80 | 7.83 ± 1.55 | 6.07 ± 1.61 | 2.00–11.00 |
| Eos% | 6.47 ± 1.70 | 6.93 ± 1.76 | 6.47 ± 0.74 | 8.13 ± 1.05 | 0.50–9.00 |
| Bas% | 0.08 ± 0.30 | 0.87 ± 1.21 | 0.77 ± 0.15 | 0.80 ± 0.36 | 0.00–1.00 |
| Neu (109/L) | 6.61 ± 0.31 | 6.91 ± 0.69 | 6.80 ± 0.42 | 6.04 ± 1.24 | 2.00–7.00 |
| Lym (109/L) | 7.59 ± 0.80 | 7.65 ± 0.46 | 8.13 ± 0.45 | 9.74 ± 2.04 | 7.00–10.00 |
| Mon (109/L) | 1.17 ± 0.19 | 1.07 ± 0.20 | 1.39 ± 0.35 | 1.11 ± 0.23 | 0.12–1.20 |
| Eos (109/L) | 1.08 ± 0.35 | 1.19 ± 0.36 | 1.14 ± 0.12 | 1.50 ± 0.10 | 0.02–0.50 |
| Bas (109/L) | 0.13 ± 0.04 | 0.15 ± 0.04 | 0.14 ± 0.03 | 0.15 ± 0.06 | 0.00–0.10 |
| RBC (1012/L) | 8.07 ± 2.02 | 6.80 ± 0.73 | 7.18 ± 0.95 | 7.51 ± 0.74 | 6.00–8.50 |
| HGB (g/L) | 151.67 ± 20.31 | 131.67 ± 15.37 | 119.67 ± 6.66 | 119.33 ± 6.81 | 110.00–160.00 |
| HCT (%) | 47.23 ± 1.85 | 43.33 ± 4.21 | 40.37 ± 1.96 | 40.27 ± 1.76 | 37.00–54.00 |
| MCV (fL) | 56.00 ± 10.65 | 63.83 ± 0.70 | 56.93 ± 2.30 | 54.03 ± 7.58 | 80.00–100.00 |
| MCH (pg) | 17.77 ± 2.72 | 19.33 ± 0.58 | 16.87 ± 2.75 | 15.97 ± 2.47 | 27.00–34.00 |
| MCHC (g/L) | 320.20 ± 33.06 | 303.00 ± 9.85 | 296.00 ± 5.20 | 295.67 ± 4.93 | 320.00–360.00 |
| RDW-CV (109/L) | 17.63 ± 1.72 | 16.07 ± 0.55 | 17.93 ± 1.93 | 18.23 ± 1.62 | 11.00–16.00 |
| RDW-SD (fL) | 34.27 ± 3.98 | 36.57 ± 0.80 | 35.83 ± 2.30 | 34.90 ± 2.88 | 35.00–56.00 |
| PLT (109/L) | 303.00 ± 28.05 | 307.33 ± 50.36 | 305.00 ± 80.22 | 302.00 ± 37.24 | 100.00–400.00 |
| MPV (fL) | 7. 97 ± 0.95 | 7.97 ± 1.10 | 7.33 ± 0.61 | 7.37 ± 0.67 | 6.50–12.00 |
| PDW (%) | 16.17 ± 1.12 | 17.47 ± 0.49 | 16.43 ± 0.67 | 16.03 ± 0.49 | 15.00–17.00 |
| PCT (%) | 0.24 ± 0.10 | 0.27 ± 0.04 | 0.22 ± 0.07 | 0.20 ± 0.04 | 0.11–0.29 |
| Parameter | Control | pFTH1 | MEPs | MEP-pFTH1 | Normal Reference Range |
|---|---|---|---|---|---|
| TP (g/L) | 65.67 ± 2.36 | 63.67 ± 4.25 | 65.00 ± 2.50 | 63.33 ± 3.25 | 60.00–80.00 |
| ALB (g/L) | 34.57 ± 1.78 | 33.40 ± 2.54 | 33.70 ± 1.93 | 32.23 ± 1.22 | 18.00–38.00 |
| GLO (g/L) | 31.10 ± 1.51 | 30.27 ± 1.72 | 31.30 ± 0.69 | 31.77 ± 1.79 | 22.00–62.00 |
| A/G | 1.11 ± 0.08 | 1.10 ± 0.02 | 1.08 ± 0.05 | 1.02 ± 0.02 | 1.50–2.50 |
| TBIL (μmol/L) | 3.53 ± 1.03 | 3.70 ± 0.43 | 3.72 ± 0.85 | 3.44 ± 0.79 | 0.00–17.10 |
| ALT (U/L) | 36.33 ± 4.04 | 36.67 ± 2.08 | 37.33 ± 2.08 | 35.67 ± 3.51 | 9.00–58.00 |
| AST (U/L) | 34.33 ± 9.02 | 36.33 ± 2.08 | 36.00 ± 3.61 | 38.33 ± 3.06 | 16.00–84.00 |
| AST/ALT | 0.90± 0.20 | 1.00 ± 0.11 | 0.96 ± 0.06 | 0.79 ± 0.01 | 0.80–1.50 |
| GGT (U/L) | 42.83 ± 3.19 | 41.80 ± 3.20 | 44.30 ± 4.50 | 42.13 ± 3.43 | 31.00–52.00 |
| ALP (U/L) | 82.67 ± 13.58 | 81.00 ± 17.06 | 83.33 ± 12.06 | 85.33 ± 18.61 | 41.00–176.10 |
| TBA (μmol/L) | 0.79 ± 0.32 | 0.86 ± 0.40 | 0.81 ± 0.19 | 0.77 ± 0.22 | 0.00–15.00 |
| CK (U/L) | 301.33 ± 27.79 | 314.67 ± 43.84 | 287.67 ± 18.15 | 296.67 ± 27.06 | 50.00–689.40 |
| AMY (U/L) | 1823.67 ± 564.1 | 1906.67 ± 291.5 | 1934.00 ± 190.31 | 1858.62 ± 239.55 | 43.50–176.00 |
| TG (mmol/L) | 0.66 ± 0.11 | 0.82 ± 0.10 | 0.81 ± 0.09 | 0.76 ± 0.01 | 0.46–0.94 |
| CHOL (mmol/L) | 2.35 ± 0.57 | 2.34 ± 0.45 | 1.95 ± 0.08 | 2.10 ± 0.24 | 1.30–3.60 |
| GLU (mmol/L) | 5.57 ± 1.06 | 5.46 ± 0.67 | 4.92 ± 0.91 | 5.06 ± 0.27 | 4.72–8.89 |
| CRE (μmol/L) | 108.67 ± 18.50 | 111.00 ± 24.58 | 106.33 ± 16.17 | 108.67 ± 9.61 | 44.00–186.00 |
| BUN (mmol/L) | 4.30 ± 0.24 | 4.23 ± 0.24 | 4.42 ± 0.31 | 4.56 ± 0.41 | 2.10–10.70 |
| tCO2 (mmol/L) | 20.33 ± 0.58 | 20.33 ± 1.53 | 20.00 ± 1.00 | 20.00 ± 1.73 | 17.00–28.00 |
| Ca (mmol/L) | 2.48 ± 0.09 | 2.46 ± 0.11 | 2.28 ± 0.27 | 2.45 ± 0.14 | 1.63–2.90 |
| P (mmol/L) | 1.60 ± 0.09 | 1.62 ± 0.23 | 1.43 ± 0.36 | 1.41 ± 0.26 | 1.16–3.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Ding, Y.; Niu, J.; Li, Y.; Chen, Z. A Multiepitope Nanovaccine Candidate Adjuvanted with Porcine Ferritin Scaffold for African Swine Fever Virus. Vaccines 2025, 13, 585. https://doi.org/10.3390/vaccines13060585
Sun L, Ding Y, Niu J, Li Y, Chen Z. A Multiepitope Nanovaccine Candidate Adjuvanted with Porcine Ferritin Scaffold for African Swine Fever Virus. Vaccines. 2025; 13(6):585. https://doi.org/10.3390/vaccines13060585
Chicago/Turabian StyleSun, Lidan, Yuping Ding, Jingqi Niu, Yingjun Li, and Zeliang Chen. 2025. "A Multiepitope Nanovaccine Candidate Adjuvanted with Porcine Ferritin Scaffold for African Swine Fever Virus" Vaccines 13, no. 6: 585. https://doi.org/10.3390/vaccines13060585
APA StyleSun, L., Ding, Y., Niu, J., Li, Y., & Chen, Z. (2025). A Multiepitope Nanovaccine Candidate Adjuvanted with Porcine Ferritin Scaffold for African Swine Fever Virus. Vaccines, 13(6), 585. https://doi.org/10.3390/vaccines13060585






